Dietary fibre consumption may influence appetite and energy intake through various mechanisms, including reducing the energy density of foods, increasing sensory satiety through increased mastication, increasing stomach distension, delaying gastric emptying and nutrient absorption, altering the secretion of gut hormones and increasing production of SCFA through fermentation in the colon( Reference Howarth, Saltzman and Roberts 1 – Reference Clark and Slavin 3 ). Moreover, fibre type and physiochemical properties such as viscosity, solubility and fermentability may be more influential on satiety compared with total dietary fibre intake alone( Reference Wanders, van den Borne and de Graaf 2 ).
Inulin-type fructan is a generic term that encompasses native inulin (chain length 2–60, average degree of polymerisation (DP)=10–12), long-chain inulin (chain length 11–60, average DP=25) and oligofructose (chain length 2–10, average DP=4, aka short-chain fructo-oligosaccharides)( Reference Raninen, Lappi and Mykkänen 4 , Reference Roberfroid 5 ). Owing to their β(2–1) linkages, these fructose-based oligomer and polymer molecules are non-digestible( Reference Niness 6 ). They act as prebiotics and are rapidly and selectively fermented in the colon( Reference Roberfroid 5 ). Oligofructose can be used as a sweetener and is produced synthetically or by hydrolysing inulin( Reference Niness 6 ) and was not the focus of this study. The longer chain molecules of inulin are generally water soluble but contribute minimal viscosity without off-flavours. These properties make inulin ingredients attractive for enriching the dietary fibre contents of foods and beverages. Oligofructose has shown promise in reducing appetite( Reference Cani, Joly and Horsmans 7 , Reference Whelan, Efthymiou and Judd 8 ) and decreasing food intake( Reference Cani, Joly and Horsmans 7 , Reference Parnell and Reimer 9 – Reference Hess, Birkett and Thomas 11 ) in most but not all( Reference Peters, Boers and Haddeman 12 ) studies. However, inulin specifically is under-represented in the literature. Few interventions have studied the effect of consumption of inulin alone on satiety, and more research is needed to elucidate its specific role in acute and chronic studies.
Chronic consumption of inulin has been studied extensively with respect to glycaemia, lipaemia, colonic health and body weight( Reference Raninen, Lappi and Mykkänen 4 , Reference Roberfroid 5 , Reference Meyer and Stasse-Wolthuis 13 , Reference Liber and Szajewska 14 ). Possible influences have also been suggested in terms of appetite and satiety. For example, the production of colonic SCFA from inulin fermentation( Reference Tarini and Wolever 15 ) may impact satiety by increasing plasma satiety hormones peptide YY( Reference Cani, Lecourt and Dewulf 16 ) and glucagon-like peptide-1( Reference Tarini and Wolever 15 , Reference Delzenne, Cani and Daubioul 17 ) and decreasing circulating plasma ghrelin( Reference Tarini and Wolever 15 ). However, human evidence of a colonic SCFA effect on future appetite and energy intake is inconclusive( Reference Darzi, Frost and Robertson 18 ), and the potential for chronic consumption of inulin, specifically, to impact satiety has been insufficiently studied and the results remain equivocal. Of the few studies that have tested inulin alone or in combination with oligofructose, there is no consensus on the dose or duration to see an effect on satiety or energy intake in healthy adults. A parallel study of daily consumption of 16 g mixed inulin–oligofructose supplements for 2 weeks showed reduced Hunger ratings at 180 min compared with baseline only and had no effect on total energy intakes in ten healthy young adults( Reference Cani, Lecourt and Dewulf 16 ). In contrast, Tulk et al.( Reference Tulk, Blonski and Murch 19 ) observed that consumption of 4 g of inulin added to a commercial yogurt for 15 d reduced total energy intakes compared with yogurt alone in healthy young adults, although appetite was not evaluated. Systematic reviews on prebiotics and satiety have concluded that long-term consumption of inulin-type fructans (inulin or oligofructose) is associated with reductions in energy intake and body weight( Reference Wanders, van den Borne and de Graaf 2 , Reference Liber and Szajewska 14 ) or decreased self-reported satiety( Reference Kellow, Coughlan and Reid 20 ). However, the studies included in those reviews were also heterogeneous in terms of fructan type and dose, complicating the ability to discern which molecules are efficacious. In particular, Wanders et al.( Reference Wanders, van den Borne and de Graaf 2 ) and Kellow et al.( Reference Kellow, Coughlan and Reid 20 ) pooled inulin and oligofructose study results in their analyses. The necessary pooling of inulin and oligofructose interventions exemplifies how scarce the research is on inulin in particular. Therefore this study aimed to focus on the distinct contributions of inulin on satiety.
