Non-alcoholic fatty liver disease (NAFLD) affects up to 40 % of the adult population( Reference Browning, Szczepaniak and Dobbins 1 – Reference Angulo 3 ), but this can exceed 85 % in obese individuals( Reference Angulo 3 ). NAFLD is defined by hepatic fat≥5·5 % in the absence of excessive alcohol consumption or other genetic, viral or drug-induced causes of liver fat accumulation( Reference Szczepaniak, Nurenberg and Leonard 4 ). Although for some patients, the clinical course of ‘simple steatosis’ is benign, approximately 10–61 % progress to non-alcoholic steatohepatitis( Reference Neuschwander-Tetri and Caldwell 5 , Reference Wong, Wong and Choi 6 ) – which can then lead to cirrhosis( Reference Krawczyk, Bonfrate and Portincasa 7 ) – and to hepatocellular carcinoma( Reference Starley, Calcagno and Harrison 8 ). At present, NAFLD can only be conclusively diagnosed by biopsy, and proton magnetic resonance spectroscopy (1H-MRS) is used for quantification in research settings( Reference Ali and Cusi 9 ). In clinical practice, increased levels of serum aminotransferases are commonly used to infer risk of NAFLD, although blood liver enzyme levels are an unreliable measure of liver damage( Reference Mofrad, Contos and Haque 10 ), and thus alternative useful markers are needed.
NAFLD is an independent predictor of CVD( Reference Targher and Arcaro 11 ) and the metabolic syndrome( Reference Cortez-Pinto, Camilo and Baptista 12 ), and thus strategies to reduce liver fat are required. Weight loss by surgery or lifestyle therapy reduces liver fat( Reference Neuschwander-Tetri and Caldwell 5 , Reference Dixon, Bhathal and Hughes 13 ); however, the required weight loss is difficult to achieve and maintain( Reference Franz, Vanwormer and Crain 14 ). Furthermore, there are safety concerns about the long-term use of current pharmacological agents to reduce liver fat( Reference Mahady, Webster and Walker 15 ).
Published studies have suggested a potential role for n-3 PUFA in the treatment of NAFLD( Reference Parker, Johnson and Burdon 16 ); however, the effect of current n-3 PUFA status on NAFLD risk is unknown. Given that erythrocytes have a lifespan of approximately 120 d, the Omega-3 Index, or the erythrocyte membrane content of EPA plus DHA, expressed as a percentage of total membrane fatty acids, is likely to be a robust marker of medium- to long-term n-3 intake that is unaffected by recent diet( Reference Harris 17 , Reference Harris, Varvel and Pottala 18 ). The index is a promising biomarker for CVD( Reference Harris and Von Schacky 19 ), and therefore may be a useful indicator of NAFLD risk.
NAFLD may exist undiagnosed in overweight and obese, but otherwise healthy, men and women. This group does not fulfil the traditional NAFLD target group criteria of morbid obesity and multiple comorbidities, and the Omega-3 Index may therefore assist in identifying those at increased risk for NAFLD and CVD. This study aimed to assess whether the index as a marker of long-term PUFA intake is a robust marker of hepatic steatosis in apparently healthy, overweight and obese men and women.
Methods
Participants
From June 2011 to September 2013, a community sample of men and women were recruited via notice boards and electronic bulletins. To be eligible, participants had to be non-smokers, aged between 18 and 60 years with a BMI>25·0 kg/m2 and with a waist circumference( Reference Alberti, Zimmet and Shaw 20 ) that exceeded the cut-off point for ‘increased waist circumference’ (>94 and 80 cm for males and females, respectively)( Reference Alberti, Zimmet and Shaw 20 ). Volunteers were excluded if they had biochemistry indicative of overt disease, had been previously diagnosed with diabetes mellitus or liver disease (other than NAFLD), were taking medication for hyperlipidaemia or if they reported taking supplements containing n-3 PUFA within the last 6 months. Other exclusion criteria included a change in antihypertensive medication within the last 12 months (if taking such medication), a regular alcohol intake exceeding 20 g alcohol/d and reported recent (within 3 months) significant changes to diet and exercise habits or body weight (>5 % weight change). This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving humans were approved by the Sydney Local Health District Ethics Review Committee (RPAH Zone), protocol number X10-0115, clinical trial registration number ANZCTRN12610000351011. Written informed consent was obtained from all the subjects.
