In the natural environment, seasonal variations in food supply cause many species of fish to endure periods of starvation(Reference Turano, Borski and Daniels1). During food shortages and nutritional deficiency, fish may experience growth stagnation or even negative growth. However, when the food supply is adequate, the weight increases to different degrees and this phenomenon is called compensatory growth (CG). According to the degree of growth, CG can be divided into over, full, partial and no compensation (Fig. 1). CG is a special survival strategy adopted by animals to adapt to changes in the natural environment and has been extensively studied in many fish species, such as rainbow trout (Oncorhynchus mykiss)(Reference Quinton and Blake2,Reference Sevgili, Hoşsu and Emre3) , Siberian sturgeon (Acipenser baerii)(Reference Chen4), channel catfish (Ictalurus punctatus)(Reference Kim and Lovell5), Amur sturgeon (Acipenser schrenckii)(Reference Gao, Chen and Zhao6), Persian sturgeon (Acipenser persicus)(Reference Yarmohammadi, Shabani and Pourkazemi7), hybrid striped bass (Morone chrysops ×M. saxtilis)(Reference Turano, Borski and Daniels1), black sea bream (Acanthopagrus schlegelii)(Reference Xiao8) and Dabry’s sturgeon (Acipenser dabryanus)(Reference Yang, He and Yan9). However, the mechanism of weight change in A. dabryanus is not clear.
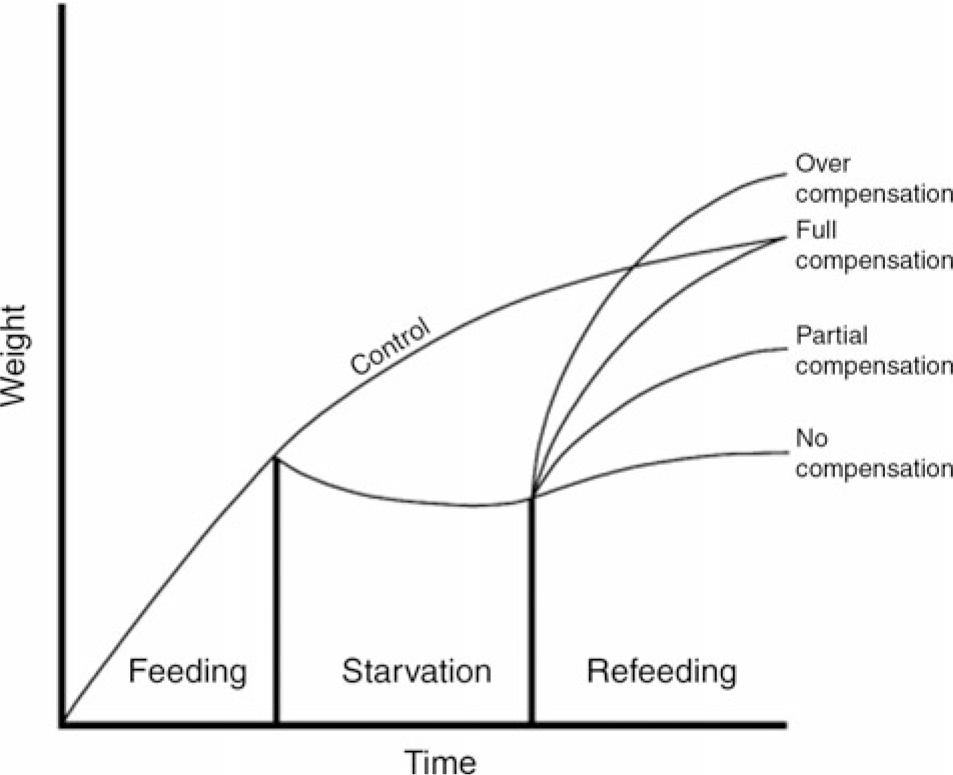
Fig. 1. Idealised patterns of growth compensation.
Starvation and refeeding constitute an important experimental model to study CG in fish. The proper mode of the model can achieve full compensation or even overcompensation(Reference Chen4,Reference Gao, Chen and Zhao6,Reference Whitledge, Hayward and Noltie10) . Most people believe that CG in fish is achieved mainly by increasing feed intake (FI) or improving feed utilisation(Reference Xiao8,Reference Shen and Shou11) , and it was reported that the appetite of fish significantly increased when feeding was resumed after starvation(Reference Xiao8,Reference Oh, Noh and Kang12) . In fish, as in all vertebrates, the regulation of food intake is mediated by signals from neuropeptides via the central nervous system, which receive input regarding metabolic status and changes in energy homoeostasis as well as hunger and satiety signals from the digestive tract(Reference Volkoff, Canosa and Unniappan13,Reference Hoskins and Volkoff14) . Neuropeptide Y (npy) plays an important role in the neural regulation of food intake in fish and acts as an orexigenic factor(Reference Zhou, Liang and Yuan15). Peptide YY (pyy) has been shown to inhibit FI in goldfish (Carassius auratus)(Reference Gonzalez and Unniappan16). As reported in yellow catfish (Pelteobagrus fulvidraco)(Reference Cao17), grass carp (Ctenopharyngodon idellus)(Reference Zhang18) and red-bellied piranha (Pygocentrus nattereri)(Reference Volkoff19), npy and pyy play major roles in the regulation of feeding and the expression of npy and pyy is changed by starvation and refeeding. Whether there are corresponding changes in A. dabryanus remains to be studied.
The growth hormone (GH) and insulin-like growth factor (IGF) axis plays a pivotal role in the neuroendocrine regulation of vertebrate growth(Reference Duan20). A study in juvenile Atlantic salmon (Salmo salar)(Reference Wilkinson, Porter and Woolcott21) showed that GH, IGF-1 and IGF-2 were regulated by nutritional regulation, which may indicate that changes in body weight are affected by fluctuations in GH–IGF. Whether this phenomenon is regulated by GH–IGF in the CG of A. dabryanus has not been reported.
Food deprivation and refeeding of fish cause changes in weight, body composition, organ mass, blood metabolites, etc.(Reference Yarmohammadi, Shabani and Pourkazemi7). These aspects have been investigated in many fish to explore how physiological and structural responses mobilise to adapt to metabolism during fasting and refeeding(Reference Xiao8,Reference Chen22–Reference Tian, Tu and Zeng24) . The utilisation of food in fish is closely related to digestive capacity. The development of digestive tract tissues and the activity of digestive enzymes are important indicators of fish digestive capacity. In channel catfish(Reference Luo, Tan and Wang25), the visceral indexes are significantly decreased in fish during fasting, and after refeeding, the hepatosomatic values were recovered in Persian sturgeon(Reference Yarmohammadi, Shabani and Pourkazemi7). These indicators are widely used in the study of nutrients to reflect the utilisation efficiency of fish feed and may be used to help us explore the mechanism of CG of A. dabryanus.
