Obesity is associated with impaired endothelial function assessed by flow-mediated dilatation (FMD)Reference Hashimoto, Akishita, Eto, Kozaki, Ako, Sugimoto, Yoshizumi, Toba and Ouchi1 but the evidence that weight loss improves FMD is contradictory. There are several studies showing that reductions in LDL-cholesterol (LDL-C) and fasting glucose concentrations but not weight loss per se were associated with improvements in FMDReference Bergholm, Tiikkainen, Vehkavaara, Tamminen, Teramo, Rissanen and Yki-Jarvinen2, Reference Raitakari, Ilvonen, Ahotupa, Lehtimaki, Harmoinen, Suominen, Elo, Hartiala and Raitakari3. The effect on FMD of dietary composition has not been extensively examined and has largely focused on the effect of dietary fat on FMD in a weight-stable settingReference Fuentes, Lopez-Miranda and Sanchez4–Reference Keogh, Grieger, Noakes and Clifton7. To our knowledge there is only one study of the effect of a low-carbohydrate diet on endothelial function during weight loss and this was conducted using a very-low-caloric dietReference Raitakari, Ilvonen, Ahotupa, Lehtimaki, Harmoinen, Suominen, Elo, Hartiala and Raitakari3. Weight loss per se is usually associated with reductions in LDL-CReference Poobalan, Aucott, Smith, Avenell, Jung, Broom and Grant8 and reducing saturated fat also decreases LDL-CReference Mensink, Zock, Kester and Katan9. We therefore developed a diet low in saturated fat and low in carbohydrate to maximise LDL-C reduction and reduce glucose concentrations designed to achieve moderate weight loss in order to test whether the combined effects with weight loss would improve FMD.
Obesity is independently associated with increased levels of cellular adhesion molecules and weight loss has been shown to reduce themReference Ferri, Desideri, Valenti, Bellini, Pasin, Santucci and De Mattia10, Reference Miller and Cappuccio11. There are few studies of the effect of diet on adhesion molecules and these have examined dietary fatReference Bemelmans, Lefrandt, Feskens, Broer, Tervaert, May and Smit12, Reference Perez-Jimenez, Castro and Lopez-Miranda13. Adiponectin is an insulin sensitising agent which is reduced in obesityReference Ukkola and Santaniemi14, Reference Bluher, Fasshauer, Tonjes, Kratzsch, Schon and Paschke15. It has been shown to increase after large amounts of weight loss after gastroplasty for morbid obesity and after weight loss in subjects with diabetes but not after moderate weight loss in a short-term loss programme in non-diabetic womenReference Kopp, Krzyzanowska, Mohlig, Spranger, Pfeiffer and Schernthaner16–Reference Abbasi, Chu, Lamendola, McLaughlin, Hayden, Reaven and Reaven19. Macronutrient composition may also have an effect on adiponectin. Kasim-Karakas et al. Reference Kasim-Karakas, Tsodikov, Singh and Jialal20 observed a reduction in adiponectin on a eucaloric low-fat diet which returned to baseline after 6 kg weight loss on a low-fat diet but overall there was no improvement in adiponectin levels.
The aim of the present study therefore was to examine the effect of a low-carbohydrate compared to a conventional high-carbohydrate diet on endothelial function as assessed by FMD and levels of adhesion molecules, and adiponectin after short-term weight loss with long-term follow-up.
We hypothesised that a low-carbohydrate weight-loss diet in comparison with a conventional high-carbohydrate, low-fat diet would be associated with improvements in FMD and adiponectin.
Methods
Study design and subjects
Subjects (101) were recruited by newspaper advertisement. Inclusion criteria were age 20–65 years and BMI 27–40 kg/m2. Exclusion criteria were diabetes mellitus or fasting glucose >7·0 mmol/l, resting blood pressure >150/95 mmHg, medications affecting study measurements, active liver or kidney disease or malignancy, current gastrointestinal disease, pregnancy or lactation, and more than 50 g alcohol/d. The study protocol was approved by the Human Ethics Committee of the Commonwealth Scientific Industrial Research Organisation (CSIRO) and subjects provided informed written consent. The trial was registered with the Australian Clinical Trials Registry (N012605000614695).