Experimental design
Participants were screened for eligibility via telephone, and those deemed likely to be eligible attended the initial visit. At the first visit, measurements included anthropometry (height, weight, waist and total body fat) and a fasting blood test (8 h fast). The second visit included an MRI and MRS scan, which were completed within 7 d of visit one.
Measurements
Anthropometry assessment
Standing height was recorded to the nearest 0·5 cm. Weight was measured in light clothing using a digital platform scale accurate to 0·1 kg (Tanita BC-418 Body Composition Analyzer; Tanita Corporation); total body fat was measured by bioelectrical impedance analysis at the same time. Waist circumference was measured to the nearest millimetre at the midpoint between the twelfth rib and the iliac crest, according to International Diabetes Federation guidelines( Reference Alberti, Zimmet and Shaw 20 ).
Blood pressure
Resting systolic (SBP) and diastolic (DBP) blood pressure and heart rate were measured in duplicate after 5 min of rest (seated) using an automated blood pressure monitor (Omron M5; Omron Healthcare Co. Ltd).
Biochemical parameters
Following an overnight (>8 h) fast, a 20 ml EDTA venous blood sample was collected and the plasma and erythrocyte portions were separated. The erythrocytes were stored at –80°C in an antioxidant (1 mg butylated hydroxytoluene) until fatty acid analysis.
Erythrocyte fatty acid derivatisation
The fatty acid profile was analysed via direct transesterification of the washed erythrocyte fraction of blood followed by GC( Reference Lepage and Roy 21 ). In brief, methanol–toluene 2 ml (4:1, v/v) (containing C19 : 0 (20 µg/ml) as internal standard) was added to the sample. Acetyl chloride (200 µl) was added while vortexing and then heated (1 h, 100°C). The tubes were cooled in water and K2CO3 6 % (5 ml) was subsequently added and centrifuged (3000 g , 5 min, 4°C). The upper toluene phase was collected and stored in a GC vial at –20°C for GC analysis.
Gas chromatography analysis
Methylated fatty acid samples were analysed by GC using a fixed carbon–silica column 30 m×0·25 mm (DB-225; J and W Scientific). The injector and detector ports were set at 250°C and the oven temperature was programmed as follows: 170°C for 2 min, increased by 10°C/min up to 190°C where it remained stationary for 1 min. The temperature was then increased by 3°C/min up to 220°C, which was maintained for a total run time of 30 min per sample. A split ratio of 10:1 and an injection volume of 3 ml were used. A known fatty acid mixture was used to compare with the analysed samples in order to identify peaks according to retention time, and their concentrations were determined using a Hewlett Packard 6890 Series GC (Hewlett Packard) with ChemStation version A.04.02.
The Omega-3 Index was calculated as the %EPA+%DHA content of erythrocyte cell membranes. The total n-3 content was calculated as the summed total of n-3 PUFA in the erythrocyte membranes: C18 : 3n-3, C20 : 5n-3, C22 : 5n-3 and C22 : 6n-3. Total n-6 content was calculated as the total of C18 : 2n-6, C18 : 3n-6, C20 : 2n-6, C20 : 3n-6 and C20 : 4n-6 contents.
Other biochemical parameters
Biochemical assessments were carried out by a commercial laboratory (Royal Prince Alfred Hospital Pathology). Glucose, albumin, serum aminotransferases (alanine aminotransferase (ALT) and aspartate aminotransferase) and lipids (total cholesterol, TAG, LDL-cholesterol and HDL-cholesterol) were analysed by photometric reactions using a C702 Cobas8000 Autoanalyser (Roche Diagnostics). Insulin was analysed by immunoassay using electrochemiluminescence on an E602 Cobas8000 Autoanalyser (Roche Diagnostics). HbA1c was analysed by HPLC (Biorad), homocysteine was analysed from plasma by HPLC (Schimazu) and high-sensitive C-reactive protein (hsCRP) was analysed by rate nephelometry on a BN II (Siemens).
Magnetic resonance imaging and proton magnetic resonance spectroscopy
Magnetic resonance imaging and proton magnetic resonance spectroscopy data acquisition
All MRI and 1H-MRS measurements were acquired using a 1·5-Tesla Achieva whole-body system (Philips Medical Systems). Visceral and subcutaneous adipose volumes were measured by MRI with the patient in the supine position. Axial T1-weighted fast field echo images were acquired from the diaphragm to the pelvis (TR=11 ms, TE=4·5 ms, flip angle=40°), with slice thickness of 10 mm and inter-slice gap of 10 mm. Images were acquired during suspended end-expiration with breath-hold duration of approximately 10 s per acquisition.