As a first-class protected animal in China, A. dabryanus shows extremely reduced numbers due to overfishing and water pollution. Therefore, the survival state of A. dabryanus in the wild is of great concern. Our purpose in this study was to simulate the effects of wild food shortage on the physiological and biochemical characteristics of A. dabryanus through starvation and to explore the possible mechanism of CG of A. dabryanus through a 2-week refeeding strategy.
Materials and methods
Experimental design
The F2 generation of A. dabryanus (initial weight 60·532 (sem 0·284) g), reproduced by The Fishery Institute of the Sichuan Academy of Agricultural Sciences, was used. Before the trial, the fish were acclimatised to the experimental conditions for 2 weeks. The trial lasted 28 d, including 14 d of refeeding after all fasting treatments. A total of 120 fish were randomly divided into four groups, and each group had three tanks (diameter of 1·5 m and water depth of 0·8 m): 0D: control group, 0 d of fasting and 14 d of refeeding; 3D: 3 d of fasting and 14 d of refeeding; 7D: 7 d of fasting and 14 d of refeeding and 14D: 14 d of fasting and 14 d of refeeding. The commercial feed contained 41·31 % protein, 10 % lipid, 9·14 % moisture and 13·51 % ash (Table 1). The fish were fed twice a day (08.00 and 18.00 hours). Uneaten feed was collected 30 min after feeding, dried and weighed to calculate FI. The water temperature was 18·5 (sem 2)°C, dissolved oxygen content was 6·24 (sem 0·53) mg/l, pH was 7·0 (sem 0·5), NH4 +-N content was 0·066 (sem 0·003) mg/l and NO2-N content was 0·001 (sem 0·000) mg/l. The feeding trial was completed under a natural light and dark cycle. All experimental protocols were approved by the Animal Care Advisory Committee of the Sichuan Academy of Agricultural Sciences (20180929001A).
Table 1. Nutrient content of diets

Sample collection
At the end of fasting and 14-d refeeding, the fish were anaesthetised by 3-aminobenzoic acid ethyl ester methanesulphonate (MS-222, 60 mg/l) and then counted, weighed and sampled (the samples of the control group were selected before and after refeeding). Three fish were randomly collected from each group (one fish per tank) and were stored at −20°C for the determination of body composition. Blood was collected from the tail vein of three fish from each tank with a 2·5 ml syringe and placed in a centrifuge tube, which was placed at 4°C for 12 h. Then, the brain, muscle, liver, stomach and intestine were sampled for determination of muscle composition, visceral indexes, enzyme activity and quantitative tests, and the samples were stored at −80°C.
Biochemical analysis
The proximate compositions of the diets, fish carcass and muscle were analysed using standard methods of the Association of Official Analytical Chemists (Horwitz, 2000)(Reference Khan, Jafri and Chadha26). Moisture was determined by drying the sample (105°C for 24 h) to a constant weight. The method of Kjeldahl nitrogen determination and Soxhlet extraction is used to measure crude protein and crude lipid, respectively. Ash was determined by incinerating the dried sample at 550°C for 12 h. The blood was centrifuged at 3000 rpm for 10 min, and the supernatant was collected and stored at −80°C for testing. The alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) activities and the concentrations of total protein (TP), albumin (ALB), globulin (GLO), cholesterol (CHOL) and TAG in serum were analysed by a CL-8000 clinical chemistry analyser (Shimadzu). The GH, IGF-1 and IGF-2 levels were analysed by using fish ELISA kits (the Nanjing Jiancheng Bioengineering Research Institute), which were used in competitive method to detect the content of GH, IGF-1 and IGF-2 in samples. Stomach and intestine samples were each homogenised in ten volumes (w/v) of ice-cold physiological saline solution and centrifuged at 6000 g for 20 min at 4°C. The supernatant was collected for enzyme activity analysis. The protein content of all the tissues was determined by the Bradford method. The pepsin, trypsin, chymotrypsin, lipase and amylase activities were assayed as described by Jiang et al. (Reference Jiang, Feng and Tang27).
RNA extraction and quantitative real-time PCR
The procedures of RNA isolation, reverse transcription and quantitative real-time PCR were similar to those previously described by Jiang et al. (Reference Jiang, Wu and Zhou28). Total RNA was extracted from the brain and intestine using the RNAiso Plus Kit (TaKaRa) according to the manufacturer’s instructions followed by DNAse I treatment. Samples were subjected to electrophoresis on 1 % agarose gels to confirm the integrity of the 28S and 18S rRNA bands, and RNA purity was assessed by the 260/280 nm absorbance ratio. Subsequently, 1 μg of total RNA was used to synthesise cDNA using the PrimeScript™ RT Reagent Kit (TaKaRa) according to the manufacturer’s instructions. Specific primers for npy (in brain), pyy (in intestine) and β-actin were designed according to the unigene sequence obtained from transcriptome sequencing of A. dabryanus in our laboratory. All of the real-time PCR analyses were performed in a Bio-Rad CFX Connect System (Bio-Rad) (Table 2). The PCR mixture consisted of 2 μl of the first strand cDNA sample, 1 μl each of forward and reverse primers from 20 μm stocks, 6 μl of RNase-free dH2O and 10 μl of 2× TB Green Premix Ex TaqII (TakaRa). The cycling conditions were 98°C for 2 min, followed by forty cycles of 98°C for 5 s, annealing at a different temperature (Table 2) for each gene for 10 s, and 72°C for 15 s. Melting curve analysis was performed following each real-time quantitative PCR assay to check and verify the specificity and purity of all PCR products. Each sample was amplified in triplicate. The target gene mRNA concentration was normalised to the mRNA concentration of the reference gene β-actin; for detailed methods, refer to Jiang et al. (Reference Jiang, Wu and Zhou28). The target and housekeeping gene amplification efficiencies were calculated according to the specific gene standard curves generated from 10-fold serial dilutions. The expression results were calculated using the 2−ΔΔCT method after verification that the primers were amplified with an efficiency of approximately 100 %.
Table 2. Primer sequence, optimal annealing temperatures (OAT) of genes selected for analysis by real-time PCR
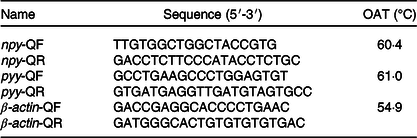
npy, neuropeptide y; pyy, peptide yy.
Statistical analysis
All data are presented as mean values with their standard errors. All data were subjected to a one-way ANOVA by Tukey’s multiple range tests to determine significant differences among the treatments at P < 0·05 with SPSS Statistics version 19.0 (SPSS Inc.).