Forty-four subjects were selected and matched for age, gender and BMI; seven withdrew before the study commenced for personal reasons. Twenty-five subjects (seventeen female, eight male; mean age 48·7 (sem 1·5) years, weight 94·2 (sem 3·5) kg, BMI 32·9 (sem 0·9) kg/m2, glucose 5·9 (sem 0·1) mmol/l, insulin 14·8 (sem 2·2) mIU/l) completed the active weight-loss part of the study (12 weeks; Table 1). Two subjects were lost to follow-up, seven withdrew for personal reasons, two were unable to comply with the protocol and one withdrew for medical reasons unrelated to the study. Thirteen subjects attended for final assessments at 52 weeks.
Table 1 Baseline characteristics of subjects

DBP, diastolic blood pressure; SBP, systolic blood pressure.
Dietary methodology
In a randomised parallel design subjects were allocated to a weight-loss diet that was either low carbohydrate (LC; ~33 % energy as carbohydrate, 27 % energy as fat, 40 % energy as protein, 7 % energy from saturated fat, 6 % energy from PUFA and 13 % energy from MUFA and 26 g fibre) or high carbohydrate (HC), low fat (~20 % energy as fat, 20 % energy as protein and 60 % energy as carbohydrate, 4 % energy from saturated fat, 5 % from PUFA and 7 % from MUFA and 40 g fibre). Both diets were designed to be ~6000 kJ so that weight loss would approximate 0·5–1 kg per week. Records of daily food intake were maintained in order to monitor food intake and volunteers saw a dietitian individually at the start of weight loss and every 2 weeks. Volunteers attended the CSIRO clinic monthly during follow-up for a consultation with the same qualified dietitian. The dietary data were analysed using FoodWorks version 3.1 dietary analysis software (Xyris Software, Highgate Hill, Australia). In order to ensure that the energy restriction was approximately 30 % below estimated usual intake a FFQ was completed at baseline to assess usual energy and nutrient intakeReference Hodge, Patterson, Brown, Ireland and Giles21 (Table 1).
Weight and height
Fasting body weight (Mercury digital scales, model AMZ14, Tokyo, Japan) was recorded in light clothing without shoes at baseline and every 2 weeks during weight loss and monthly during follow-up. Height was measured on a stadiometer (Seca, Hamburg, Germany) at baseline. BMI was calculated by weight (kg)/height (m2).
Resting (seated) blood pressure
Resting blood pressure (mean of three measurements) was measured by automated oscillometry (845XT/XT-IEC, Dinamap™, Tampa, FL, USA) with subjects in a seated position for 5 min at baseline and every 4 weeks and also at 6 weeks after the start of weight loss when FMD was measured.
Flow-mediated dilatation
Subjects underwent assessment of endothelium-dependent FMD of the right brachial artery, as previously describedReference Anderson, Uehata and Gerhard22, at baseline, after 6 weeks of intensive weight loss when dietary adherence would be at its best and glucose and LDL-C were predicted to be at their lowest level, and at 52 weeks. Subjects were kept quiet for 5 min before vessel diameter measurements which were taken in the morning after an overnight fast, using a 7·5 MHz linear array transducer of an Acuson Aspen ultrasound (Siemens, Denver, CO, USA) before and after forearm ischaemia caused by inflation of a blood pressure cuff, which had been placed around the subjects' forearm approximately 2 cm distal to the olecranon, to 200 mmHg for 5 min. Measurements were also recorded for 4 min at 15 s intervals after a 300 μg tablet of nitro-glycerine was administered sublingually. The operator performing the FMD procedure was blind to the dietary treatment. All FMD measurements were evaluable and none were repeated. Measurements were also recorded for 4 min at 15 s intervals after a 300 μg tablet of nitro-glycerine was administered sublingually. Based on assessments performed on two separate days in nine subjects by the operator, the within-subject CV of the endothelium-dependent response was 8·4 %.
Pulse wave velocity
Aortic pulse wave velocity was measured via doppler recordings at the carotid and femoral arteries at baseline, 6, 12 and 52 weeks. Approximately ten consecutive beats were recorded to cover a complete respiratory cycle. A simultaneous electrocardiogram recording was used to calculate the interval between the R-wave and the upstroke of each sound wave. The difference between the average intervals for each artery was calculated. Pulse wave velocity was then determined by dividing the measured surface distance by this difference.