Liver fat (intrahepatic lipid (IHL)) concentration and composition were measured in vivo by 1H-MRS according to the methods detailed previously( Reference Johnson, Walton and Sachinwalla 22 ). Image-guided, localised (3·0×2·0×2·0 cm voxel) 1H-MRS data were acquired using the whole-body (Q body) (transmit) coil and a circular polarised surface (flex M multi-channel surface) (receive) coil, with volumes of interest centred within the right lobe of the liver. The subjects lay in the supine position and spectra were acquired with respiratory gating (end-expiration). Spectra were acquired using the PRESS (point resolved spectroscopy) technique (TR=5000 ms, TE=45 ms, 64 measurements, 1024 sample points). Fully automated high-order shimming was performed on the volume of interest to ensure maximum field homogeneity. Excitation water suppression was used to suppress the water signal during data acquisition. Unsuppressed water spectra were acquired in vivo for use as the internal standard.
Cross-sectional areas of both the visceral ( Reference Pepe, Marasco and Haas 23 ) and subcutaneous adipose tissue (VAT and SAT) depots were computed by automated software (Hippo FatTM version 2.11)( Reference Positano, Gastaldelli and Sironi 24 ) with manual editing of contour lines and Gaussian curves as necessary. Volumes of VAT and SAT from the diaphragm to the pelvis were calculated by the summation of VAT and SAT area from the abdominal slices, adjusted for slice thickness and inter-slice gap.
Magnetic resonance imaging and proton magnetic resonance spectroscopy data processing
Spectral data were post-processed by the magnetic resonance user interface software (jMRUI version 3.0, EU Project). After Fourier transformation and manual phasing of the spectra, the water peak was identified and nominated 4·69 ppm and the signal amplitude was measured using HLSVD (Hankel Lanczos Squares Singular Values Decomposition). For the water-suppressed signal in vivo, the remaining water resonance (if evident) was first removed by the SVD (singular values decomposition) filter after which a five-resonance model was used to fit the lipid peaks: hepatic lipid olefinic methene protons ((CH)n; approximately 5·3 ppm), bulk methylene protons (-(CH2)n; approximately 1·3 ppm), methylenic protons in the α (-CH2; approximately 2·3 ppm) position relative to the carboxyl group, allylic protons (-(CH2)n; approximately 2·0 ppm) and methyl protons (-CH3; approximately 0·9 ppm) with metabolite signal amplitude quantified using the QUEST algorithm (QUantitation based on QUantum ESTimation), as we have described previously( Reference Johnson, Walton and Sachinwalla 22 , Reference Ryan, Itsiopoulos and Thodis 25 ).
To ensure consistency across subjects, all MRI and MRS analyses were performed by a single investigator who was blinded to treatment allocation.
Calculations
In vivo IHL% and hepatic fat composition were calculated as we have described recently( Reference Ryan, Itsiopoulos and Thodis 25 ). In brief, IHL% was measured as the ratio of hepatic methylene fatty acid amplitude to hepatic water amplitude corrected for T 2 effects, and hepatic lipid saturation was measured as the inverse of the hepatic allylic fatty acid resonance as a fraction of total hepatic fatty acids( Reference Johnson, Walton and Sachinwalla 22 ). Participants were subsequently grouped as those with NAFLD (IHL≥5·5 %) and those without NAFLD (IHL<5·5 %)( Reference Szczepaniak, Nurenberg and Leonard 4 ).
Statistical analysis
Data were analysed using Statistical Package for the Social Sciences (SPSS; Release 17.0; SPSS Inc.). Data are reported as the mean values with their standard errors from the mean or frequencies as appropriate. Groups were compared using the χ 2 and the unpaired two-tailed t tests. Pearson coefficients (R) were used for all correlations. Hierarchical regression analyses were used to examine the contribution of variables to IHL%. Simple anthropometric and demographic variables (waist, age) were entered into block 1. Clinically relevant biochemical variables (TAG, HDL, hsCRP and ALT) were entered into block 2 of the regression analysis, and, finally, the novel marker, erythrocyte content of %EPA+%DHA (Omega-3 Index), was entered into the regression in block 3. Pairwise exclusion for missing data was employed in all analyses. Significance was set at P<0·05.