Results
Growth performance, whole-body protein composition and protein retention value
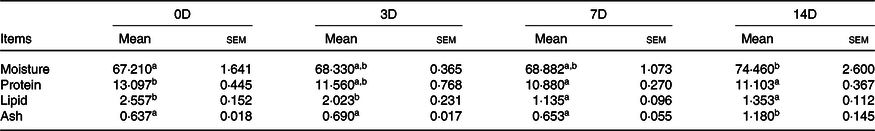
The effects of fasting and refeeding on the growth parameters of A. dabryanus are given in Table 3. The middle body weight was higher in 0D than in the other groups (P < 0·05). Percentage weight gain, specific growth rate, feed efficiency (FE) and protein efficiency ratio were highest in 0D and 3D, thereafter decreased (P < 0·05). Final body weight and FI were the lowest in 14D, and there was no significant difference between 0D and 3D. The whole-body composition and correlation indexes during the fasting phase are displayed in Table 4. During fasting, the moisture content significantly improved from 0D to 14D. The protein content in 14D was the lowest among all the groups (P < 0·05), and there was no difference among the other groups. The lipid content was highest in 0D and then significantly decreased. No significant difference was found in ash content. The whole-body composition and correlation indexes after the refeeding phase are displayed in Table 5. After 14 d of refeeding, the moisture content was minimal for fish in 0D (P < 0·05) and there was no difference among the other groups. The protein content was significantly lower in 14D than in 0D and was not significantly different from that in the other groups. The maximum values of lipids, protein production value and lipid production value were observed in 0D, gradually declining thereafter (P < 0·5). The opposite trend was observed for the ash and ash production value levels.
Table 3. Initial body weight (IBW, g/fish), middle body weight (MBW, g/fish), final body weight (FBW, g/fish), percentage weight gain (PWG), specific growth rate (SGR, %), feed intake (FI, g/fish), feed efficiency (FE, %) and protein efficiency ratio (PER) of Acipenser dabryanus during fasting and refeeding*
(Mean values with their standard errors)

0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups.
† PWG (%) = 100 × (FBW (g/fish) − MBW (g/fish))/MBW (g/fish).
‡ SGR (%) = 100 × (ln FBW − ln MBW)/d.
§ FI (g/fish) = total dry feed intake (g)/number of fish.
|| FE (%) = 100 × (FBW (g/fish) − IBW (g/fish))/FI (g/fish).
¶ PER = weight gain (g)/protein intake (g).
Table 4. Whole body composition (%, wet-basis) of Acipenser dabryanus during fasting*
(Mean values with their standard errors)

0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b,c,d Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups with two fish in each group.
Muscle composition
The effects of fasting and refeeding on the muscle protein composition of A. dabryanus are shown in Tables 6 and 7. During fasting, the levels of moisture and ash were significantly increased in 14D and no significant change was observed among 0D, 3D and 7D. The protein and lipid levels were maximum for fish in the control group and were significantly higher than those for fish in the 7D and 14D groups. During refeeding, the moisture and ash levels in 14D were higher than those in the control group (P < 0·05). The protein and lipid levels in 14D were significantly decreased compared with the control group.
Table 5. Whole-body composition (%, wet-basis), protein production value (PPV), lipid production value (LPV) and ash production value (APV) of Acipenser dabryanus during refeeding*
(Mean values with their standard errors)

0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups with two fish in each group.
† PPV = fish protein gain (g)/total protein intake (g) × 100.
‡ LPV = fish lipid gain (g)/total lipid intake (g) × 100.
§ APV = fish ash gain (g)/total ash intake (g) × 100.
Table 6. Muscle composition (%, wet-basis) of Acipenser dabryanus during fasting*
(Mean values with their standard errors)
0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups with two fish in each group.
Table 7. Muscle composition (%, wet-basis) of Acipenser dabryanus during refeeding*
(Mean values with their standard errors)

0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups with two fish in each group.
Serum biochemical indexes
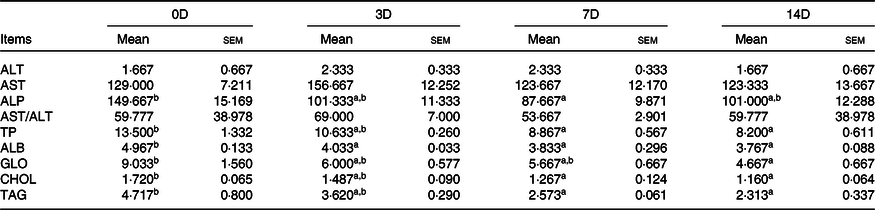
The effects of fasting and refeeding on serum biochemical indexes are shown in Tables 8 and 9. During fasting, ALT, AST and AST/ALT were not significantly different among the groups (P > 0·05). The highest ALP value was observed in 0D, and the lowest was observed in 7D (P < 0·05). The maximum values of TP, ALB, GLO, CHOL and TAG were in the control group, and there was no statistically significant difference among the other groups. During refeeding, ALT, AST, ALP and AST/ALT were not significantly different among the groups. The minimum values of TP, TAG, ALB and CHOL were observed in 14D (P < 0·05), and there were no statistically significant differences in the other groups. The GLO value in 3D was higher than that in 7D and 14D (P < 0·05); however, there was no significant difference between 0D and 3D.
Table 8. Alanine aminotransferase (ALT, U/l), aspartate aminotransferase (AST, U/l), alkaline phosphatase (ALP, U/l), total proteins (TP, g/l), albumin (ALB, g/l), globulin (GLO, g/l), cholesterol (CHOL, mmol/l) and TAG (mmol/l) of Acipenser dabryanus during fasting*
(Mean values with their standard errors)
0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups with two fish in each group.
Table 9. Alanine aminotransferase (ALT, U/l), aspartate aminotransferase (AST, U/l), alkaline phosphatase (ALP,U/l), total proteins (TP, g/l), albumin (ALB, g/l), globulin (GLO, g/l), cholesterol (CHOL, mmol/l) and TAG (mmol/l) of Acipenser dabryanus during refeeding*
(Mean values with their standard errors)
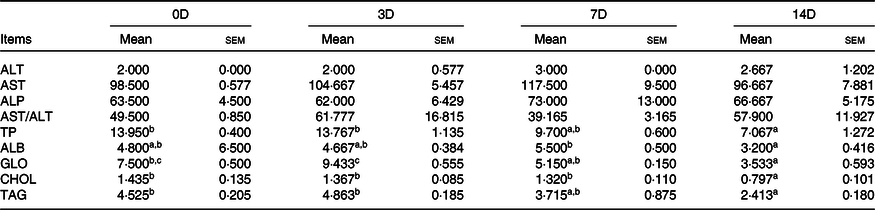
0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups with two fish in each group.