Short-term effects of inulin consumption on appetite and energy intake, although suggested, also have not been concluded( Reference Wanders, van den Borne and de Graaf 2 , Reference Clark and Slavin 3 , Reference Liber and Szajewska 14 , Reference Darzi, Frost and Robertson 18 ). The few human studies in this area have shown mixed results. For example, 6 g of inulin added to yogurt increased postprandial feelings of fullness and reduced subsequent ad libitum energy intake in healthy young adults( Reference Perrigue, Monsivais and Drewnowski 21 ). About 5 g of inulin added to 100 g water reduced ad libitum lunch and total day energy intakes in slightly overweight women( Reference Harrold, Hughes and O’Shiel 22 ), and 24 g of inulin added to a sausage breakfast patty as a fat-replacer reduced 24-h energy intake in healthy men( Reference Archer, Johnson and Devereux 23 ). However, no acute effects on appetite ratings or energy intakes were found after one-time consumption of chocolate bars containing 10 g inulin in healthy young women( Reference Karalus, Clark and Greaves 24 ) or 22·4 g long-chain inulin split between standard breakfast and lunch meals in healthy adults( Reference Darzi, Frost and Robertson 18 ). There have been no systematic reviews on the effects of acute inulin consumption alone on postprandial satiety and subsequent food intake, and transparent, well-controlled clinical trials are needed.
Therefore, although inulin is touted as having the potential to modulate satiety, controlled studies are lacking. Despite being easily incorporated into food products, it may not be as effective as other fibre types in terms of enhancing satiety and attenuating energy intake. The objective of this study was to determine the influence of inulin on satiety measures using a crossover preload study design, with repeated at-home consumption. This was accomplished by adding inulin to a commercially available yogurt served as part of a breakfast meal and determining Day 1 and Day 8 appetite ratings and subsequent energy intakes in healthy young women. It was hypothesised that 8-d consumption of the yogurt with 6 g added inulin would result in significantly lower scores of Hunger, Desire to Eat and Prospective Food Consumption (PFC) and significantly higher Fullness scores, with associated decreases in energy intakes compared with the control yogurt.
Methods
Participants
Healthy young female participants were recruited from the University of Guelph and surrounding area through poster, online and newspaper advertisements. Young women are not commonly studied alone in satiety research, particularly with consideration of influences of female hormones on appetite and food intake. Initial eligibility was assessed by phone. Before the in-person screening visit, participants completed a 3-d food record (2 weekdays and 1 weekend day), which was reviewed with a study coordinator.
Participants met the following inclusion criteria: aged 20–35 years, healthy, BMI≥18·9 and ≤26·1 kg/m2, regular yogurt consumers (≥3 servings/week) and typical breakfast consumers (before 09.00 hours 5 d/week) with an average breakfast energy intake of 837–2092 kJ (200–500 kcal). Participants were also required to consume at least 15 % of daily energy from protein and to have been consistently using combination hormonal contraceptives for at least 3 months with regular monthly menstrual cycles.
Exclusion criteria consisted of the following: presence of any medical condition, including gastrointestinal disorders, regular medication use (besides hormonal contraceptives), any food allergies and all anaphylactic allergies, smoking and recreational drug use. Elite or training athletes were excluded, along with persons trying to lose or gain weight or whose body weight had changed >5 kg within the previous 6 months. Volunteers were excluded if they scored ≥11, 9 or 8 on the Cognitive Restraint, Disinhibition or Hunger scales, respectively, on the Three-Factor Eating Questionnaire( Reference Stunkard and Messick 25 ), disliked the study foods or had unusual dietary or sleep patterns. Additionally, persons regularly consuming a high number of caffeinated (>4 drinks/d) or alcoholic drinks (>14 drinks/week), <12 or >50 g of dietary fibre/d or taking fibre supplements or who were pregnant or breast-feeding were also excluded. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the University of Guelph Research Ethics Board (#11AP036). The study was registered with clinicaltrials.gov registry (NCT01379911), and all participants provided written informed consent.