Measurement of Augmentation Index
Vascular measurements were performed as previously describedReference van Trijp, Beulens, Bos, Uiterwaal, Grobbee, Hendriks and Bots23 using the SphygmoCor™ blood pressure analysis system (AtCor Medical, Sydney, Australia) at baseline, 6, 12 and 52 weeks.
Blood collections
At baseline, 6, 12 and 52 weeks subjects had fasting bloods for the measurement of plasma glucose and insulin, serum total cholesterol, TAG, HDL-cholesterol (HDL-C) and LDL-C. At baseline, 12 and 52 weeks subjects had fasting blood samples taken for high-sensitivity C-reactive protein (hs-CRP), vascular cell adhesion molecule-1 (VCAM1), soluble intracellular adhesion molecule-1 (sICAM1), E-selectin, P-selectin and total adiponectin.
Laboratory analysis
Total cholesterol, HDL-C, TAG and glucose were measured using a Boehringer Mannheim/Hitachi 902 automatic analyser (Boehringer Mannheim GmbH, Mannheim, Germany) and LDL-C concentrations in serum were calculated using the Friedwald equation [(total cholesterol − HDL-C) – (TAG × 0·45)]. High-sensitivity CRP concentrations were measured using a Tina-quant C-reactive protein (latex) high-sensitivity kit (Roche Diagnostics, Rotkreuz, Switzerland), with 0·03 mg/l as the lowest detection limit and an interassay CV of 10 %. Serum insulin was measured by AxSYM microparticle enzyme immunoassay, with 1·0 mIU/l as the lowest limit of detection (Mercodia Insulin ELISA cat. no. 10-1113-10' Mercodia AB, Uppsala, Sweden). Concentrations of sICAM-1, sVCAM-1, E-selectin, P-selectin and adiponectin were determined by an ELISA (ImmunoKontact, Wiesbaden, Germany).
Statistics
ANOVA with repeated measures was used. All analyses were performed using SPSS for Windows version 10.0 (SPSS Inc., Chicago, IL, USA) and statistical significance was set at P < 0·05. All values are means with their standard errors unless otherwise stated.
Power
With twelve people in each group based on a CV for FMD of 10 %, we had sufficient power to measure a change of 12 % in FMD with 80 % probability and a change of 14 % with 90 % probability.
Results
Weight, body composition and waist circumference
When FMD was repeated at 6 weeks subjects had lost 6·1 (sem 0·6) % (P < 0·01) of their initial body weight with no difference between diets. After 12 weeks they had lost 8·7 (sem 0·7) % (P < 0·01) (Table 2). Those volunteers who remained in the study for 52 weeks (n 13) lost 5·6 (sem 1·4) % of their initial body weight with no difference between diets (P < 0·01) (Table 2).
Table 2 Anthropometric variables at 6 and 12 weeks (n 25) and 52 weeks (n 13) (Mean values with their standard errors)
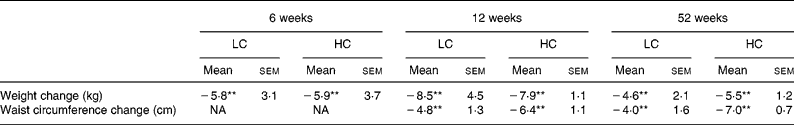
HC, high-carbohydrate diet; LC, low-carbohydrate diet; NA, not applicable.
Mean values were significantly different from those of the baseline: **P < 0·01 (no diet effect).
Waist circumference was also significantly reduced after 12 weeks (and remained reduced at the end of study; P < 0·01 for time with no diet effect).
Dietary intake
Compliance with the protocol was confirmed by the dietary analysis. Energy intake and percentage energy from saturated fat were not different between the groups during the short-term part of the study, 6262 (sem 323) v. 6242 (sem 352) kJ, 7·4 (sem 0·3) v. 3·7 (sem 0·5), HP v. LC, respectively.
As planned, percentage energy from carbohydrate was statistically different between the groups (37·7 (sem 1·5) v. 53·4 (sem 1·1) %, HP v. LC, respectively, P < 0·001). Percentage energy from protein (35·7 (sem 1·1) v. 22·6 (sem 0·7) %, HP v. LC, respectively, P < 0·001) and total fat were also different (24·6 (sem 0·8) v. 20·9 (sem 1·0) %, HP v. LC, respectively, P < 0·01).