Results
Participants
A total of eighty eligible volunteers (70 % male) participated in the study (Fig. 1). Participant characteristics are summarised in Table 1. Study participants were aged 38·8±1·3 years (range: 18–59 years), with BMI of 29·6±0·4 kg/m2 (range: 25·3–45·7 kg/m2) and waist circumference of 99·8±0·9 cm (males: 101·9±0·9 cm; females: 94·9±2·3 cm). Liver fat was 5·1±0·5 % (range: 0·2–18·9 %). In all, thirty participants (37·5 % of the cohort) were classified as having NAFLD (liver fat≥5·5 %)( Reference Szczepaniak, Nurenberg and Leonard 26 ), while the remaining fifty were not; fifteen participants satisfied the criteria for diagnosis of the metabolic syndrome( Reference Cleeman, Grundy and Becker 27 ), eleven of these participants had NAFLD .

Fig. 1. Flow diagram of participant screening and recruitment. eGFR, estimated glomerular filtration rate.

Fig. 2. Intrahepatic lipid concentration (IHL%) v. Omega-3 Index for those with (○) and without (●) non-alcoholic fatty liver disease.
Table 1 Baseline participant characteristics (Mean values with their standard errors)

NAFLD, non-alcoholic fatty liver disease; BIA, bioelectrical impedance analysis; SBP, systolic blood pressure; DBP, diastolic blood pressure; MetSyn, metabolic syndrome; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; ALT, alanine aminotransferase; AST, aspartate aminotransferase; hsCRP, high-sensitive C-reactive protein; 1H-MRS, proton magnetic resonance spectroscopy; IHL%, intrahepatic lipid per cent; SI, saturation index.
* P value for NAFLD v. non-NAFLD using Student’s t test for continuous variables and the χ 2 test for categorical variables.
† Metabolic syndrome criteria( Reference Cleeman, Grundy and Becker 27 ) – individuals must satisfy three or more of the following criteria: fasting glucose ≥5·6 mmol/l; fasting TAG >1·7 mmol/l; fasting HDL <1·29 or <1·03 mmol/l for females and males, respectively; blood pressure ≥130/85; waist circumference (midpoint between iliac crest and twelfth rib) >88 or >94 cm for females and males, respectively.
‡ Visceral and subcutaneous adipose tissue measurements at the level of the umbilicus.
§ Normal reference ranges: glucose, 3·0–5·4 mmol/l; insulin, 10–96 pmol/l; ALT, 5–55 U/l; AST, 5–55 U/l; hsCRP 25/50/75/100th percentiles: 0·72/1·47/2·96/9·00 mg/l; total cholesterol, ≤5·2 mmol/l; TAG, ≤2·5 mmol/l; HDL, 1·0–2·5 mmol/l; LDL, ≤3·5 mmol/l.
Compared with those without NAFLD, participants with NAFLD had higher mean BMI, DBP and visceral adipose tissue (P<0·05 for all; Table 1). Waist circumference was also higher in the group with NAFLD (P=0·004); however, when males and females were studied separately, this observation was statistically significant for females only (P=0·003 and 0·082 for females and males, respectively). As expected, participants with NAFLD had higher fasting glucose, insulin, hsCRP and TAG and lower HDL (P<0·05 for all; Table 1). The IHL saturation index (the level of SFA in liver TAG) tended to be higher in the NAFLD group compared with the non-NAFLD group (P=0·058; Table 1). There were no differences in serum aminotransferases or erythrocyte fatty acid composition between those with and without NAFLD (Table 1).
Bivariate correlations
Bivariate correlations between IHL and biochemical and anthropometric variables are summarised in Table 2. Higher IHL% was associated with higher BMI, waist circumference, glucose and TAG levels, as well as with lower HDL levels (Table 2). IHL% was positively correlated with the Omega-3 Index (Fig. 2) and negatively correlated with the erythrocyte n-6:n-3 ratio. The Omega-3 Index was positively correlated with BMI and glucose level, but not other components of the metabolic syndrome. Waist circumference, BMI, glucose and insulin were all moderately, positively correlated with one another, and these correlations were highly significant (Table 2).