Study design and protocol
A double-blind controlled crossover design with simple randomisation was used. Separate parties enrolled participants in the study and allocated the intervention. Both treatment periods consisted of 8-d yogurt consumption with study visits on Days 1 and 8. Treatment periods were separated by a 3-week washout so that all four study visits were scheduled during the follicular phase of each participant’s menstrual cycle. For the duration of the study, participants were asked to avoid yogurt products other than those provided. The day before each visit, participants were asked to avoid alcohol and physical exercise and to record their gastrointestinal function and food intake in study documents.
On study visit days, participants arrived at the Human Nutraceutical Research Unit (HNRU) at the University of Guelph after a 12-h overnight fast. Participants consumed the yogurt breakfast (at 08.50 hours) and remained in the HNRU for the duration of the study visit (4 h). Appetite was assessed periodically using paper 100 mm visual analogue scales (VAS), and participants consumed an ad libitum pizza lunch (at 11.50 hours) from which food intake was covertly measured. To avoid influencing appetite ratings and food intake, participants were instructed not to discuss food during the study visits and were not aware that satiety was being measured. They were told that they were participating in a yogurt liking study investigating whether yogurt preference had an effect on food choice. After completion of the study, participants attended a follow-up visit where the true objective was disclosed, at which point they were provided the option of removing their data from the analysis. The follow-up visits took place between May 2013 and April 2014. Food intake was recorded for the remainder of each study day using provided food records, and a gastrointestinal questionnaire (GIQ) was completed the following day.
Breakfast meal
The control yogurt (yogurt-control (YC)) was a commercially available vanilla-flavoured yogurt (Activia; Danone). A quantity of 6 g of TIC Pretested® Inulin LV 110 Powder from chicory root (Nealanders International Inc.) was added to each 100 g serving of treatment yogurt (yogurt-inulin (YI)). As per the manufacturer’s specifications, the inulin was a low viscosity product with a DP of approximately 10. The quantity of 6 g of inulin was chosen to be comparable to other studies where a satiating effect was observed with a yogurt matrix( Reference Tulk, Blonski and Murch 19 , Reference Perrigue, Monsivais and Drewnowski 21 ) and as an acceptable dose for gastrointestinal tolerance( Reference Bonnema, Kolberg and Thomas 26 ). In pilot studies, 6 g inulin was undetectable by taste or texture (results not shown). Yogurt portions in 200 ml opaque coded plastic containers were prepared 3–5 d in advance of each study visit by a third party who was not involved with data collection or analysis.
The nutritional information for the breakfast meal is shown in Table 1. A commercially available strawberry-flavoured breakfast bar (Kellogg’s Special K Bar®; Kellogg Canada Inc.) and 125 ml of cool water were consumed with the study yogurt. Participants were asked to consume the entire meal within 10 min.
Table 1 Nutritional information for the yogurt-control and yogurt-inulin products and breakfast barFootnote *
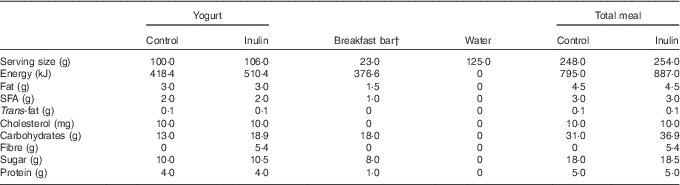
* As per product label Nutrition Facts tables, including the inulin soluble fibre energy density of 17 kJ/g (4 kcal/g) as per Food and Drug Administration regulations.