Flow-mediated dilatation
At baseline, brachial artery diameter before inflation of the cuff was 4·53 (sem 0·21) mm and 4·63 (sem 0·28) mm, LC and HC, respectively. There was an increase to 4·87 (sem 0·21) mm and 4·8·4 (sem 0·28) mm after the cuff was released, LC and HC, respectively (P < 0·001). After weight loss neither baseline nor post-ischaemic change in FMD were different, 45·8 (sem 1·6) mm LC v. 46·1 (sem 1·3) mm HC, increasing to 48·1 (sem 1·8) mm LC v. 48·3 (sem 1·3) mm HC (P < 0·001), with no difference between the diet groups.
FMD did not change after weight loss of 6·1 (sem 0·6) % (Table 3). FMD was negatively correlated with age (r − 424, P < 0·05) but there was no correlation with any other variable in particular lipid or glucose values.
Table 3 Vascular measures at 0, 6 and 12 weeks (n 25) (Mean values with their standard errors)
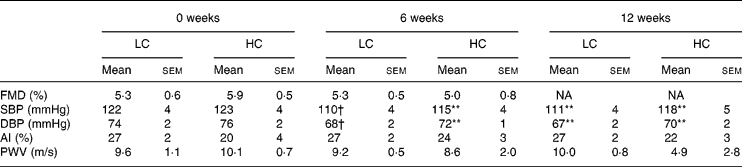
AI, Augmentation Index; DBP, diastolic blood pressure; FMD, flow-mediated dilatation; HC, high-carbohydrate diet; LC, low-carbohydrate diet; PWV, pulse wave velocity; SBP, systolic blood pressure.
Mean values were significantly different from those of the baseline (0 weeks): **P < 0·01; †P < 0·05.
FMD was not different from baseline after sustained weight loss of 5·6 (sem 1·4) % at the end of the study (Table 4).
Table 4 Vascular measures at 0 and 52 weeks (n 13) (Mean values with their standard errors)

AI, Augmentation Index; DBP, diastolic blood pressure; FMD, flow-mediated dilatation; HC, high-carbohydrate diet; LC, low-carbohydrate diet; PWV, pulse wave velocity; SBP, systolic blood pressure.
Mean values were significantly different from those of the baseline (0 weeks): *P < 0·05.
† SBP was different by diet at baseline and at 52 weeks in the thirteen volunteers who participated in the study until 52 weeks.
Resting (seated) blood pressure
Resting systolic and diastolic blood pressure were both lower after 6 weeks of weight loss (P < 0·01) and remained reduced at 12 weeks (P < 0·01) with no further change and no difference between diets (Table 3). At the end of the study diastolic blood pressure remained reduced but systolic blood pressure was not different (Table 4).
Augmentation Index and pulse wave velocity
Augmentation Index, pulse wave velocity and arterial compliance were not different compared to baseline at 12 (Table 3) or 52 weeks (Table 4).
Glucose
There was a small but statistically significant reduction in fasting glucose at 6 weeks ( − 0·22 (sem 0·08) mmol/l), with no effect of diet ( − 0·28 (sem 0·09) v. − 0·13 (sem 0·08) mmol/l LC v. HC, P < 0·05)), which had rebounded somewhat at 12 weeks ( − 0·18 (sem 0·09) mmol/l, P = 0·06; Table 5) but there was no reduction at the end of the study (Table 6).
Table 5 Glucose, insulin and lipids at 0, 6 and 12 weeks (n 25) (Mean values with their standard errors)

HC, high-carbohydrate diet; LC, low-carbohydrate diet.
Mean values were significantly different from those of the baseline (0 weeks): *P < 0·05; **P < 0·01; ***P < 0·001.
Table 6 Glucose, insulin and lipids at 0 and 52 weeks (n 13) (Mean values with their standard errors)
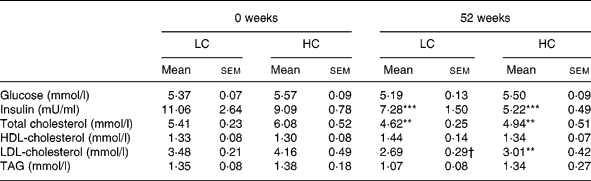
HC, high-carbohydrate diet; LC, low-carbohydrate diet.