Concentrations of growth hormone, insulin-like growth factor 1 and insulin-like growth factor 2 in serum
The concentrations of GH, IGF-1 and IGF-2 in serum during fasting and refeeding are shown in Fig. 2(A) and (B), respectively. During fasting, GH was significantly increased in the 14D group compared with the control group and no significant difference was found in the other groups. The IGF-1 concentration significantly declined with prolonged starvation, and no obvious change was found among 3D, 7D and 14D. No significant change in IGF-2 concentration was found among the four groups (P > 0·05). During refeeding, the concentration of GH was significantly up-regulated in 3D and 7D and then declined slightly (P > 0·05). The highest IGF-1 concentration was observed in 0D, and no significant change was found in other groups. There was also no significant difference in the IGF-2 concentration.
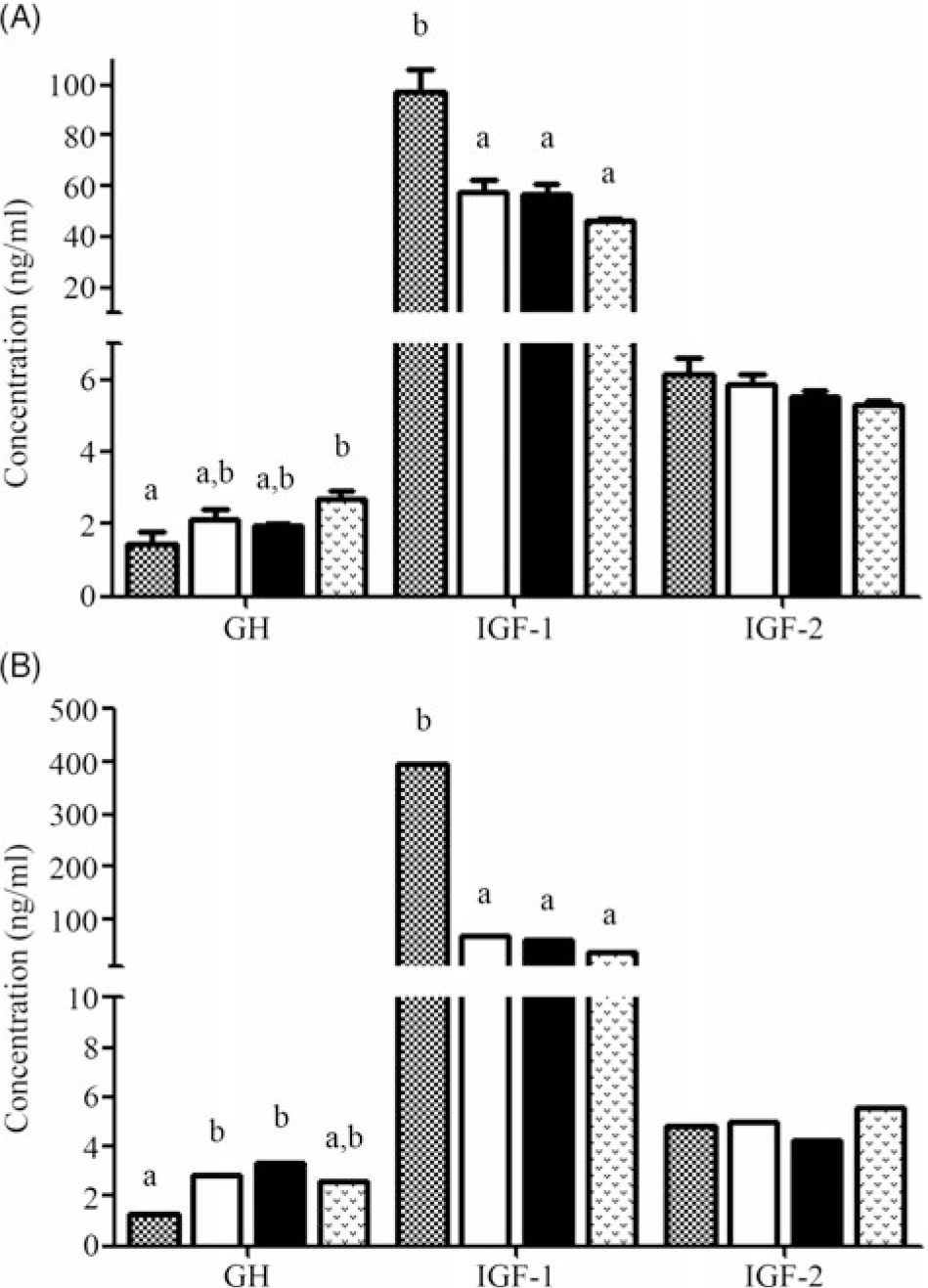
Fig. 2. Concentration of growth hormone (GH, ng/ml), insulin-like growth factor 1 (IGF-1, ng/ml) and insulin-like growth factor 2 (IGF-2, ng/ml) in serum of Acipenser dabryanus during fasting (A) and refeeding (B). Data are mean values with their standard errors, of six replicates. a,b Mean values with unlike letters are significantly different (P < 0·05). ![]() , 0 d of fasting and 14 d of refeeding (0D);
, 0 d of fasting and 14 d of refeeding (0D); ![]() , 3 d of fasting and 14 d of refeeding (3D);
, 3 d of fasting and 14 d of refeeding (3D); ![]() , 7 d of fasting and 14 d of refeeding (7D);
, 7 d of fasting and 14 d of refeeding (7D); ![]() , 14 d of fasting and 14 d of refeeding (14D).
, 14 d of fasting and 14 d of refeeding (14D).
Visceral index
The visceral indexes of fasting and refeeding in A. dabryanus are presented in Tables 10 and 11, respectively. During fasting, there was no significant difference in stomach weight with prolonged starvation. Stomach somatic index, liver weight, intestinal weight, intestinal length, intestinal somatic index and relative gut length exhibited the same trend, with the lowest values observed for 14D compared with the control group, and there was no difference between 3D and 7D. The liver somatic index in 0D and 3D was significantly higher than that in 14D. After 14 d of refeeding, the values of stomach weight, liver weight, liver somatic index, intestinal weight and intestinal somatic index in the 14D group were significantly lower than those in the other groups and the intestinal length and relative gut length in the 14D group were not significantly different from those in the control group. The lowest value of stomach somatic index was in 14D, and there was no significant change in other groups.
Table 10. Stomach weight (SW, g/fish), liver weight (LW, g/fish), intestinal weight (IW, g/fish), intestinal length (IL, cm/fish), stomach somatic index (SSI), liver somatic index (LSI), intestinal somatic index (ISI) and relative gut length (RGL) of Acipenser dabryanus during fasting*
(Mean values with their standard errors)

0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups with two fish in each group.
† LSI = wet liver weight (g)/wet body weight (g) × 100.
‡ ISI = wet intestine weight (g)/wet body weight (g) × 100.
§ RGL = whole intestine length (cm)/body length (cm) × 100.