† The last nine participants consumed a slightly different formulation of the Special K breakfast bar, due to manufacturer changes. Product energy content did not change, but SFA and fibre were, respectively, 0·5 and 1 g in the new bars.
Appetite ratings
VAS were used to measure feelings of appetite (Hunger, Fullness, Desire to Eat and PFC) every 30 min (30, 60, 90, 120, 150 and 180 min). Each consisted of a horizontal 100 mm line with opposing anchors at each end (e.g. ‘not hungry at all’ and ‘extremely hungry’)( Reference Blundell, de Graaf and Hulshof 27 ). To distract participants from the real purpose of the study, VAS questions relating to characteristics of the yogurt and participants’ current state, for example, tiredness or thirst, were included but are not presented.
Ad libitum lunch
Participants were served an ad libitum pizza lunch and instructed to eat until comfortably full. Delissio four-cheese thin crust frozen pizza (Nestlé) was prepared according to the directions and served to participants on three different plates (220 g/plate) for a total of 660 g. The pizza had no crust around the edges and was cut into pieces of different sizes and shapes. Participants were given 6 min with each plate that was weighed before and after each serving. A 500 ml glass of cool water was provided with the lunch. Pizza and water intake were covertly measured. Energy intakes were calculated using the product label nutritional information presented in Table 2.
Table 2 Nutritional information for the lunch pizzaFootnote *

* The last three participants consumed a slightly different pizza formulation (containing 50 kJ less/109 g portion) at the second study visit, due to manufacturer changes.
Total day energy intakes
After leaving the HNRU on Days 1 and 8, participants recorded their food intake for the remainder of each day. Participants were thoroughly instructed on how to complete detailed entries and were provided with references to estimate serving sizes. The submission of homemade recipes and food labels was encouraged to improve accuracy. Food records were reviewed with a research coordinator and analysed by a consistent research coordinator for determination of energy intakes (kcal; converted to kJ using factor of 4·184 kJ/kcal) using ESHA Food Processor Software (The Food Processor® version 10.3.0.0). Total day energy intakes were the sum of the breakfast meal, pizza lunch and remainder of day.
At-home yogurt consumption
Participants consumed the same breakfast meal as above but at home on Days 2–7. On Day 1, participants were provided with the at-home breakfast meals. The yogurt was provided in pre-portioned opaque 200 ml cups and breakfast bars in their original packaging and packaged in a large brown paper bag, with instructions to refrigerate as soon as possible. Participants were asked to measure 125 ml of water to consume with the meal. For at-home data collection, participants completed one study diary sheet per day for an estimate of compliance. A period of 8 d was chosen so that study visits would occur on the same day of the week, for example, Tuesdays, to minimise variation from changes in routine between weekend and weekdays and to allow for menstrual cycle consistency between Days 1 and 8 visits.
Gastrointestinal symptoms
Participants completed a GIQ for comparison of gastrointestinal symptoms between YC and YI for baseline (Day 0), acute (Day 2) and chronic (Day 7) tolerance. The paper GIQ consisted of 100 mm VAS to assess abdominal discomfort, bloating, cramping, rumbling, flatulence, bowel movement number/function/consistency and overall gastrointestinal function.
Rheological analysis of yogurts
Rheological analysis, a unique addition to this study, was performed to rule out impacts of the inulin on viscosity and subsequent feelings of fullness. A controlled stress rheometer (AF 2000; TA Instruments) fitted with a 4 cm cone (truncation gap of 50·8 µm and 2° angle) was used to study the flow behaviour of YI and YC. Continuous shear rate sweeps were performed on samples from 1 to 120 s−1 and apparent viscosity values compared at 50 s−1 to correspond with reported shear rates associated with swallowing( Reference Borwankar 28 ). Samples of YI and YC (5 g) were also mixed with 5 ml simulated gastric fluid( Reference Malaki Nik, Corredig and Wright 29 ) (pH 2·0, 3·2 mg/ml pepsin (Sigma Chemical Co.), 37°C), and flow behaviour after 40 min studied using a recessed-end concentric cylinder and 200 µm gap geometry and testing parameters as above. Samples were analysed in triplicate.