Mean values were significantly different from those of the baseline (0 weeks): **P < 0·01; ***P < 0·001; P < 0·05.
Insulin
Insulin was lower after 6 weeks of weight loss ( − 2·87 (sem 0·10) mIU/l, P < 0·01, no diet effect) and was further reduced at 12 weeks ( − 4·43 (sem 1·05) mIU/l, P < 0·001; Table 5). At the end of the study insulin remained reduced from baseline ( − 4·29 (sem 1·12) mIU/l, P < 0·001; Table 6).
Lipids
Total cholesterol fell at 6 weeks ( − 0·88 (sem 0·13) mmol/l, P < 0·01) with no difference between diets and remained reduced at 12 weeks ( − 0·81 (sem 0·14) mmol/l, P < 0·01; Table 5) and at the end of the study ( − 0·95 (sem 0·28) mmol/l, P < 0·01; Table 6).
HDL-C fell from baseline at 6 weeks ( − 0·10 (sem 0·04) mmol/l, P < 0·05; Table 5) but was not different from baseline after 12 or 52 weeks (Table 6).
LDL-C was lower at 6 weeks ( − 0·63 (sem 0·19) mmol/l, P < 0·01, no effect of diet) and remained reduced at 12 ( − 0·71 (sem 0·15) mmol/l, P < 0·01) and 52 ( − 0·95 (sem 0·27) mmol/l, P < 0·01) weeks (Tables 5 and 6).
TAG was reduced at 6 ( − 0·33 (sem 0·01) mmol/l, P < 0·01) and remained reduced at 12 weeks ( − 0·44 (sem 0·11) mmol/l, P < 0·01) with no effect of diet but was not reduced at the end of the study (Tables 5 and 6).
Adhesion molecules: soluble intracellular adhesion molecule-1, soluble vascular cell adhesion molecule-1, E-selectin and P-selectin
There were reductions in sVCAM1 (P < 0·05), sICAM (P < 0·05) and E-selectin (P < 0·01) after weight loss with no diet effect (Table 7), sICAM remained reduced at the end of the study (P < 0·05) whereas the change in sVCAM1 did not reach statistical significance (P = 0·08; Table 5). E-selectin remained reduced at the end of the study (P < 0·05; Table 7). P-selectin did not change after 12 weeks of weight loss but was reduced at the end of the study (P < 0·05).
Table 7 Adhesion molecules soluble intracellular adhesion molecule-1 (sICAM), soluble vascular cell adhesion molecule-1 (sVCAM), E-selectin, P-selectin, adiponectin and C-reactive protein (CRP) at 0 and 12 weeks (n 25) and 0 and 52 weeks (n 13) (Mean values with their standard errors)
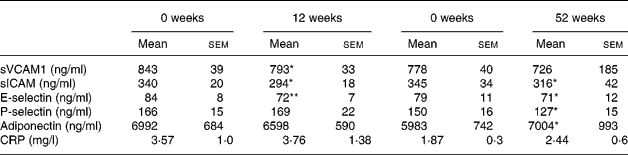
Mean values were significantly different from those of the baseline (0 weeks): *P < 0·05; **P < 0·01.
Adiponectin
Adiponectin did not change significantly after 12 weeks of weight loss although it was lower than the initial level (P = 0·1; Table 5) but it increased at the end of the study (P < 0·05). Adiponectin was negatively correlated with insulin at baseline (r − 0·485, P < 0·05) but not at the end of the study, while HDL was positively correlated at the start and the end of the study (r 0·630, P < 0·01, r 0·778, P < 0·01, respectively). Change in adiponectin was correlated with change in insulin at the end of the study (r 0·552, P = 0·05) and the correlation remained significant but was slightly weaker after adjustment for baseline BMI (r 0·579, P < 0·05). Change in adiponectin was also correlated with change in HDL (r 0·797, P < 0·01, corrected for baseline BMI) negatively with change glucose (r − 0·617, P < 0·05, corrected for baseline BMI). Adiponectin was correlated with systolic blood pressure at baseline (r − 0·427, P < 0·05) and 52 weeks (r − 0·554, P < 0·05) and with diastolic blood pressure at 52 weeks (r − 0·610, P < 0·05) but not at baseline. There were also trends towards negative correlations between change in adiponectin and change in VCAM (r − 0·524, P = 0·08) and intracellular adhesion molecule-1 (ICAM; r − 0·570, P = 0·05).