Table 11. Stomach weight (SW, g/fish), liver weight (LW, g/fish), intestinal weight (IW, g/fish), intestinal length (IL, cm/fish), stomach somatic index (SSI), liver somatic index (LSI), intestinal somatic index (ISI) and relative gut length (RGL) of Acipenser dabryanus during refeeding*
(Mean values with their standard errors)

0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups with two fish in each group.
Digestive enzyme activities
The effects of fasting and refeeding on digestive enzyme activities of A. dabryanus are given in Tables 12 and 13. During fasting, the activity of pepsin was significantly decreased in 14D compared with 0D and 3D. Trypsin was the highest for fish in the control group, and there was no significant difference among 3D, 7D and 14D. Fish in 0D, 3D and 7D had significantly higher levels of chymotrypsin than those in 14D (P < 0·05). Lipase activities were decreased in 7D and 14D compared with 0D and 3D. The activity of amylase in 14D was lowest for fish in 3D (P < 0·05). After 14 d of refeeding, the activities of pepsin, trypsin and lipase in 14D were significantly lower than those in the control group and there was no significant difference between 3D and 7D. The chymotrypsin activity was significantly decreased in 7D and 14D. There was no significant difference in amylase activity.
Table 12. Activities of pepsin (U/mg protein), trypsin (U/mg protein), chymotrypsin (U/mg protein), lipase (U/g protein) and amylase (U/mg protein) of Acipenser dabryanus during fasting*
(Mean values with their standard errors)

0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups with two fish in each group, while quadratic regressions were run with the triplicate data points.
Table 13. Activities of pepsin (U/mg protein), trypsin (U/mg protein), chymotrypsin (U/mg protein), lipase (U/g protein) and amylase (U/mg protein) of Acipenser dabryanus during refeeding*
(Mean values with their standard errors)

0D, 0 d of fasting and 14 d of refeeding; 3D, 3 d of fasting and 14 d of refeeding; 7D, 7 d of fasting and 14 d of refeeding; 14D, 14 d of fasting and 14 d of refeeding.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Values are means with standard errors of three replicate groups with two fish in each group, while quadratic regressions were run with the triplicate data points.
Expression of the neuropeptide Y and peptide YY genes
Relative mRNA expression levels of npy and pyy during fasting and refeeding are displayed in Fig. 3(A) and (B). During fasting, the expression level of npy in 7D was significantly increased compared with that in 0D and 3D and was not significantly different from that in 14D. The pyy mRNA level was highest in 0D (P < 0·05) and was significantly down-regulated thereafter. During refeeding, no significant difference was found in npy and pyy mRNA levels.
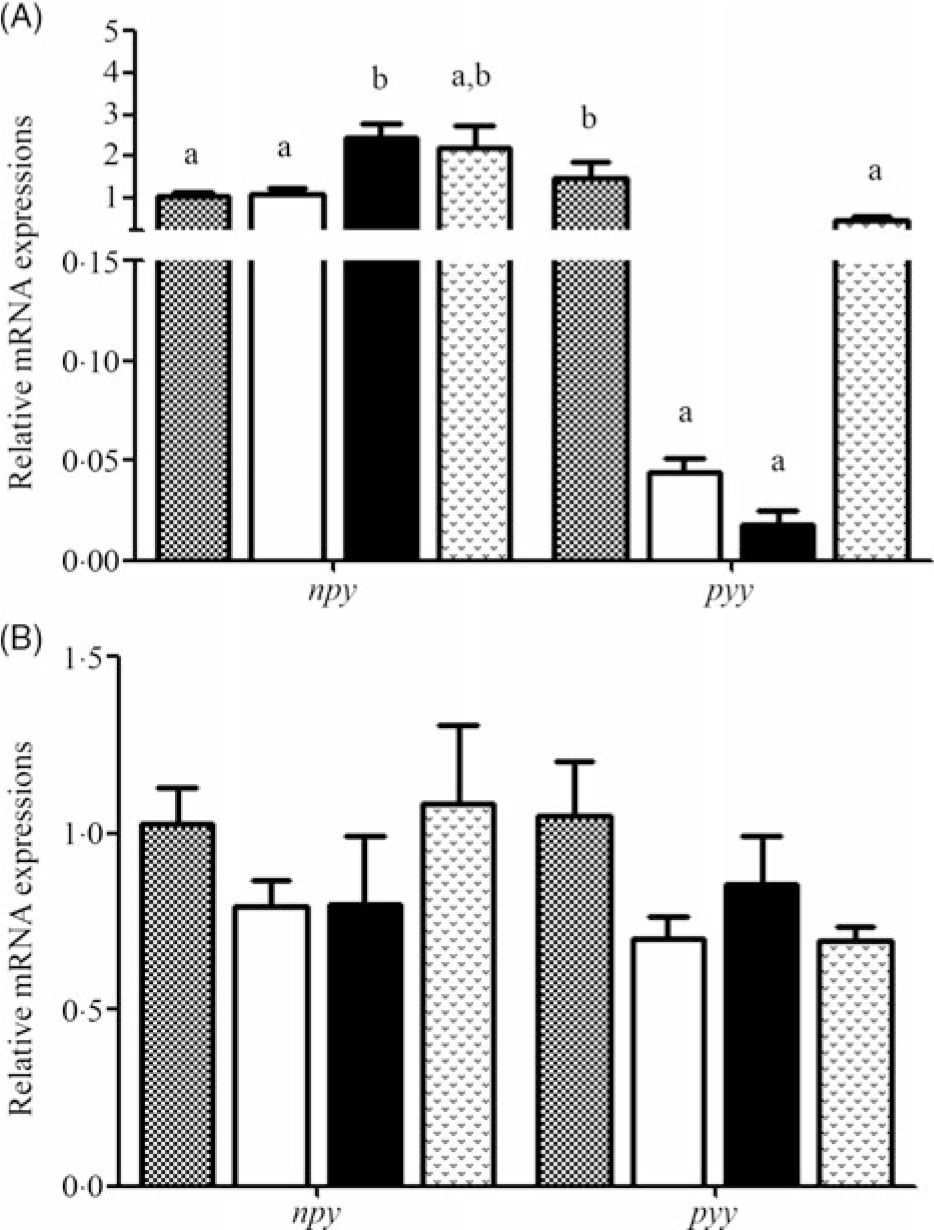
Fig. 3. Relative expression of neuropeptide Y (npy) in the brain and peptide YY (pyy) in the intestine of Acipenser dabryanus during fasting (A) and refeeding (B). Data are mean values with their standard errors, of six replicates. a,b Mean values with unlike letters are significantly different (P < 0·05). ![]() , 0 d of fasting and 14 d of refeeding (0D);
, 0 d of fasting and 14 d of refeeding (0D); ![]() , 3 d of fasting and 14 d of refeeding (3D);
, 3 d of fasting and 14 d of refeeding (3D); ![]() , 7 d of fasting and 14 d of refeeding (7D);
, 7 d of fasting and 14 d of refeeding (7D); ![]() , 14 d of fasting and 14 d of refeeding (14D).