Statistical analysis
Sample size was determined on the basis of previous recommendations for seventeen participants in a paired design with a study power of 0·80 to detect both a 10 mm difference in mean postprandial appetite ratings and 502 kJ (120 kcal) difference in ad libitum energy intake( Reference Horner, Byrne and King 30 ). Total AUC was calculated from 0–180 min VAS appetite ratings (Hunger, Fullness, Desire to Eat and PFC) using GraphPad Prism version 5 (GraphPad Software Inc.). Statistical analysis was performed using SAS version 9.3 (SAS Institute). Data are presented as arithmetic means and standard deviations, unless otherwise noted. P≤0·05 were considered statistically significant.
Appetite rating AUC values for Day 1 were compared according to Blundell et al.( Reference Blundell, de Graaf and Hulshof 27 ), between YI and YC by ANCOVA, using time point zero score (mm) as a covariate. Appetite rating AUC values for Day 8 were compared between YI and YC by ANCOVA, using Day 1 AUC (mm×min) as a covariate.
Day 1 ad libitum lunch energy intakes and total day energy intakes were compared between YI and YC using ANOVA. Day 8 ad libitum energy intakes and total day energy intakes were compared between YI and YC by ANCOVA, using Day 1 AUC (mm×min) as a covariate.
Differences in GIQ scores were compared at Day 0, Day 2 and Day 7 between YI and YC by ANOVA for overall GI and bowel movement consistency and non-parametric Kruskal–Wallis for all other GIQ scores (abdominal, bloating, cramping, rumbling, flatulence, bowel movement number, bowel movement function, bowel movement urgency), as they were not normally distributed.
Unpaired t tests were used to compare the apparent viscosities at 50 s−1 for YI v. YC, with and without dilution in the simulated gastric fluid.
Results
Participants
In all, 208 women were screened from February 2013 to January 2014, and twenty-one women were enrolled in the study. One participant was unable to complete the study because of unrelated medical issues, and a second was excluded for starting a new medication during the study period (Fig. 1). Participant characteristics at the time of study enrolment for the nineteen participants included in the analysis are presented in Table 3. Compliance to consuming the breakfast meal on Days 2–7 was reported at 100 %, with approximately 60 % of participants consuming the meal before 08.50 hours and, on average, eating within 36·6 (YI) and 31·9 min (YC) from waking.

Fig. 1 Consolidated Standards of Reporting Trials (CONSORT) flow diagram. YC, yogurt-control.
Table 3 Participant characteristics (n 19) (Mean values and standard deviations)
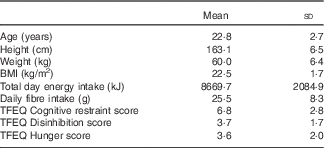
TFEQ, Three-Factor Eating Questionnaire.
Appetite ratings
Day 1 and Day 8 mean VAS and 180 min AUC values are presented in Fig. 2. Day 1 AUC values for Hunger (P=0·76), Fullness (P=0·32), Desire to Eat (P=0·62) and PFC (P=0·59) did not differ significantly between treatments. However, AUC values were significantly lower for Day 8 YI v. YC for Desire to Eat (P=0·04) and PFC (P=0·02) but not for Hunger (P=0·15) or Fullness (P=0·07).

Fig. 2 Appetite ratings of Hunger (a, b), Fullness (c, d), Desire to Eat (e, f), and Prospective Food Consumption (PFC; g, h), on Day 1 (a, c, e) and Day 8 (b, d, f) for yogurt-control (YC, ![]() ) and yogurt-inulin (YI,
) and yogurt-inulin (YI, ![]() ). Insets are corresponding AUC values with their standard errors represented by vertical bars. * Statistical significance between YC and YI (P<0·05), for ANCOVA using Day 1 as a covariate. n 19 except for YC Day 8 data (n 18).
). Insets are corresponding AUC values with their standard errors represented by vertical bars. * Statistical significance between YC and YI (P<0·05), for ANCOVA using Day 1 as a covariate. n 19 except for YC Day 8 data (n 18).