C-reactive protein
CRP did not change after 12 weeks of weight loss or at the end of the study and this remained true when subjects with CRP>10 mg/l were excluded from the analysis (Table 7).
Discussion
The main finding of the present study was that weight loss on a low-carbohydrate diet which brought about reductions in glucose, insulin and LDL-C did not improve FMD either after short-term weight loss or long-term weight maintenance. Irrespective of diet composition weight loss had beneficial effects in the short term on adhesion molecules and blood pressure and in the longer term on adiponectin and P-selectin. There appears to be a delay in improvement in both adiponectin and P-selectin as these molecules did not in improve until weight loss had been maintained for a year.
Lack of change in FMD in the present study confirms our previous findings that weight loss does not improve FMDReference Clifton, Keogh, Foster and Noakes24. In our previous study FMD did not change with weight loss of approximately 6 kg on two different low-fat diets, with reductions in TAG, insulin, CRP, plasminogen activator inhibitor-1 and sICAM1. Similarly to our results, Brook et al. Reference Brook, Bard, Glazewski, Kehrer, Bodary, Eitzman and Rajagopalan25 did not demonstrate improvement in FMD following weight loss on orlistat with a reduction in LDL-C of approximately 7 % and weight loss of a similar amount.
However, these findings are in contrast to studies showing a benefit on FMD of weight lossReference Bergholm, Tiikkainen, Vehkavaara, Tamminen, Teramo, Rissanen and Yki-Jarvinen2, Reference Raitakari, Ilvonen, Ahotupa, Lehtimaki, Harmoinen, Suominen, Elo, Hartiala and Raitakari3, Reference Hamdy, Ledbury and Mullooly26, Reference Williams, Chowienczyk, Wheatcroft, Patel, Sherwood, Momin, Shah and Kearney27. Differences between the present study and other studies include an exercise component and a much greater weight loss of 23 kg which may also have had an impact on the improvement in FMDReference Hamdy, Ledbury and Mullooly26, Reference Williams, Chowienczyk, Wheatcroft, Patel, Sherwood, Momin, Shah and Kearney27. Improvements in endothelial function, measured as the blood flow responses to intra-arterial acetylcholine have been observed following similar weight loss of 7 kg on orlistat which correlated with a reduction in LDL-CReference Raitakari, Ilvonen, Ahotupa, Lehtimaki, Harmoinen, Suominen, Elo, Hartiala and Raitakari3. One of our goals with the dietary intervention was a reduction in LDL-C which we achieved, 18 % at 6 weeks and nearly 30 % at the end of the study with no effect on FMD. Improvements in FMD which correlated with reductions in fasting glucose of 8 % but not with weight loss per se or the reduction in LDL-C have also been reportedReference Raitakari, Ilvonen, Ahotupa, Lehtimaki, Harmoinen, Suominen, Elo, Hartiala and Raitakari3. The present study was also designed to achieve a reduction in glucose in a 6-week weight-loss intervention on a more moderate diet of 6000 kJ and we achieved this but with no effect on FMD. It is unclear why these conflicting results occur. Different study design and methods to assess endothelial function make it difficult to compare the studies directly.
It is perplexing that in a large (n>2000) longitudinal study in young Finns there was a direct correlation between FMD and BMI whereas most studies report that obesity is associated with impaired FMDReference Raitakari, Ilvonen, Ahotupa, Lehtimaki, Harmoinen, Suominen, Elo, Hartiala and Raitakari3, Reference Benjamin, Larson and Keyes28–Reference Suwaidi, Higano and Holmes30. The authors suggest that an increase in body size in the non-obese range is associated with physiological changes resulting in enhanced FMD. However, BMI is a surrogate measure of body fatness and there are people for whom BMI is not an appropriate measure of obesity and more direct measures of fat mass are neededReference Nevill, Stewart, Olds and Holder31, Reference Prentice and Jebb32.
We acknowledge that weight loss is an intervention that leads to changes in many physiological systems but the extent to which each system changes varies between individuals. A complex physiological response such as FMD may be related to LDL and glucose cross-sectionally and in post hoc analyses but these may not be causally related but correlate in some circumstances with the real unmeasured mediator of change. For instance, oxidative stress may be a major factor in reducing NO bioactivity but reducing LDL levels may have no effect on this even though the endothelial cell is clearly healthier as judged by reduced adhesion molecules.