Table 2 Descriptive statistics and correlations of metabolic, anthropometric and erythrocyte outcomes (Mean values with their standard errors)

IHL%, intrahepatic lipid per cent; ALT, alanine aminotransferase; AST, aspartate aminotransferase; hsCRP, high-sensitive C-reactive protein.
* P<0·05, ** P<0·01, *** P<0·001.
Hierarchical multiple regression analysis
Demographic and anthropometric variables (waist circumference, age) accounted for 31 % of the variance in liver fat (P<0·001; Table 3), although only waist circumference was a statistically significant predictor of IHL%. The addition of biochemical measures (TAG, HDL, hsCRP and ALT) accounted for a further 11 % (P=0·009) of the variance in liver fat (∆R 2=0·11). Finally, adding Omega-3 Index into the model (block 3; Table 3) raised the total variance explained by the model to 46 % (P=0·049).
Table 3 Hierarchical regression model for prediction of liver fat
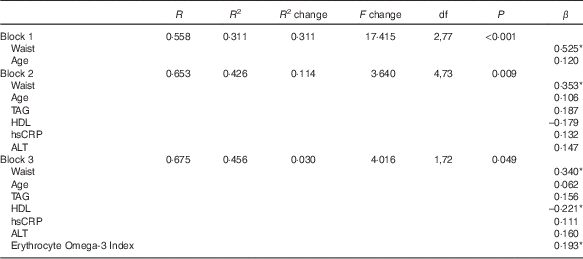
hsCRP, high-sensitive C-reactive protein; ALT, alanine aminotransferase.
* P<0·05.
Discussion
This study sought to examine the associations between traditional anthropometric, biochemical markers and a novel marker – the Omega-3 Index – and liver fat level in a group of apparently healthy, but overweight, adults. We found that all the components of the metabolic syndrome( Reference Alberti, Zimmet and Shaw 20 ) except blood pressure were associated with IHL%, and the relationships were as expected: glucose, TAG and waist circumference were positively correlated and HDL was negatively correlated with IHL%. The Omega-3 Index was significantly associated with IHL% in bivariate correlations, but did not meaningfully improve the statistical power of the regression model for predicting NAFLD. These data from a small pilot sample suggest that measurement of the Omega-3 Index does not improve prediction of NAFLD in overweight individuals compared with previously established simple anthropometric and biochemical risk factors.
Observational studies have linked lower n-3 PUFA status in liver tissue( Reference Araya, Rodrigo and Videla 28 ) and low intake of n-3 PUFA( Reference Zelber-Sagi, Nitzan-Kaluski and Goldsmith 29 , Reference Cortez-Pinto, Jesus and Barros 30 ) with the presence of NAFLD, and experimental research has shown that n-3 PUFAs regulate mRNA expression of genes involved in hepatic lipid storage, such as PPAR- α, SREBP-1c and ChREBP ( Reference Jump 31 – Reference Sampath and Ntambi 33 ). Furthermore, human studies of n-3 supplementation have demonstrated that n-3 PUFA can ameliorate fatty liver( Reference Parker, Johnson and Burdon 16 ). The Omega-3 Index, or the percentage of erythrocyte cell membrane content of EPA+DHA( Reference Harris and Von Schacky 19 ), is a robust long-term marker (≥3 months) of n-3 intake( Reference Harris and Von Schacky 19 ), and therefore is a potentially useful marker of NAFLD risk. As NAFLD commonly exists with few or no symptoms, readily available single biomarkers are needed, particularly given that current methods used to identify those at risk and to ascertain disease progression are unsuitable for the growing prevalence of NAFLD. Thus, liver function tests are poor predictors of liver disease( Reference Mofrad, Contos and Haque 10 ), which is supported by the findings of this study, and quantification of liver fat, fibrosis and inflammation by invasive liver biopsy carries a risk for complications. The only non-invasive method validated to quantify liver fat – MRS – is expensive and generally restricted to research( Reference Ali and Cusi 9 ). In this study, anthropometric and biochemical variables such as BMI, waist circumference and glucose and insulin levels were all correlated with one another and also with IHL% in both men and women (Tables 2 and 3). Taken together with the correlations also observed between TAG, HDL and IHL%, it would appear that increased NAFLD risk may be best identified via a cluster of anthropometric and biochemical markers suggestive of a ‘sub-clinical’ or pre-metabolic syndrome.