Energy intakes
Day 1 and Day 8 lunch and total day energy intakes are presented in Fig. 3. There were no differences observed in terms of energy throughout the study. Energy intakes on Day 1 at the ad libitum lunch were not significantly different (P=0·40) after consumption of the YI compared with YC. Lastly, Day 1 total daily energy intakes (sum of breakfast meal, lunch meal and remainder of day) were not significantly different (P=0·61) with YI v. YC.

Fig. 3 Energy intakes at the ad libitum lunch and total day on Day 1 v. Day 8. Values are means with their standard errors represented by vertical bars. n 17 except for yogurt-control (![]() ) Days 1 and 8 lunch (n 18) and yogurt-inulin (
) Days 1 and 8 lunch (n 18) and yogurt-inulin (![]() ) Days 1 and 8 lunch (n 19).
) Days 1 and 8 lunch (n 19).
Ad libitum lunch energy intakes on Day 8 also were not significantly different (P=0·09) after consumption of YI compared with YC. Lastly, Day 8 total daily energy intakes (sum of breakfast meal, lunch meal and remainder of day) were not significantly different (P=0·74) after YI compared with YC.
Gastrointestinal symptoms
No significant differences were observed between Days 0, 2 or 7 in terms of abdominal discomfort, bloating, cramping, rumbling, flatulence or overall gastrointestinal function after consuming the YI or YC yogurts (data not shown). When GIQ scores were compared between YI and YC on post-treatment days, no significant differences were seen for any symptoms (data not shown, P>0·05).
Discussion
The primary purpose of this study was to investigate the effect of 8-d repeated 6 g inulin consumption on satiety measures in young healthy female adults. Postprandial breakfast Desire to Eat and PFC ratings were significantly lower after 8-d yogurt with inulin consumption, compared with yogurt alone. Therefore, repeated inulin consumption was associated with appetite reductions. This is consistent with a previous 2-week study on 16 g inulin–oligofructose supplementation in healthy adults( Reference Cani, Lecourt and Dewulf 16 ). In the present study, however, the effect on appetite ratings did not translate into decreases in food intake, either at the ad libitum pizza lunch or throughout the day. This is inconsistent with other studies reporting that chronic inulin consumption lowered daily energy intakes( Reference Cani, Lecourt and Dewulf 16 , Reference Tulk, Blonski and Murch 19 ). Notably, the addition of only 4 g inulin/d to a synbiotic yogurt reduced reported energy intakes by 937 kJ (224 kcal) in healthy men and women after 15 d of consumption( Reference Tulk, Blonski and Murch 19 ). In another study, daily supplementation of 11 g inulin for 5 weeks was found to slow gastric emptying by 30 min compared with a control diet or baseline state in healthy young men( Reference Russo, Clemente and Linsalata 31 ). Kellow et al.( Reference Kellow, Coughlan and Reid 20 ) observed that, of the clinical trials in their meta-analysis with prebiotic interventions that showed an effect on energy intake, 2 weeks of prebiotic consumption was the minimum study duration associated with reductions in energy intake. Therefore, study duration longer than 8 d may be needed to observe effects of inulin on energy intake, although Day 8 modulations in appetite were observed in the present study. Interestingly, Kolida et al.( Reference Kolida, Meyer and Gibson 32 ) showed that 14-d daily consumption of 5 or 8 g inulin with a DP similar to that of the present study (i.e. 9–10) increased bifidobacteria levels, with baseline levels influencing the bifidogenic effect.