, 14 d of fasting and 14 d of refeeding (14D).
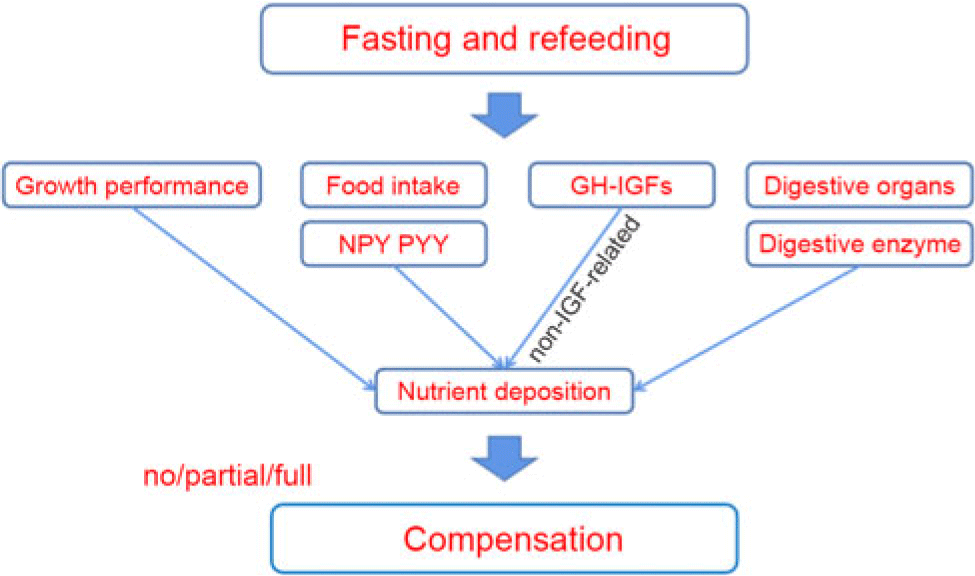
Fig. 4. The mechanism of compensatory growth during fasting and refeeding. NPY, neuropeptide Y; PYY, peptide YY; GH, growth hormone; IGF, insulin-like growth hormone.
Discussion
Growth performance
In the present study, a significant decrease in middle body weight was observed during constant starvation. Similar results have been found in channel catfish(Reference Luo, Tan and Wang25) and gilthead seabream(Reference Peres, Santos and Oliva-Teles29) subjected to 21 and 28 d of fasting, respectively. It is remarkable that after 14 d of refeeding, the final body weight, percentage weight gain and specific growth rate in 7D and 14D were still significantly lower than those in the control group; however, during the 14 d of refeeding, the final body weight in 3D showed no significant difference compared with that in the control group. This result suggests that full compensation is evoked by 3-d starvation and 14-d refeeding, after which A. dabryanus exhibits partial CG.
Based on the above conclusion, we preliminarily explored the CG mechanism of A. dabryanus. At present, most scholars believe that CG is caused by the enhancement of food intake and food conversion efficiency, individually or collaboratively(Reference Hang, Li and Tang30). In our study, the FI and FE changed with our starvation–refeeding pattern and the trends were similar to those of Siberian sturgeon(Reference Chen4) and tongue sole (Cynoglossus semilaevis)(Reference Fang31). The FI and FE during the 3-d starvation and 14-d refeeding were the same as those in the control group; however, even refeeding could not improve the values of FI and FE after 3-d starvation in A. dabryanus. Moreover, correlation analysis showed that percentage weight gain was positively related to FI (r 0·981, P < 0·05) and FE (r 0·980, P < 0·05). This may indicate that the increase in body weight (the compensation growth) in A. dabryanus during starvation and 14-d refeeding was due to the enhancement of FI and FE.
Appetite regulation
It has been reported that npy and pyy play key roles in the control of food intake and body weight(Reference Aldegunde and Mancebo32,Reference Cristina, Blanco and Suraj33) . In goldfish, it has been demonstrated that npy acts centrally to stimulate food intake(Reference Aldegunde and Mancebo32) and pyy exhibits a wide presence and anorexigenic effects(Reference Gonzalez and Unniappan16). Hence, we speculated that npy and pyy might regulate the change in FI in A. dabryanus. Our results showed that during fasting, the expression of npy was significantly increased in 7D and then slightly decreased in 14D. The npy mRNA levels were similar to those in yellow catfish, which were markedly enhanced during 96 and 168 h of starvation(Reference Cao17). The levels were significantly increased in grass carp(Reference Zhang18) and goldfish(Reference Narnaware and Peter34) after 72 h of fasting. Fasting induced a significant decrease in pyy expression in the intestine of red-bellied piranha (P. nattereri)(Reference Volkoff19), and pyy mRNA levels in the brains of goldfish markedly declined after 3 and 7 d of starvation(Reference Gonzalez and Unniappan16). In our study, the expression of pyy in the intestine significantly declined during starvation, although it was slightly up-regulated in 14D, and there was no significant difference compared with the control group. We speculated that this phenomenon is coordinated with the slight down-regulation of npy mRNA levels. This suggested that the expression of npy was increased and that of pyy was decreased during starvation in A. dabryanus, and the appetite of fish increases when they are hungry.
After 14 d of refeeding, our results showed that the expression of npy and pyy was not significantly different among the groups. This may suggest that appetite regulation is rapid under the condition of refeeding and that the expression of npy and pyy remained stable after 14 d of refeeding. In yellow catfish, the npy mRNA levels returned to those in the control group after 3 h of refeeding(Reference Cao17). The npy transcript was found to be dramatically decreased following refeeding in Argus snakehead (Channa argus)(Reference Yang, Wen and Zou35). Similar results were also found at 47 d post-first feeding in Atlantic halibut (Hippoglossus hippoglossus)(Reference Gomes, Jordal and Olsen36), and there was no significant response in the expression levels of pyy between before feeding and 1 and 3 h after feeding(Reference Gomes, Jordal and Olsen36). This implies that the recovery of food may lead to the rapid recovery of npy and pyy mRNA levels in other species; however, whether this phenomenon occurs in A. dabryanus needs further study.