Although regression analyses did not find the Omega-3 Index to be predictive of fatty liver in this group of individuals, the evidence linking NAFLD with low n-3 PUFA (intake and status) is strong, and more research in large cohorts using quantitative MRS is, therefore, warranted.
Strengths and limitations
Potentially limiting the strength of associations found in the present study between Omega-3 Index and liver fat was that the mean Omega-3 Index was relatively high in this group of participants compared with other free-living populations( Reference Harris and Von Schacky 19 , Reference von Schacky 34 ). The healthy but overweight population studied was recruited from an affluent area within a capital city and had ‘high’ mean Omega-3 Index levels( Reference Harris and Von Schacky 19 ), with 64 % of participants having Omega-3 Index above 8·0 %, which is considered to be the level for optimum cardiovascular protection( Reference Harris and Von Schacky 19 ). In addition, the observed variance in IHL% was relatively low, which may also have reduced the capacity to examine the strength of this association. Although a moderately large sample size was achieved, given the unexpectedly low variance in Omega-3 Index and IHL% between participants, a larger sample with a wider range of individuals from various demographic and socio-economic spectra may yield further insights into the relationship between Omega-3 Index and liver fat. Furthermore, the gender split was not even, with only twenty-four females included in the analyses, preventing sub-analysis of the major outcomes by sex, which may have affected the relationship between liver fat, Omega-3 Index and other variables.
As NAFLD is likely to occur in apparently healthy individuals as well as in obese and/or those with comorbidities, it would be beneficial to examine the utility of the Omega-3 Index and other biomarkers across the spectrum of both Omega-3 Index and liver health/disease.
Although further research on the Omega-3 Index is warranted, clinicians should continue to investigate the potential for NAFLD in all overweight and obese patients, particularly those who exhibit anthropometry and biochemistry suggestive of, or approaching, metabolic syndrome. The data from this study reinforce the usefulness of simple markers, particularly waist circumference, age, TAG, HDL and hsCRP in predicting those at risk of NAFLD.
Acknowledgements
The authors would like to acknowledge staff at The Heart Research Institute and the Boden Institute of Obesity, Nutrition, Exercise and Eating Disorders for technical and practical support provided during data collection for this research.
This research was supported by funding from the Diabetes Australia Research Trust (Establishment Grant: N. A. J.) and Blackmores Australia Ltd. J. G. is supported by the Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney; a National Health and Medical Research Council of Australia (NHMRC) Program Grant (1053206) and a Project grant (1049857). The funding bodies had no role in the design of the study, collection and analysis of data or decision to publish. The authors declare that there are no competing financial interests in relation to the work described.
The authors’ contributions are as follows: H. M. P. was involved in the study design, subject recruitment, data collection, statistical analysis, data interpretation and drafting of the manuscript. H. T. O. was involved in the study design, data interpretation and drafting of the manuscript. S. E. K. was involved in the study design, subject recruitment and data collection. J. S. C. was involved in obtaining the required funding, study design and manuscript preparation. M. L. G. was involved in data analysis and manuscript preparation. I. D. C. and J. G. were involved in obtaining the required funding and manuscript preparation. N. A. J. was involved in obtaining the required funding, study design, statistical analysis, data interpretation and drafting of the manuscript. N. A. J. had overall responsibility for the study; H. M. P. and N. A. J. had full access to all the data regarding the study and took responsibility for the integrity of the data and the accuracy of data analysis.
H. M. P., H. T. O., S. E. K., J. S. C. and M. L. G. declare no conflicts of interest. N. A. J. has received honoraria for speaking engagements for Merck Sharp & Dohme. I. D. C. has performed and still performs clinical trials for obesity treatment and prevention, some of which have been funded by the government, but others by the pharmaceutical industry. Current trials are funded by the NHMRC (3), NovoNordisk, Amylin Corporation, the Egg Board. He serves on the steering committees of international trials (SCOUT and EXSCEL) and has received honoraria for this. He has given talks for NovoNordisk, Servier Laboratories, Pfizer and iNova pharmaceuticals in the last 3 years. He serves on the scientific advisory board of the Sansom Institute for Health Research, University of SA, the board of the Children’s Medical Research Institute, and chairs the Executive Management Committee of the bariatric surgical register for the Obesity Surgery Society of Australia and New Zealand. J. G. has no conflicts to declare in relation to this submission.