Adiponectin rose at the end of the present study but not after short-term weight loss. With weight loss of 5 kg sustained at 12 months we observed a 16 % increase in adiponectin, suggesting that it is sensitive to small amounts of weight loss.
Several studies have examined the effect of weight loss on adiponectin with conflicting resultsReference Kopp, Krzyzanowska, Mohlig, Spranger, Pfeiffer and Schernthaner16–Reference Dvorakova-Lorenzova, Suchanek, Havel, Stavek, Karasova, Valenta, Tintera and Poledne18, Reference Abbasi, Lamendola, McLaughlin, Hayden, Reaven and Reaven33. Adiponectin concentrations increased by approximately 28 % after large amounts of weight loss over a long period (39 kg) while diet and physical activity achieved 7 kg weight loss and an increase in adiponectin in eight diabetic subjects but not in the group as a wholeReference Kopp, Krzyzanowska, Mohlig, Spranger, Pfeiffer and Schernthaner16, Reference Monzillo, Hamdy and Horton17. Other studies found that adiponectin levels increased in a rosiglitazone-treated group but not in a weight loss-only group or that there was no improvement in adiponectin after 7 kg of weight lossReference Dvorakova-Lorenzova, Suchanek, Havel, Stavek, Karasova, Valenta, Tintera and Poledne18, Reference Abbasi, Chu, Lamendola, McLaughlin, Hayden, Reaven and Reaven19. A low-fat diet in energy balance has been shown to decrease adiponectin by about 13 % while weight loss of 6 kg on a low-fat diet brought adiponectin back to baseline but with no overall increaseReference Kasim-Karakas, Tsodikov, Singh and Jialal20. Therefore adiponectin did not change in three short-term and one longer-term study with moderate weight loss but increased in two long-term studies, including our own suggesting that it takes time for adiponectin production to adjust to weight loss.
We observed reductions in sVCAM1, sICAM and E-selectin after short-term weight loss and sICAM, E-selectin and P-selectin were reduced at the end of the study whereas VCAM1 was not but this may be due to the small sample size. This has also been observed by othersReference Ferri, Desideri, Valenti, Bellini, Pasin, Santucci and De Mattia10, Reference Pontiroli, Pizzocri, Koprivec, Vedani, Marchi, Arcelloni, Paroni, Esposito and Giugliano34, Reference Ziccardi, Nappo, Giugliano, Esposito, Marfella, Cioffi, D'Andrea, Molinari and Giugliano35.
Limitations of the study
We estimated that for the short-term part of the study we had sufficient power to measure a change of 12 % in FMD with 80 % probability. However, this is still a relatively small study and we had a higher than anticipated dropout rate during the follow-up, making the long-term results less robust. The numbers of subjects are similar to the subject numbers in other studies in which FMD improved with weight loss (e.g. Hamdy et al. Reference Hamdy, Ledbury and Mullooly26, n 24; Vazquez et al. Reference Vazquez, Pazos, Berrazueta, Fernandez-Escalante, Garcia-Unzueta, Freijanes and Amado36, n 26) but smaller that other studies (e.g. Raitakari et al. Reference Raitakari, Ilvonen, Ahotupa, Lehtimaki, Harmoinen, Suominen, Elo, Hartiala and Raitakari3, n 67; Shechter et al. Reference Shechter, Freimark, Beigel, Matetzky and Feinberg37, n 80). In one study the subject numbers are smaller (n 6) than in the long-term part of the present study, however weight loss, 23 kg, was much greaterReference Williams, Chowienczyk, Wheatcroft, Patel, Sherwood, Momin, Shah and Kearney27.
We acknowledge that a relatively small sample size and higher than anticipated dropout rate is a limitation of the study.
In conclusion, weight loss on a low-carbohydrate, low-saturated fat diet, does not improve FMD despite improvement in cardiovascular risk factors. The improvement in adiponectin was delayed.
Acknowledgements
We thank the Diabetes Australia Research Trust (DART) for partial financial support for the trial. We also acknowledge the contribution to the conduct of the trial of Anne McGuffin, Kathryn Bastiaans, Julia Weaver, Rosemary McArthur, Candita Sullivan and Julie Turner.