Most human satiety studies utilise a preload design in an acute setting. Therefore, the secondary purpose of this study was to determine whether 6 g of inulin added to a commercially available yogurt served as part of a breakfast meal would influence acute subjective ratings of appetite and same-day energy intake. There were no observed differences with one-time consumption of YI v. YC on measures of appetite, ad libitum lunch intakes or total day energy intakes. This lack of association between inulin consumption and acute satiety agrees with the finding of Karalus et al.( Reference Karalus, Clark and Greaves 24 ) who reported that one-time consumption of 10 g inulin-enriched bars had no effect on appetite ratings or subsequent meal intake. This study similarly included only young healthy female participants with consideration of menstrual cycle and excluded restrained eaters. In contrast, Perrigue et al. ( Reference Perrigue, Monsivais and Drewnowski 21 ) added 6 g of inulin to high and low energy density liquid yogurt preloads. In that study with healthy men and women, inulin addition was associated with significantly lower ad libitum meal energy intakes. Harrold et al.( Reference Harrold, Hughes and O’Shiel 22 ) tested a commercially available inulin similar to that used in the present study (5 g in 100 g water) and observed an acute main effect in terms of decreased ad libitum lunch and total day energy intakes in slightly overweight young women. Of note, that study was unique in that it included two fibre doses – namely, 15 min before the preload breakfast and 15 min before the ad libitum lunch served 4-h later – and utilised a unique statistical approach( Reference Harrold, Hughes and O’Shiel 22 ). In another study on healthy overweight men, half the fat in a breakfast sausage patty was replaced with 24 g of inulin. Appetite was not affected, but total day energy intake was significantly lower compared with full-fat patties( Reference Archer, Johnson and Devereux 23 ). Inulin, particularly with DP approximately 25, can be used as a fat substitute because of its potential mouthfeel properties( Reference Niness 6 ). However, the DP utilised in the current study was relatively low, and participants did not detect the presence of 6 g inulin in YI (data not shown). The follow-up questionnaire also revealed that roughly half of the participants (n 10) reported no detectable difference between the two yogurts. Moreover, although nine participants reported some differences between the yogurts (i.e. texture, thickness and sweetness), the reports were inconsistent (e.g. YI was reported to be both thicker and thinner compared with YC). Rheological testing also confirmed there were no differences between YI and YC in terms of apparent viscosity values (i.e. 0·53 (sem 0·03) and 0·48 (sem 0·02) Pa×s at 50 s−1; P>0·05, data not shown). The presence of inulin was also not associated with differences in apparent viscosity at 50 s−1 of simulated gastric fluids containing the yogurts (P>0·05, data not shown). These results support the non-viscous nature of the inulin utilised and are interesting considering that dietary fibre-induced meal viscosity( Reference Marciani, Gowland and Spiller 33 ) and in vitro gut content viscosity( Reference Logan, Wright and Goff 34 ) have been associated with feelings of Fullness, which were not impacted in the present study. Although the scales are collectively meant to assess appetite, each is intrinsically asking about unique feelings and motivations to eat, and it is not uncommon to see changes in some appetite ratings and not others. In the present study, the preloads had similar viscosities and energy contents. These factors may have contributed to the lack of differences between ratings of Fullness, which is a physical feeling( Reference Drapeau, Blundell and Therrien 35 ) and associated Hunger. Perhaps the impact of inulin consumption on appetite, reflected in the Day 8 differences in Desire to Eat and PFC, was related to alterations in underlying mechanisms not quantified in this study.
The physiological effects of inulin appear to be dramatically influenced by inulin quality and type, including DP. As above, comparisons between human studies are complicated by various factors. This includes the fact that some studies have not disclosed the inulin source( Reference Perrigue, Monsivais and Drewnowski 21 ) or have utilised a combination of short- and long-chain molecules( Reference Cani, Lecourt and Dewulf 16 ). Others have utilised longer chain products (average DP approximately 25, depending on the source( Reference Darzi, Frost and Robertson 18 , Reference Archer, Johnson and Devereux 23 , Reference Karalus, Clark and Greaves 24 , Reference Russo, Clemente and Linsalata 31 )) or, as in the present study, native inulin (DP approximately 10)( Reference Tulk, Blonski and Murch 19 , Reference Harrold, Hughes and O’Shiel 22 ). Inulin-type fructans with shorter chain lengths are generally fermented more quickly in the caecum and proximal colon, thereby potentially enhancing the release of appetite-suppressing hormones( Reference Delzenne, Cani and Daubioul 17 ). However, longer chain inulins are generally better tolerated because of less fermentation( Reference Bonnema, Kolberg and Thomas 26 ). In the present study, 6 g of inulin did not affect gastrointestinal symptoms after 2 or 7-d consumption. Similarly, Bonnema et al.( Reference Bonnema, Kolberg and Thomas 26 ) reported that up to 10 g of native inulin (average DP of 10–12) consumed in a breakfast meal was well tolerated by healthy men and women. Therefore, relatively low-dose consumption of inulin with DP approximately 10 may maximise long-term physiological effects on appetite, while avoiding undesirable side effects.
