The domestic cat (Felis silvestris catus) is adopted as a pet in millions of homes and can be considered one of the most popular pet animals worldwide. Cats were domesticated approximately 9000–10 000 years ago in the Near East(Reference Driscoll, Menotti-Raymond and Roca1) and are thought to originate from at least five distinctive subspecies of F. silvestris from across the Near East region, namely F.s. silvestris, F.s. lybica, F.s. ornate, F.s. cafra and F.s. bieti (Reference Driscoll, Menotti-Raymond and Roca1). After domestication, descendants were dispersed across the world with human assistance, and gave rise to today's domestic cat. A small subset of these domesticated cats has undergone intensive selection directed at specific aesthetic traits, leading to the development of so-called pedigree cats. Nowadays, forty-one breeds are recognised by the Cat Fanciers' Association, including sixteen ‘natural breeds’(2). These natural breeds are thought to be regional variants originating from domesticated F. silvestris subspecies(Reference Wastlhuber and Pederson3).
The initiation of domestication of wildcats is believed to have coincided with the change from the hunter–gatherer lifestyle of man in the Palaeolithic to the agricultural lifestyle in the Fertile Crescent about 12 000 years ago(Reference Driscoll, Menotti-Raymond and Roca1, Reference Vigne, Guilaine and Debue4). The permanent human settlements stored grains and middens, providing a new ecological niche for commensal species such as mice and rats. These rodents became peridomestic and provided a reliable food source for native wildcats. Wildcats then became adapted to the urban environment and became commensals like rodents(Reference Serpell, Turner and Bateson5). Wildcats in the urban environments were tolerated by people and, over time and space, gradually diverged from their ‘wild’ relatives by natural selection(Reference Driscoll, Macdonald and O'Brien6).
Overall, the domestic cat genome organisation is remarkably conserved compared with the human genome, with cats displaying the fewest number of chromosomal changes relative to man(Reference Murphy, Sun and Chen7, Reference O'Brien and Yuhki8). Domestic cats also have retained a behavioural repertoire, for example, the ability to hunt effectively(Reference Bradshaw9), which makes them very successful in the feral environment. The modern domestic cat still seems to largely resemble its wild ancestors genomically, morphologically and behaviourally. The formation of distinct breeds and the selection for breed-specific exterior traits over the past 50 years are unlikely to have resulted in major changes in the physiology and metabolism of certain breeds, as pedigree breeds are described as simple single-gene variants of natural breeds(Reference Lipinski, Froenicke and Baysac10).
The domestic cat's wild ancestors are known to be obligatory carnivores, consuming predominantly prey. The consumption of a diet composed of animal tissues throughout evolution has led to unique digestive and metabolic adaptations (often referred to as idiosyncrasies)(Reference Morris11–Reference Zaghini and Biagi14). Reduction of redundant enzymes and modification of enzyme activities will have had specific advantages in terms of energy expenditure(Reference Morris11). Examples of these adaptations include:
(a) The high dietary protein requirement as a consequence of a limited ability to decrease the enzyme activity of amino acid-catabolising enzymes below a certain threshold in response to a lowered protein intake(Reference Morris11). The fact that other carnivorous animals, including fish and birds, have developed the same adaptations in protein metabolism(Reference Migliori, Linder and Moura15–Reference Cowey, Cooke and Matty17) indicates an advantage to carnivorous species in general.
(b) An inability for de novo arginine synthesis because of reduced activity of two enzymes in the intestinal pathway of citrulline synthesis (pyrroline-5-carboxylate synthase and ornithine aminotransferase)(Reference Morris11).
(c) Two key enzymes in the pathway for taurine synthesis, namely cysteine dioxygenase and cysteinesulfinic acid decarboxylase, show low activities, thereby greatly reducing the endogenous synthesis of taurine and making this sulfonic amino acid an essential dietary nutrient for cats(Reference Morris11). In addition, cats and dogs use taurine almost exclusively as a source for bile acid conjugation, unlike other animals, which can use glycine when taurine is limiting(Reference Morris11).
(d) Cats are unable to use carotenoids to synthesise retinol because of a lack of carotene dioxygenase(Reference Morris11).
(e) Synthesis of vitamin D3 is prevented by the high activity of 7-dehydrocholestrol reductase, an enzyme that reduces the availability of the precursor for 25-hydroxyvitamin D(Reference Morris18).
(f) Cats are not able to synthesise niacin from tryptophan because of an extremely high activity of picolinic carboxylase. The activity of this enzyme is inversely related to niacin synthesis(Reference Morris11).
(g) Cats have a limited ability to synthesise arachidonic acid from linoleic acid, attributed to a low activity of Δ-6 and Δ-8-desaturase(Reference Morris11, Reference Macdonald, Anderson and Rogers19).