Body and muscle components
Fish weight gain is primarily attributed to the accretion of protein and fat(Reference Bureau, Azevedo and Tapiasalazar37). Starvation can induce metabolic depression(Reference Hidalgo, Morales and Arizcun38). After 14 d of starvation, the changes in the protein and lipid levels were, respectively, reducing 28·05 and 76·89 % in the whole body, and 15·22 and 47·09 % in the muscle. However, the opposite trends were observed for the levels of moisture and ash. Similar results were shown in channel catfish(Reference Luo, Tan and Wang25) and gilthead seabream(Reference Peres, Santos and Oliva-Teles29). It is said that under nutrient depletion, the lipids of fish degrade before the proteins(Reference Xiao8,Reference Bar, Volkoff and McCue39) . This may be the reason for the decline rates of lipids in the whole body and muscle being higher than those of proteins. During refeeding, an obvious downward trend was observed, although the protein content in the whole body was not significantly different among the control group, 3D and 7D. However, our result is different from that for gilthead seabream(Reference Peres, Santos and Oliva-Teles29), which showed no significant difference in protein, lipid, moisture and ash levels after refeeding for 10 weeks. In combination with the values of final body weight and FI, the decline in protein production value in 7D and 14D can be explained. In 0D and 3D, the value of protein production value showed no significant change. As protein synthesis is repressed in both skeletal muscle and liver after short-term fasting and is rapidly stimulated in response to feeding(Reference Kimball, Jefferson and Nguyen40), refeeding can cause proteins in A. dabryanus to accumulate rapidly and reach the level in the control group after 3 d of starvation. However, there was a significant decrease in the lipid production value in the 3D group compared with the control group. This suggests that a large number of lipids are degraded during starvation and that our refeeding cycle is not enough to restore the lipid utilisation efficiency. On the other hand, the results for muscles seem to lag behind those for the whole body, which may be due to the order of different tissues in metabolism.
Serum biochemistry
The nutritional status of fish can be reflected by their serum biochemical indexes, which are closely related to the response of fish to environmental factors(Reference Jahanbakhshi, Imanpoor and Taghizadeh41,Reference Zhang, Wan and Kun42) . ALT and AST are tissue enzymes that may be enriched in blood as a result of leakage from cells in injured tissue. Because the liver is rich in ALT and AST and liver damage could result in the liberation of high levels of these enzymes into the blood, ALT and AST are used as indicators of liver dysfunction(Reference Zaghloul, Hassan and Abdel Kader43). In the present study, we did not find any significant changes in ALT or AST activity or in the AST:ALT ratio in the starvation and refeeding groups. This suggests that the liver is not affected by starvation or refeeding. ALP is a phospholipase that can accelerate the uptake and transport of substances and is related to the metabolism of DNA, RNA, proteins and lipids(Reference Sun23). In our study, the activity of ALP was significantly decreased in the 7D and 14D groups during fasting, similar to the results in Nile tilapia(Reference Tian, Tu and Zeng24). This suggests that the transport efficiency of serum substances is significantly decreased from 7D in A. dabryanus. ALB is an important protein in serum that can repair tissue and regulate plasma colloid osmotic pressure(Reference Wang, Zhu and Xu44). Our results show that the ALB content was significantly decreased at the beginning of starvation. GLO is mainly involved in the non-specific immune response and was decreased in 14D. The TP content includes ALB and GLO and was significantly decreased in 7D and 14D. The levels in yellow catfish(Reference Sun23) were reduced after 12 d of starvation, which is slightly different from our data. This may be caused by differences between the species. In general, these results may imply that starvation can rapidly reduce the abilities of tissue repair and plasma colloid osmotic pressure regulation, and long-term starvation may reduce fish immunity. The levels of TAG and CHOL in serum represent the levels that the body can ingest or synthesise, respectively. In the present study, the levels of TAG and GHOL were significantly decreased in 7D and 14D. These changes are similar to those observed in large yellow croaker(Reference Zhang, Wan and Kun42), yellow catfish(Reference Sun23) and southern catfish(Reference Chen22). During starvation, the lipid content in the whole body and muscle significantly declined, which may indicate that lipids were rapidly used by A. dabryanus to replenish energy. Nevertheless, lipid levels in serum were still lower than those in the control group, which may be closely related to the decrease in body metabolism. Overall, serological indicators also confirm that lipids are quickly used for energy under starvation conditions; however, the effect of short-term fasting (3D) on these indicators is not obvious. This may explain why refeeding after 3 d of starvation can fully compensate for growth.
During refeeding, the results show that no significant difference was observed in ALT, AST, ALP activity and AST/ALT among the four groups. This result suggested that the liver function and transport efficiency of serum substances in A. dabryanus could be retained under normal conditions under refeeding. The TP, TAG, ALB, GLO and CHOL levels were relatively stable in 3D and 7D but were significantly decreased in 14D compared with the control group. This result suggested that even refeeding did not reverse the declines in TP, TAG, ALB, GLO and CHOL levels, which were caused by the 14-d starvation. The results for TAG, TP and CHOL in grass crap(Reference Li, Li and Tan45) had the same trend as those observed in our data. However, the TAG and CHOL levels in Nile tilapia (Oreochromis niloticus)(Reference Tian, Tu and Zeng24) were not significantly different after 14 or 21 d of refeeding, but the activities of ALT and AST significantly declined. These differences suggest that the effects of starvation on metabolism in different fish are diverse, which may be related to the nutrients accumulated by the fish and the ability to withstand hunger stress. In addition, long-term starvation slows down the ability of tissue repair, colloidal osmotic pressure regulation, and immune and fat metabolism in fish.
Growth hormone–insulin-like growth factor axis
It is generally accepted that the GH–IGF axis plays a pivotal role in the neuroendocrine regulation of vertebrate growth(Reference Duan20). Moreover, this progress is directly or indirectly affected by nutritional status. It was reported that 30-, 28-, 15- and 15-d fasting in rainbow trout(Reference Gabillard, Kamangar and Montserrat46), channel fish(Reference Small and Peterson47), Atlantic salmon(Reference Wilkinson, Porter and Woolcott21) and black seabream(Reference Deng, Zhang and Lin48) can significantly increase serum GH, respectively. These results are similar to our results, which exhibited a marked improvement in GH content after 14 d of fasting. However, the value for channel fish in Small’s(Reference Small and Peterson47) research indicated little or no effect of 21-d fasting on plasma GH levels(Reference Small49). The reasons for the difference may be related to the species, and the fluctuation in GH levels in the body exhibits rebalancing over time(Reference Fox, Breves and Hirano50). The level of IGF-1 was significantly reduced in many teleost species, such as tilapia(Reference Fox, Breves and Davis51), Atlantic salmon(Reference Wilkinson, Porter and Woolcott21) and channel fish(Reference Small and Peterson47). It has been reported that fasting typically has the opposite effect on circulating concentrations of IGF-1 in fish and mammals(Reference Small49). The IGF-1 content in our study was significantly decreased by starvation, similar to the results in channel fish(Reference Small49), Atlantic salmon(Reference Wilkinson, Porter and Woolcott21), juvenile chum salmon(Reference Taniyama, Kaneko and Inatani52) and tilapia(Reference Fox, Breves and Hirano50). The mechanism underlying the regulatory effect of fasting on GH and IGF in fish is not clear, but there are two points of view regarding the increased GH levels and decreased IGF-1 levels. One view supports the idea that the reduced hepatic binding capacity for GH during fasting was one of the mechanisms responsible for the decline in circulating IGF-1, and the other hypothesises that the observed increase in circulating GH after fasting was due to low plasma IGF-1 levels, resulting in reduced negative feedback effects on GH synthesis and release(Reference Small49). The level of IGF-2 showed no significant difference during starvation in our results; however, this is different from the results for rainbow trout and Atlantic salmon(Reference Wilkinson, Porter and Woolcott21). In juvenile Atlantic salmon(Reference Wilkinson, Porter and Woolcott21), starvation for a period of 15 d caused a 19 % decrease in circulating IGF-2 levels, but there was no significant difference in 22-d starvation. In fact, our results for GH, IGF-1 and IGF-2 during the 14-d starvation are most similar to those observed in juvenile Atlantic salmon during a 22-d starvation(Reference Wilkinson, Porter and Woolcott21,Reference Dalbagni53) . Due to the lack of research on IGF-2, the mechanism is not clear.
