Ethiopia has passed through a long and troubled history of famines due to frequent drought and desertification(Reference Ramakrishna and Demeke1). Notably, a widespread famine affected the country from 1983 to 1985, where its epicentre was the northeast region of the country namely, Tigray and Wollo(Reference Dercon and Porter2–Reference Keller4). In a famine scale, it was ranked as ‘Great Famine’ due to its global catastrophic impact in the country compared with other famine that had ever happened in Ethiopia or Africa(Reference Gráda5). In the memory of the famine survivors, it was named ‘Kefu Qan’ translated to ‘Evil days’(Reference Watch6).
Due to the short-term catastrophic consequences of the famine, mass migrations of people, social breakdown, loss of personal pride or value and massive morality had hugely occurred(Reference Scrimshaw7). However, the long-term impact or far-reaching consequences of the famine exposure are yet to be explored. Famine exposure in the early life, particularly in the prenatal period, which is a critical time for growth and development, could result in various adverse health consequences such as non-communicable diseases (NCD)(Reference Barker8).
Among the NCD, the metabolic syndrome is the commonest consequences of malnutrition during early life(Reference Stanner9). The metabolic syndrome includes different metabolic changes such as elevated blood pressure, dyslipidaemia, hyperglycaemia, BMI and waist circumference(Reference Alberti, Zimmet and Shaw10). Earlier studies have documented adult lifestyles such as cigarette smoking, alcohol drinking, physical inactivity and unhealthy dietary habits as a risk factor for the development of the metabolic syndrome(Reference Bigna and Noubiap11–Reference Yusuf, Hawken and ⓞunpuu13). Accordingly, healthcare policymakers, programmers and planners have used the distribution of these factors to formulate healthcare interventions and gauge their outcome. Subject-specific intrinsic attributes of one’s biology, however, are usually neglected, as they often are considered as non-modifiable risk factors(Reference Baird, Jacob and Barker14).
Barker et al. have introduced a theory called ‘Fetal origin of adult diseases (FOAD)’ or ‘developmental origins of health and disease (DOHaD)’ to explain the impact of prenatal undernutrition on adult health and diseases such as the metabolic syndrome(Reference Baird, Jacob and Barker14-–Reference Barker18). Empirical understanding of the link between the metabolic syndrome with prenatal adverse environment requires natural experiments such as famine, an epidemic or a disastrous events that occurred during the early life of an individual(Reference Moraru, De Almeida and Degryse19–Reference Almond21). Earlier famine studies conducted in Dutch(Reference de Rooij, Painter and Holleman22), China(Reference Wang, Wang and Li23–Reference Zheng, Wang and Ren25), Bangladesh(Reference Finer, Iqbal and Lowe26), Nigeria(Reference Hult, Tornhammar and Ueda27) and Holocaust survivors(Reference Bercovich, Keinan-Boker and Shasha28) have contributed substantial evidence on the current understanding of developmental origins of health and disease including the metabolic syndrome.
Intra-uterine exposure to different adverse risk factors including malnutrition, however, could be modified or even get worsen during later life, indicating that earlier exposure to famine is not the only factor in shaping adult health outcomes(Reference Amuna and Zotor29,Reference Fall30) . This is well documented in different studies that had indicated the impact of childhood environment in modifying the impact of risks acquired during fetal life(Reference Cohen, Stern and Rusecki20,Reference Li, Jaddoe and Qi24,Reference Fall30–Reference Wells32) .
One of the opportunities to better understand the long-term consequence of prenatal risk exposure is natural famine such as the Chinese great famine(Reference Almond, Edlund and Li33), the 1983–1985 Ethiopian great famine(Reference Dercon and Porter2) and the Dutch famine(Reference Roseboom, Van Der Meulen and Ravelli31). Presumably, such studies could explain the recent increment of NCD in Ethiopia and also give more insights on the fetal origin of adult diseases or developmental origins of health and disease hypothesis. In Ethiopia, attempt was not yet made to document the effect of the great famine on the health and well-being of the survivors. Hence, we conducted this study to evaluate the effects of prenatal famine exposure on the metabolic syndrome in adults among survivors of the 1983–1985 famine in Wollo province, Ethiopia.
Subjects and methods
Study setting and design
The study was conducted in North Wollo Zone, Raya Kobo district, Northern Ethiopia. The district was the epicentre for the 1983–1985 Ethiopian great famine(Reference Dercon and Porter2). The district is estimated to have a total population of 228 798 resided over thirty-two rural and four urban kebeles (lowest administrative unit)(Reference Muluken34). A historical cohort study design was employed from 15 March to 30 April 2019 to collect data from the survivors of the famine to investigate the effect of prenatal famine exposure on adulthood metabolic syndrome.