(h) Cats show several adaptations in the metabolism of starch and glucose, including a lack of salivary amylase activity, low activity of pancreatic and intestinal amylases(Reference Kienzle20, Reference Kienzle21), low hepatic glucokinase activity(Reference Washizu, Tanaka and Sako22), lack of hepatic fructokinase activity, necessary for metabolism of simple sugars(Reference Kienzle21, Reference Kienzle23) and a non-functional Tas1R2 receptor resulting in an inability to taste sugar(Reference Li, Li and Wang24).
The above-mentioned adaptations are thought to have evolved from nutrition solely based on animal tissues and highlight the carnivorous nature of cats. Although the latter is well recognised, there is a paucity of information on the precise dietary nutrient profile responsible for these physiological and metabolic adaptations of the domestic cat. Many published studies have investigated the feeding habits of free-ranging cats and specified the dietary items consumed. However, there is no information in the literature of the nutrient intake of cats from consumed dietary items.
The main objective of this literature study was to assess the nutrient profile to which the domestic cat's physiological and metabolic system has adapted. For this purpose, the feeding habits of feral cats (a free-ranging representative of the domesticated house cat) were reviewed and data on the nutrient composition of the different prey species were obtained from the literature. The nutrient profile (DM, crude protein (CP), ethereal extract (EE), N-free extract (NFE), ash, minerals and energy) of the diet of feral cats was calculated.
Methods
Literature search and selection
In the period from January to May 2010, electronic literature searches were conducted in Scopus and Web of Science to identify potentially eligible studies reporting diet compositions of free-roaming cats, as well as studies reporting whole-body nutrient composition of prey species consumed by cats. The literature search yielded fifty-five potentially eligible studies (Table 1). Eligibility of studies to include in the data analysis was based on four criteria. First, studies on feral cats were included whereas studies on the diet composition of wildcats were excluded. Second, for studies that used scat samples of cats for the assessment of diet composition, a criterion was set for the minimal number of collected scats samples. Trites & Joy(Reference Trites and Joy25) stated that a minimal sample size of ninety-four scats is required when comparing diets to distinguish moderate effect sizes over time or between areas. These authors also state that collecting too few samples increases the likelihood of not finding a species in a scat sample that is consumed in low numbers and that dietary preference of a single individual becomes a larger part of the sampling error. Therefore studies with a scat sample size lower than ninety-four were not included in the present study. Third, for studies that used stomach and/or gut samples of cats for assessment of diet composition, the minimal number of collected samples was arbitrarily set at thirty per study. Fourth, to guarantee the ‘wild’ and ‘human-independent’ feeding behaviour of the cats, studies in which human-linked foods (for example, food scraps, anthropogenic refuse, human refuse, human garbage, rubbish) contributed more than 5 % of the biomass consumed were not included. Based on these four criteria, twenty-eight studies were excluded (Table 1). The remaining twenty-seven eligible studies contained dietary information of feral cats based on 6666 stomach, gut and scat samples.
Table 1 Overview of the considered studies for inclusion in the calculations to determination the nutrient composition of feral cat diets

NP, not provided.
* Sample sizes shown for studies using stomach/gut contents exclude animals with empty stomachs/guts.
Diet composition
To standardise the comparison of results among studies, dietary item groups were created (see below) based on the information provided in the twenty-seven eligible studies. The category ‘mammals’ was split into subcategories ‘rodents’ (including rats, mice, voles, and other rodents), ‘rabbits’, ‘insectivores’ and ‘other mammals’.
Twenty studies reported the percentage of weight (PW) for each consumed dietary item as part of the total biomass consumed by feral cats and these data were used as reported by in these studies. The remaining seven studies reported the frequency of occurrence (FO) of dietary items in stomach, gut and/or scat samples. Data reported as FO are generally considered to underestimate the importance of large prey and overestimate the importance of small prey(Reference Fitzgerald, Turner and Bateson26). For this reason, in the studies where data were reported as FO, the PW for each dietary item was calculated according to Fitzgerald & Karl(Reference Fitzgerald and Karl27):
where Nprey i is the number of individuals of prey i within all samples, BMprey i is the biomass of prey i obtained from the literature, and Σ(Nprey × BMprey) was the total amount of biomass consumed. The biomass of identified prey groups was as follows: rats, 125 g(Reference Fitzgerald and Karl27); mice, 15·5 g(Reference Fitzgerald and Karl27); voles, 32·5 g(Reference Malo, Lozano and Huertas28); unidentified rodents and insectivores, 50 g (estimated mean weight of rodents/insectivores, based on data from Malo et al. (Reference Malo, Lozano and Huertas28), Fitzgerald & Karl(Reference Fitzgerald and Karl27) and Harper(Reference Harper29)); rabbits, 215 g ( = calculated daily fresh matter intake (FMI), see below); birds, 50 g(Reference Harper29); reptiles/amphibians, 3 g(Reference Malo, Lozano and Huertas28); fish, 15 g(Reference Fitzgerald and Karl27); invertebrates, 0·5 g(Reference Malo, Lozano and Huertas28).