Food format may be an important contributor to satiety and also confounds the reported effects of inulin consumption. For example, there may be greater potential for fibre to enhance satiety when added to liquid v. solid foods, such as with oat bran added to orange juice v. biscuits( Reference Pentikäinen, Karhunen and Flander 36 ). Effects may also reasonably differ on the basis of the water-holding properties of a fibre and whether or not the fibre was allowed time to fully hydrate before consumption( Reference Wanders, van den Borne and de Graaf 2 ). Yogurt as a vehicle for fibre may be particularly impactful on satiety. Semi-solid and liquid yogurts were previously reported to decrease acute Hunger and increase acute Fullness ratings compared with two isoenergetic beverages (dairy products or fruit juice)( Reference Tsuchiya, Almiron-Roig and Lluch 37 ). In the same study, there also were no differences in satiety observed between the two yogurt forms – namely, one eaten with a spoon v. a drinkable form – in young men and women( Reference Tsuchiya, Almiron-Roig and Lluch 37 ). This supports that the composition of yogurt alone may contribute to feelings of satiety. Additionally, interactions of dietary fibre with other ingredients can impact effects. For example, the combination of 2·6 g guar gum and 7·9 g milk protein in a yogurt snack was found to influence energy intake( Reference Lluch, Hanet-Geisen and Salah 38 ).
The current study was unique in terms of the inclusion of young healthy females with consistent hormonal contraceptive use( Reference Tucci, Murphy and Boyland 39 ), screened for dietary restraint( Reference Stunkard and Messick 25 ) and scheduled according to menstrual cycle( Reference Buffenstein, Poppitt and McDevitt 40 ). As health status, including body weight, may impact response to treatment in satiety studies( Reference Benelam 41 ), careful screening parameters and reporting of such are important. The use of deception to collect unbiased appetite and food intake results and repeated consumption were also strengths, albeit the study duration may not have been long enough to see longer term impacts on food intake. The collection of VAS appetite ratings over 3 h, direct weighing of food intake at an ad libitum lunch and accounting for energy compensation that may occur over the remainder of the day using food records were positives in terms of a comprehensive assessment of appetite and eating behaviour effects. The study is limited by its short duration, and a 28-d intervention, considering menstrual cycle in women, is recommended for future research. Lastly, the use of self-reported food records constitutes a limitation, although the tendency for under-reporting energy intake is a particular concern in obese and restrained individuals( Reference Schoeller 42 ) who were excluded from participating in this study.
In conclusion, 6 g of the soluble fibre inulin added to a commercially available yogurt did not affect same-day appetite ratings or energy intakes but lowered Desire to Eat and PFC ratings after 8-d consumption by young healthy females. This study showed not only that repeated consumption of inulin was required for reductions in appetite to be observed but also that 8-d consumption was insufficient to affect energy intake. No effects were observed in terms of GI symptoms with the 6 g daily inulin consumption. Future studies should examine higher doses of inulin incorporation and measure satiety outcomes after a longer duration of consumption. Finally, energy compensation, participant characteristics and female menstrual cycle should be taken into account in study design, analysis and interpretation of satiety studies, although a balance between targeting evidence to support efficacy v. effectiveness is needed.
Acknowledgements
The authors thank the study participants as well as Agota Ferenc, Lois Xinjie Lin, Melissa MacNeil, Mary Coleen North, Eleanor Reid and Emily van Niekerk for their technical assistance.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
A. J. W. and A. J. T. designed the study. S. H., J. I. and M. L. recruited participants and collected study data. S. H. and A. J. T. organised the data and conducted the data analysis. S. H., A. J. T. and A. J. W. interpreted the data and wrote the manuscript. All authors edited the manuscript.
The authors declare that there are no conflicts of interest.