During refeeding, the GH level was significantly increased in the 3D and 7D groups and there was no significant difference between the 14D and control groups. This suggests that the short-term food shortage caused a significant rise in serum GH after the 14-d refeeding; however, the GH content during long-term food shortage may slowly increase even when the nutrition becomes available again. The levels of IGF-1 were significantly decreased in the refeeding groups compared with the control group, but there was no significant difference among the refeeding groups. It may be inferred that refeeding cannot alleviate the effect of serum IGF-1 under our starvation conditions. These changes in GH and IGF-1 in 14D are still most similar to those observed in juvenile Atlantic salmon starved for 15 d and refed for 7 d(Reference Wilkinson, Porter and Woolcott21). However, it has been reported that the serum IGF-1 level and the expression of igf-1 in the liver were restored to the levels in the control group with 14-d fasting and 14-d refeeding in gibel carp(Reference Shen, Ren and Zhu54). This may suggest that different fish may have different compensation mechanisms. In the present study, IGF-2 remained unchanged in the refeeding groups. It has been shown that the serum IGF-2 in A. dabryanus is not sensitive to changes in nutrients, but the underlying reason is unknown. On the other hand, full/partial CG was observed after 14 d of refeeding; however, the trend for IGF-1 did not seem to match this result. As proposed by Wilkinson et al. (Reference Wilkinson, Porter and Woolcott21), the reason could be that the non-IGF-related GH activity promotes growth following the removal of GH resistance at the receptor level(Reference Wilkinson, Porter and Woolcott21).
Digestive function
Rapid changes in both gut physiology and enzyme activity can be observed at the early stages of starvation(Reference Bar, Volkoff and McCue39). In neon damselfish (Pomacentrus coelestis)(Reference Hall and Bellwood55), gut length was reduced after a short-term starvation period. In this trial, the effect of starvation on intestinal length was the most obvious in 3D. Luo et al. (Reference Luo, Tan and Wang25) reported a more linear decrease in VSI, hepatosomatic and IPR in channel catfish for up to 80 d of starvation. Similar results were observed in white sturgeon with 10 weeks of starvation(Reference Hung, Liu and Li56). Our results show that all the visceral indexes except stomach weight were markedly decreased in 14D. Although there was no significant difference in stomach weight, there was a downward trend. These results suggest that prolonged food deprivation can inhibit the growth of digestive organs.
Our study shows that the activities of pepsin, chymotrypsin and amylase, but not those of trypsin and lipase, decreased significantly in 14D. The activity of trypsin was rapidly reduced by 81·19 % in 3D, and the activity of lipase was reduced by 65·18 % in 7D. In Adriatic sturgeon, as previously reported, the activities of amylase and protease declined continuously throughout the 72 d of starvation(Reference Furné, García-Gallego and Hidalgo57). This suggests that digestive enzyme activity was down-regulated by starvation. Interestingly, slight increases were observed in pepsin, chymotrypsin and amylase, but there were no significant differences compared with the control group. The mechanisms underlying these results are not clear.
After 14 d of refeeding, the growth and development of digestive organs, except intestinal length and RGL, still declined in 14D. However, hepatosomatic values in Persian sturgeon(Reference Yarmohammadi, Shabani and Pourkazemi7) and A. dabryanus (Reference Yang, He and Yan9) returned to the levels in the control fish after 4 weeks of refeeding(Reference Yarmohammadi, Shabani and Pourkazemi7). This suggests that the effects of prolonged starvation on the digestive organs are enormous, and even refeeding takes a long time to reverse these effects. On the other hand, the effect of refeeding on digestive enzyme activities in A. dabryanus seemed weaker than those on the development of digestive organs. While there was no significant difference in amylase activity during refeeding, the activities of trypsin and lipase were still lower than those of the control group. As reported in Atlantic cod(Reference Bélanger, Blier and Dutil58), the trypsin activity in D10f (10 weeks of food deprivation and 24 d of refeeding) did not show overcompensation, suggesting that this enzyme does not exert a substantial regulatory effect on CG(Reference Bélanger, Blier and Dutil58). This suggests that the effect of refeeding on the decrease in digestive enzyme activities caused by starvation was not significant, especially after long-term food deprivation, and that the CG in A. dabryanus is not closely related to digestive enzyme activity.
In general, we preliminarily explored the possible mechanism of compensation in A. dabryanus by starvation and refeeding (Fig. 4). Through further exploration, we showed that hunger stress can inhibit the growth of A. dabryanus; however, short-term food deprivation (3 d in our study) has little effect on the physiological metabolism and digestive capacity of the fish. Finally, full CG of A. dabryanus was observed with 3-d fasting and 14-d refeeding. This may be caused by an increase in appetite, as reflected by the expression of npy and pyy, and another reason may be the non-IGF-related GH activity observed following the removal of GH resistance at the receptor level. Although the development of digestive organs was slowly restored, digestive capacity seemed to have no substantial effect on CG. On the other hand, this may suggest that the optimal growth benefit for A. dabryanus can be achieved by adjusting its dietary rhythm during the process of artificial propagation.
Acknowledgements
Thanks for the efforts by all of the members in our project team, as well as the assistance of the base colleagues.
This work was supported by Frontier project of Sichuan Academy of Agricultural Sciences (2019QYXK-21), Sichuan Science and Technology Program (2018JY0636), Sichuan Science and Technology Program (2016NYZ0047), the Project of Research on Large-scale Artificial Breeding and Breeding Technology of Triplophysa siluroides (2016TSCY-004) and National modern agricultural industrial technology System Sichuan Freshwater fish Innovation team.
X.-Y. W., Q. G. and Z.-H. L. designed the study; J.-S. L. and M.-J. S. conducted the trial; Y.-X. W., Y.-Y. C. and Y. L. performed the experiment and analysed the data; X.-Y. W. wrote the manuscript.
The authors declare that there are no conflicts of interest.




