Although vegetation (i.e. plant material and seeds) is found in scat and stomach samples of cats, it usually represents a minor to negligible component of the diet on weight basis(Reference Catling30–Reference Triggs, Brunner and Cullen32), and as a consequence was not taken into account in the calculations to PW.
Nutrient composition of diet
For the approximation of the nutrient composition of the diet consumed by the feral cats, the PW of each prey group was combined with compositional data of each of these groups. As these prey groups may contain several prey items, a mean value was calculated based on available data. Preferably compositional data of wild whole prey items were obtained from the literature (see Tables 3 and 4). No whole-body nutrient composition data were found in the literature for wild rats. Therefore data based on captive rats were used. Each separate study/diet composition (thirty in total) was used as an individual data point and all data were analysed with SPSS 16.0 for Windows (release 16.0.2; SPSS Inc., Chicago, IL, USA), using descriptive statistics.
Calculation of dietary intake
Daily FMI of cats was calculated based on estimated mean energy requirements for an average feral cat in a population. The energy requirement data for different age and reproductive classes were obtained from van Aarde et al. (Reference van Aarde33) and the percentage distributions of age and reproductive classes within a population of free-living feral cats were derived from Scott et al. (Reference Scott, Levy and Crawford34). These data were combined to obtain a mean daily metabolisable energy (ME) requirement of 1258 kJ per cat. The estimated mean ME content of prey was calculated using modified Atwater factors(35) with 3·5 × CP, 8·5 × EE and 3·5 × NFE. NFE was determined by difference as 100 – CP – EE – ash. The data are presented in Table 3. Daily FMI was calculated as the mean ME requirement divided by mean ME content of prey (585 kJ ME/100 g as is) resulting in 215 g fresh matter/feral cat per d. For prey items with a body weight exceeding the daily FMI, i.e. rabbits, the daily FMI was used instead of actual body weight for calculation of PW of that item to conform to the calculations by Fitzgerald & Karl(Reference Fitzgerald and Karl27).
Results
Dietary profiles
The twenty-seven articles included in the data analysis were carried out on four continents (North America, Europe, Africa and Australia) and included eighteen islands (Table 1). The dietary profiles of feral cats as reported in these twenty-seven studies are reported in Table 2. The main items consumed by feral cats are mammals (78 %), followed by birds (16 %), reptiles/amphibians (3·7 %) and invertebrates (1·2 %). Fish consumption is reported in three studies, and comprises of 0·3 % of the items consumed. The consumption of plant material is reported in twenty-one studies, and fifteen studies reported consumption of human-linked food items, with one study (study 47) reporting an intake of 3·0 % on a weight basis. Major mammals consumed are rabbits and rats although there is a large variation between studies. In one study (study 16), rats contributed 95·8 % to the total consumed biomass. On study sites where rabbits were abundantly present, they form a large proportion of the diet. On islands, the prey items consumed by feral cats differ markedly from that on the continents. Birds are an important part of the feral cat diet on islands where nesting sea birds are present. Marion Island (South Africa), an island with seabird colonies, nesting seabirds contribute to 81·3 % (study 5) and 96·6 % (study 55) to total biomass consumed (Table 2).
Table 2 Data of dietary profiles of feral cats found in the literature (% of weight)

− , Food item was not mentioned; +, food item was present but not clearly quantified.
* Data of studies 4, 5, 16, 43, 44, 46 and 55 were calculated from frequency of occurrence to percentage of weight as mentioned in the Methods section. Study numbers correspond to those in Table 1.
† Data of studies 23 and 29 were divided in subsets, as data were derived from different geographic locations (see also Table 1).
‡ Large mammals (>5 kg body weight, i.e. sheep, cattle, kangaroos) were included within the ‘carrion’ category. Mammal, bird and reptile carrion was not quantified precisely and is included within the totals for each group. Cat fur, non-organic and unidentified materials were included within the ‘unidentified’ category.
Macronutrient composition prey items
The whole-body macronutrient composition of different prey species obtained from various literature sources is shown in Table 3. Nutrients are expressed on a DM basis. The energy content is expressed as kJ ME/100 g DM. Energy contents of prey items varied reasonably, ranging from 1430 kJ/100 g DM for reptiles to 1917 kJ/100 g DM for other mammals. DM contents of prey species ranged from 24·8 % (reptiles) to 34·7 % (invertebrates). The CP content of prey items was relatively constant, ranging from 55·6 % DM for other mammals to 69·1 % DM for fish. The proportion of EE varied more widely between prey items, ranging from 9·0 % (reptiles) to 31·0 % DM (other mammals). NFE content varied considerably between 0 % (rats) and 12·9 % DM (invertebrates). The ash content of mammals was broadly similar, ranging from 9·4 % (rats) to 14·9 % DM (insectivores). The ash content of birds, reptiles, fish and invertebrates was 10·6, 15·2, 6·8 and 4·8 % DM, respectively.
Table 3 Macronutrient composition of dietary ingredients of the feral cat diet

ME, metabolisable energy; CP, crude protein; EE, ethereal extract; NFE, N-free extract (100 – CP – EE –ash).
* Derived from four different squirrel species.
† Derived from moles.
‡ Derived from ten different species of bats and opossums.
§ Derived from house sparrows.
∥ Derived from two different species of reptiles, commonly eaten by cats.
¶ Derived from three different species of fish, commonly eaten by cats.
** Derived from seven different species of invertebrates, commonly eaten by cats.
Micronutrient and trace element composition of prey items
The micronutrient and trace element compositions of the different prey items are given in Table 4. The Ca concentration of vertebrate species ranged between 2·6 and 3·8 % DM, while invertebrates contained only 0·1 % Ca on a DM basis. The P content ranged from 1·0 (invertebrates) to 2·7 % DM (voles). Na and K content showed a relatively wide variance, ranging from 0·35 (mice) to 0·83 % DM (other rodents) and 0·66 (birds) to 1·33 % DM (invertebrates), respectively. Mg content was fairly constant for most species, varying from 0·10 to 0·16 % DM, with the exception of the Mg content in voles, which was only 0·04 % DM. Fe, Cu and Zn content showed a wide variation between prey species, ranging from 7·8 (invertebrates) to 50·0 mg/100 g DM (insectivores), 0·74 (mice) to 12·24 mg/100 g DM (reptiles/amphibians) and 8·6 (rabbits) to 25·7 mg/100 g DM (invertebrates), respectively.
Table 4 Micronutrient and trace element composition of dietary ingredients of the feral cat diet

* Derived from fox squirrels.
† Derived from shrews.
‡ Data from rats, mice, voles, other rodents, insectivores and rabbits pooled together.
§ Data derived from five different bird species, commonly eaten by cats.
∥ Data derived from two different species of reptiles, commonly eaten by cats.
¶ Data derived from two different species of invertebrates, commonly eaten by cats.
Nutrient profile
Data presented in Tables 2 to 4 were used to calculate the nutrient profile of the natural diet of free-ranging feral cats. Fig. 1(a) displays the calculated macronutrient composition. The mean energy content of the natural diet was 1770 (sem 13) kJ/100 g DM, with the DM content being 30·5 (sem 0·4) %. The calculated mean macronutrient composition on a DM basis was 62·7 (sem 0·30) % CP, 22·8 (sem 0·5) % EE, 11·8 (sem 0·1) % ash and 2·8 (sem 0·3) % NFE. Fig. 1(b) shows the calculated micronutrient composition of the dietary profile of free-ranging feral cats, including the Ca:P ratio. A mean mineral content (in g/100 g DM) of 2·64 (sem 0·04) was found for Ca, 1·76 (sem 0·03) for P, 0·50 (sem 0·01) for Na, and 0·93 (sem 0·01) for K. The mean Ca:P ratio was 1·51 (sem 0·02). Trace element composition is shown in Fig. 1(c). The mean trace element content (in mg/100 g DM) was 130 (sem 4) for Mg, 29·6 (sem 1·1) for Fe, 1·67 (sem 0·12) for Cu and 9·77 (sem 0·19) for Zn.

Fig. 1 (a) Calculated macronutrient composition of the natural diet of free-ranging feral cats. CP, crude protein; EE, ethereal extract; NFE, N-free extract. The upper and lower hinges represent the 75th and 25th percentiles of the dataset. The band within the box represents the median. The whiskers extend to the 5 % and 95 % CI. The calculated means are: DM, 30·5 (sem 0·4) g/100 g; CP, 62·7 (sem 0·3) g/100 g DM; EE, 22·8 (sem 0·5) g/100 g DM; NFE, 2·8 (sem 0·3) g/100 g DM; ash, 11·8 (sem 0·1) g/100 g DM; energy, 1770 (sem 13) kJ/100 g DM. (b) Calculated micronutrient composition of the natural diet of free-ranging feral cats. The upper and lower hinges represent the 75th and 25th percentiles of the dataset. The band within the box represents the median. The whiskers extend to the 5 % and 95 % CI. The calculated means are: Ca, 2·64 (sem 0·04) g/100 g DM; P, 1·76 (sem 0·03) g/100 g DM; Na, 0·50 (sem 0·01) g/100 g DM; K, 0·93 (sem 0·01) g/100 g DM; Ca:P, 1·51 (sem 0·02). (c) Calculated trace element composition of the natural diet of free-ranging feral cats. The upper and lower hinges represent the 75th and 25th percentiles of the dataset. The band within the box represents the median. The whiskers extend to the 5 % and 95 % CI. The calculated means are: Fe, 29·59 (sem 1·08) mg/100 g DM; Cu, 1·67 (sem 0·12) mg/100 g DM; Zn, 9·77 (sem 0·19) mg/100 g DM; Mg, 130 (sem 4) mg/100 g DM.
Discussion
Knowledge about the feeding strategies, food items consumed and composition of the natural diet of man and animals provides valuable insights for the formulation and selection of appropriate diets to maintain health. The natural diet of humans has received much attention over the past decades(Reference Eaton and Konner36–Reference Strohle and Hahn41) and has provided new information regarding the nutritional composition of the diet to which evolutionary forces adapted the core metabolism and physiology over a period of millions of years(Reference Eaton and Konner36, Reference Mann40, Reference Cordain, Eaton and Sebastian42). Frassetto et al. (Reference Frassetto, Schloetter and Mietus-Synder39) investigated whether a natural diet confers health benefits in human subjects and found that even short-term consumption of a Palaeolithic-type diet has proven health benefits for glucose metabolism and the cardiovascular system. In addition, the composition of breast milk has provided valuable information about the dietary nutrient profile to meet the nutrient requirement for optimal health and development of human infants(Reference Raiten, Talbot and Waters43). For many captive, endangered and domesticated animal species, the study of the natural diet has yielded data to successfully improve their nutrition(Reference Potter, Hendriks and Lentle44–Reference Cottam, Merton and Hendriks48).
Here we report the nutrient profile of free-ranging feral cats using reported rates of ingestion of various dietary (prey) items in the literature. As expected, the results of the present study clearly show that feral cats are true carnivores, with the daily energy intake of feral cats from protein being 52 %, from fat 46 % and from NFE only 2 %. Interestingly, a recent study by Hewson-Hughes et al. (Reference Hewson-Hughes, Miller and Hall49) on voluntary macronutrient selection by adult domestic cats showed that when given the choice, adult cats select an intake target of about 420 kJ/d from protein, about 280 kJ/d from fat and about 100 kJ/d from carbohydrate, representing 52 % of daily energy intake from protein, 36 % from fat and 12 % from carbohydrate. These results are highly similar to the data presented here, indicating that cats appear to have developed, in addition to the above-mentioned metabolic adaptations, sensitive metabolic regulation mechanisms to consume an overall dietary macronutrient profile close to their evolutionary diet. The nutrient profile provides information to further enhance today's feline diets.
In the present study we used feral cats as a free-ranging model for domestic cats. Feral cats are described in the literature as cats which are descended from domestic cats, but are born and live without human contact and have survived in an ecosystem for many generations(Reference Achterberg and Metzger50–Reference Pearre and Maass52). The domestic (feral) cat and wildcats are able to create fertile progeny(Reference Pierpaoli, Biro and Herrmann53), which shows the close genetic resemblance between the wildcat population and domestic feral cats. Also, the behavioural repertoire of the feral cat to hunt effectively is remarkably conserved, with feral cats displaying similar hunting methods to wildcats(Reference Corbett54). The genetic variation between domestic and feral cats is negligible and metabolic adaptations are not likely to differ, making the feral cat a highly suitable free-roaming model for the domestic house cat.
As described above, the cat's metabolism has adapted to a carnivorous lifestyle with many of the known adaptations relating to the protein, carbohydrate and vitamin component of the diet. Almost all the metabolic adaptations related to the carbohydrate component of the diet indicate the lack of this nutrient in the evolutionary diet. It could be argued that the shift from an obligatory meat-based natural diet to a meat-based and grain-based pet food rich in carbohydrates may place the cat's metabolism under stress, and might have unwanted negative health effects in the long run. Although dietary carbohydrate intake could not directly be determined in the present study, the NFE content was calculated. The fraction consists of components such as sugars, starches, mono- and disaccharides, but also water-soluble vitamins. Animal tissue itself contains small amounts of glucose, glycogen, glycoproteins, glycolipid and pentose but does not contain starch. However, when consuming whole prey, the digesta of prey items may contain some starch. These carbohydrate sources may be the reason why cats have retained a limited ability to digest starch. The starch content of prey species is difficult to assess, as it is primarily based on the diet consumed. However, as an example, the following calculation provides an indication of the magnitude of starch ingestion by feral cats. The starch content of the digesta of captive young rabbits can be up to 130 g/kg DM, depending on the starch source(Reference Blas, Gidenne, de Blas and Wiseman55). Assuming that the mean starch content of the digesta is approximately 100 g/kg DM, digesta moisture content 80 %(Reference Carabaño, Piquer, Menoyo, de Blas and Wiseman56) and the digesta mass of rabbits 10 %(Reference Livingston57), the calculated starch content of a rabbit weighing 1·5 kg is 3·0 g (0·2 % body weight). Wild rabbits forage primarily on grasses and leafy weeds, with high contents of fibre and relatively low contents of starch, making the latter a large overestimate. Prey species consumed by cats show considerable differences in digestive tract anatomy, with the digesta mass of rabbits being as high as 10 % of body mass(Reference Livingston57), while omnivorous species such as the rat have a digesta mass of 0·5–2 % of body weight(Reference Danielson, Newman and Newman58). The ability of cats to secrete pancreatic amylase may be beneficial in utilising the glycogen content of prey. Based on the above calculations, it can be concluded that the NFE content reported in the present study contains little starch and as such is composed of other fibrous material. Twenty-one of the twenty-seven studies reported small amounts of plant material being found in the scats, stomach and gut content of feral cats. Molsher et al. (Reference Molsher, Newsome and Dickman59) reported that cats frequently consume vegetation (FO of 26·3 %) consisting mostly of a few strands of grass. The authors concluded, however, that plant material is a minor component of the diet of feral cats, as ingestion is likely to occur incidentally while foraging for invertebrates.
The physiological minimum nutrient requirements of cats for growth, maintenance and late gestation/peak lactation have been accurately determined(35) and can be considered to represent the limit of the adaptation capacity of domestic cats in relation to dietary nutrient concentrations. Table 5 provides the minimum nutrient requirements and the recommended allowance of cats as provided by the National Research Council expressed in units/MJ ME. As can be seen from Table 5, there is a large difference between the recommended CP allowance and the CP content consumed by free-roaming feral cats. The data presented here on the evolutionary diet of cats do not include digestibility and bioavailability estimates of the different nutrients, making direct comparison with the recommended allowance more difficult. Estimates of macronutrient digestibility of whole prey items can be extrapolated from the literature on whole-prey assimilation by bobcats(Reference Powers, Mautz and Pekins60) and ocelots(Reference Bennett, Booth-Binczik and Steele61). In the study with bobcats, Powers et al. (Reference Powers, Mautz and Pekins60) evaluated the nutritive and energy value of winter diets of bobcats. Amongst others, a diet comprising of four species of rodents (mice and voles) was fed to four bobcats of wild origin. The apparent digestibility of CP and EE was 82·0 and 92·3 %, respectively. In the recent study by Bennett et al. (Reference Bennett, Booth-Binczik and Steele61), six diets (a commercial processed diet and five species of whole prey) were fed to a total of six ocelots to evaluate nutrient digestibility. The diets had similar digestibility values, with CP digestibility ranging from 85 to 91 %, and EE digestibility ranging from 96 to 99 %. The outcome of these studies makes the use of modified Atwater coefficients (in which protein and fat digestibility are estimated as 79 and 90 %, respectively(35)) for energy prediction of whole prey defendable but also the comparison of the recommended CP requirements of cats and the evolutionary CP intake. Data on bioavailability of micronutrients and trace elements in felids consuming whole prey items are lacking. Further research is needed to determine the precise nutrient digestibility of the natural diet, especially with respect to minerals such as Ca, P, Mg and Fe, which are consumed in relatively high concentrations compared with recommended allowances determined using empirical methods. It is likely that the absorption of minerals such as Ca and P is much lower in prey items compared with the forms used to supplement commercial feline diets.
Table 5 Recommended nutrient composition v. assessed nutrient composition of the ‘natural’ cat diet
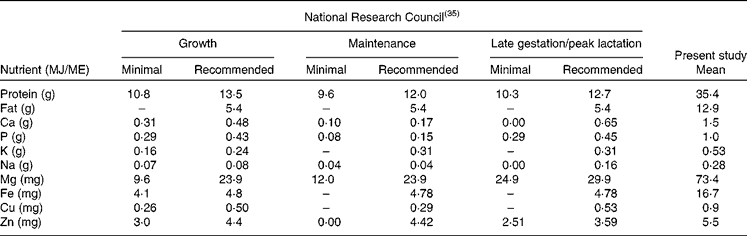
ME, metabolisable energy.
Information on the precise nutrient digestibility of the feral cat diet would allow conversion to a nutrient profile (and nutrient ratios) to which the cat's metabolism has been exposed during evolution. This nutrient profile originates from a cat population in which nutrition is a precondition for survival and procreation. In domestic cats, the nutritional goals may have gone beyond this, and are based on optimising health and longevity and as such may not be optimal. The median lifespan of a feral cat has been reported to be 4·7 years(Reference Levy, Gale and Gale62), while the domestic house cat has an average life expectancy of 12–14 years(Reference Taylor, Adams and Neville63). Although such a nutrient profile may reflect the profile to which the cat's metabolic system has adapted, the question is whether it may be considered ‘optimal’ for today's nutritional goals in pet feeding. However, as stated earlier, valuable insights may be gained by an approach of studying the diet of feral cats. For example, the fatty acid composition is known to be influenced by the nutritional fatty acid intake in both humans and animals(Reference Beynen, Hermus and Hautvast64, Reference Plantinga, Van Dijk and Van Niel65). The fatty acid composition, especially the PUFA content and the n-6:n-3 ratio, differed considerably between wild or free-ranging animals and captive or feedlot animals. The n-6:n-3 ratios in captive or feedlot animals range between 6:1 to 19:1(Reference West and Coady66–Reference Vicenti, Ragni and di Summa68). It can be calculated that a diet based on wild animal species contains a ratio about 2:1(Reference West and Coady66, Reference Rule, Broughton and Shellito67, Reference Conway, Eddleman and Simpson69, Reference Cobos, Delahoz and Cambero70). Domestic cats are fed commercially prepared foods containing lipids from captive domestic animal species and thus will consume a different fatty acid pattern compared with feral cats. For instance, the typical n-6:n-3 ratios in dog foods ranges between 5:1 to 17:1(Reference Ahlstrom, Krogdahl and Vhile71).
In addition to insights into the dietary nutrient intake, it is also important to note that non-nutritive properties, such as food consistency, texture, taste and temperature may play an important role in maintaining optimal health and function. Bond & Lindberg(Reference Bond and Lindburg72), in an investigation of the effect of feeding whole carcasses to captive cheetahs compared with feeding a commercial diet, concluded that feeding a more naturalistic diet may better meet a cheetah's physical, physiological and nutritional needs. In addition, feed consistency and texture have shown to be important in maintaining a balanced microbial population in the gastrointestinal tract in different animal species(Reference Huang, Li and Xing73, Reference Mikkelsen, Naughton and Hedemann74). Moreover, consumption of whole prey provides for a relatively high intake of raw animal-derived fermentative substances, such as cartilage, collagen and glycosaminoglycans, which may enhance gut health, stimulate growth of a different subset of microbial commensals, and optimise immune function in a different way compared with consuming foods which are for a large part derived from plant origin and heat-treated.
Compositional data of prey species frequently preyed upon by feral cats are not abundantly available in the literature. For most mammals, data from wild-living animals could be obtained, with the exception of the rat. The rat data originate from captive rat species, which might explain the somewhat higher fat content compared with wild mice and voles (30·5 v. 24·5 and 17·2 % DM, respectively). Also, with regard to the micronutrient and trace element composition of the different prey species (Table 4), some interesting results were found. First, the Fe content of insectivores (50·0 mg/100 g DM) and birds (49·6 mg/100 g DM) is nearly twice as high as for the other species. The group of insectivores includes soil-dwelling species, like moles and some shrews, which, because of their underground lifestyle, have undergone specific haematological changes. These changes include a higher serum Fe content, a higher Hb content, and a higher Fe-binding capacity of the blood(Reference Quilliam, Clarke and Salsbury75) and are thought to facilitate the uptake of O2 in an environment of reduced O2 and increased CO2 tension. The same is true for flying birds. Flying is one of the most O2-consuming activities, and facilitating O2 uptake through haematological changes enables flying birds to carry out this strenuous activity. Garcia et al. (Reference Garcia, Ramis and Planas76) found that the Fe concentration per unit body weight of starling birds ranged from 153 to 185 parts per million, two to four times higher than values for mammals and non-flying birds. Second, the relatively high Cu and Zn contents for reptiles and amphibians may be overestimated. The reported values of both minerals in Carolina anoles (35·3 and 31·5 mg/100 g DM, respectively) by Dierenfeld et al. (Reference Dierenfeld, Alcorn and Jacobsen77) differ considerably from those reported by Cosgrove et al. (Reference Cosgrove, Beermann and House78) (0·5 and 14·3 mg/100 g DM, respectively). The latter Cu and Zn contents of Carolina anoles are comparable and within the ranges of mammalian species (Table 4). Considering the finding that reptiles and amphibians only marginally contribute to the total energy intake of free-ranging feral cats (Table 2) and the fact that compositional data for reptiles and amphibians in literature are scarce, it was decided not to exclude the study of Dierenfeld et al. (Reference Dierenfeld, Alcorn and Jacobsen77). The relatively high Cu and Zn content of invertebrates is a normal pattern seen in many invertebrate species, both terrestrial and aquatic. Invertebrates are thought to be susceptible to accumulating heavy metals, especially Cd, Zn and Cu(Reference Boháč, Pospíšil and Vernet79). Nevertheless, the nutrient composition of the different species provides an indication of the range of nutrient intakes of feral cats. Further compositional data of prey items consumed in conjunction with digestibility data would provide more robust estimates and ranges of the nutrient intake and metabolic exposure to nutrients of cats.
The methods used in the present study are open to criticism. The studies that were used to assess the dietary composition of feral cats used different methods (scats v. stomach content) and expressed their results in different ways (FO v. PW). These various ways of studying the diet and expressing results might produce biases that must be kept in mind. For example, identification of prey remains is more difficult in scat analysis than in analysis of stomach content. As a consequence, prey that was consumed in lesser amounts can more easily be overlooked when using scat analysis for dietary habit assessment. However, the major features of the diet of cats are thought to be sufficiently robust to be revealed despite the differences in methodology(Reference Fitzgerald, Turner and Bateson26). In addition, results expressed as FO were converted to PW to standardise the comparison of results. In these calculations assumptions needed to be made such as the mean weight for each prey item consumed, as described by Fitzgerald & Karl(Reference Fitzgerald and Karl27). For prey items with body weights exceeding the daily FMI, i.e. rabbits, the daily FMI (215 g fresh matter) was used instead of actual body weight for calculation of PW. These calculations rely on the assumption that when a cat catches a rabbit, it consumes its full daily energy requirement in fresh matter (215 g). However, when a fasting cat catches a large prey item it may eat far more than 215 g of fresh matter. Jones & Coman(Reference Jones and Coman80) investigated the mean weights of rabbits eaten per meal by cats and calculated a mean FMI of 269–274 g. This would imply that the contribution of rabbits to the dietary profile in the present study may be underestimated. On the other hand, a cat may eat less than 215 g of fresh matter if a larger part of its daily energy requirement is already met by the previous consumption of smaller prey items. In this situation the contribution of rabbits to the dietary profile may be underestimated. In the literature, a large variation is found in the assessment of daily FMI of free-ranging cats to calculate PW. Fitzgerald & Karl(Reference Fitzgerald and Karl27) estimated the daily maximum FMI within a cat population to be 170 g, while, as previously mentioned, Jones et al. (Reference Jones and Coman80) calculated the mean FMI for rabbits to be 269–274 g. On average, the calculated daily FMI in the present study reflects the mean of the data range found in the literature. It should also be noted that most of the studies made year-round observations of the dietary habits of the feral cat, which means that seasonal fluctuation in nutrient intake were not taken into account. Also the studies used here for the calculation of the dietary nutrient profile of feral cats were carried out on different continents and islands, with the prey items consumed varying markedly between the different studies. For example, birds were a more important part of the feral cat diet on islands compared with continents (Table 2). These differences are probably related to latitude, climate and species diversity, and show that cats are general, opportunistic predators, exploiting a wide range of prey(Reference Fitzgerald, Turner and Bateson26). However, a dietary nutrient composition was developed from each individual study (n 30), and a mean (and standard error) nutrient composition calculated. The differences in prey profile between studies are thus reflected in the standard error, which is remarkably small for most nutrients. Overall, the approach taken in assessing the nutrient intake of feral cats can be criticised. However, the relatively large number of studies used (thirty data points from twenty-seven studies, yielding a total of 6666 samples) to calculate the nutrient intake, combined with the small range in nutrient composition between prey items makes the current estimates relatively robust.
Conclusion
The present study provides estimates of the gross nutrient intake of feral cats based on literature data of thirty different food consumption patterns. The calculated diet consists of 69·5 % water, and contains 62·7 % CP, 22·8 % EE, 11·8 % ash and 2·8 % NFE on a DM basis. The starch content of the NFE fraction is low. The fatty acid profile consumed by feral cats has a ratio of n-6:n-3 in the order of 2:1, which differs from the ratio consumed by pet cats (ranging between 5:1 to 17:1). Additional data on specific prey item composition combined with estimates of the nutrient availability of prey items or a composite diet would provide more accuracy to derive a metabolic nutrient profile to which cats have adapted throughout evolution. Future research focus on the nutritive as well as non-nutritive value of consuming a natural diet of whole prey may gain valuable insights into how the nutrition of domestic cats can be further enhanced to increase health and longevity.
Acknowledgements
All authors contributed fundamentally to the present study. E. A. P. contributed to all facets, including research design, data collection, statistical analysis, interpretation and manuscript preparation. G. B. and W. H. H. contributed to research design, data interpretation and manuscript preparation.
The present study received no specific grant from a funding agency in the public, commercial or not-for-profit sectors, and therefore the present study is free of any conflicts of interest.