Study subjects
The study subjects were adult men and women aged 30–36 years who had a history of prenatal exposure to famine. Self-reported birth date and age of the subjects were used to classify the status of famine exposure. Windows for famine exposure were determined using the start and end dates of the famine(Reference Dercon and Porter2). Accordingly, the study subjects were categorised into two groups: exposed, age from 34 to 36 years with date of birth ranging from 8 August 1983 to 30 August 1985; non-exposed, age from 30 to 32 years with date of birth ranging from 8 September 1987 to 8 October 1988. Subjects born immediately after the end of the famine were not considered in this study. This is to get an optimal washout period between exposed and non-exposed groups. Thus, subjects born between 8 September 1986 and 30 August 1987 were excluded from the study (online Supplementary File 1). Adults who were displaced to other area of the country and those who were in other location during the famine, physically disabled subjects with deformity (Kyphosis, Scoliosis and limb deformity) and pregnant women were excluded from the study.
The sample size was calculated by applying two population proportion formulas using Epi-Info version 7 and taking type I error 5 %, 80 % power, a design effect of 1·5, 5 % non-response rate and a 1:1 ratio of exposed group:non-exposed group (r 1). Assuming the prevalence of diabetes mellitus in prenatal exposed group (22·6 %) and non-exposed group (9·8 %) from a study conducted in China(Reference Wang, Cheng and Han35), the total calculated sample size was 456 (228 exposed and 228 non-exposed). A multistage stratified random sampling technique was used to select the study subjects (Fig. 1).

Fig. 1. Flow diagram representing sample recruitment.
Data collection
The data were collected through questionnaire, anthropometric and biochemical measurements. A pretested structured questionnaire was used to collect data on subjects’ status to main exposure variable, background and socio-economic characteristics. Four trained and experienced clinical nurses collected the questionnaire, anthropometric data and blood pressure measurements. Three laboratory technologists experienced in lipid profile analysis collected the biochemical measurements.
Measurements
Outcome
The outcome variable, the metabolic syndrome, was measured using the International Diabetes Federation criterion of 2005(Reference Alberti, Zimmet and Shaw36). Based on the International Diabetes Federation, the definition of the metabolic syndrome must include central obesity (waist circumference ≥83·7 cm in Ethiopian men and ≥78·0 cm in Ethiopian women)(Reference Sinaga, Worku and Yemane37) plus any two of the following: (1) elevated blood pressure: systolic ≥ 130 mmHg, diastolic ≥ 85 mmHg or use of antihypertensive medication; (2) hypertriacylglycerolaemia: fasting serum TAG ≥ 1·7 mmol/l or on TAG treatment; (3) reduced HDL-cholesterol: fasting HDL-cholesterol <1·03 mmol/l in men and <1·30 mmol/l in women or on HDL-cholesterol treatment; and (4) hyperglycaemia: ≥5·6 mmol/l or previously diagnosed diabetes.
Anthropometric measurements
Height of the study subjects was measured to the nearest 0·1 cm using a stadiometer (Seca®) with the subjects positioned at the Frankfurt Plane and the four points (heel, calf, buttocks and shoulder) touching the vertical stand and their shoes taken off. Weight was measured using a portable battery-operated Seca® digital scale. Waist and hip circumference were measured in centimetres using constant tension tapes (Seca®). All anthropometric measurements were done in triplicate, and the average value was used for further analyses(Reference Riley, Guthold and Cowan38). Standardisation exercise was done to reduce inter-observer error. The scale was checked, read zero and standardised using an object of known weight before measurement.
Blood pressure measurement
Blood pressure was measured in triplicate using digital blood pressure measurement after 5 min of rest. The subsequent measurements were done 5 min apart. During data analysis, the mean of the second and third readings was calculated(Reference Riley, Guthold and Cowan38).
Biochemical measurements
Biochemical measurements included fasting total cholesterol, plasma glucose, TAG and HDL. Five millilitres of venous blood were collected from each subject in plane test tubes after overnight fasting (8–12 h), and serum was separated immediately, put in ice bag and safely transported to Amhara Public Health Institute, Dessie branch reference laboratory. The extracted serum was used to determine the level of HDL-cholesterol, TAG and fasting glucose using an A-25 bio-system® clinical chemistry analyser. LDL level was determined using the Freidwald formula as follows: LDL-cholesterol (mmol/l) = total cholesterol – (HDL-cholesterol + (TAG/5))(Reference Hata and Nakajima39). Standard operating procedures were followed to collect blood samples and perform laboratory analysis(Reference Riley, Guthold and Cowan38).
Covariates
In addition, different sets of covariates to reflect subject’s sex, age, educational status, marital status, residence, occupational status, wealth index, dietary pattern, substance use and physical activity were collected. Household wealth index was assessed by using the principal component analysis(Reference Vyas and Kumaranayake40). Physical activity was assessed by using the International Physical Activity Questionnaire(Reference Craig, Marshall and Sjöström41). Subjects who use the specified substance (smoking, drinking and Khat chewing) in the past 3 months and once in their life time were considered as current users and ever users, respectively(Reference Humeniuk, Ali and Babor42). Dietary pattern was assessed using a qualitative FFQ composed of thirty-eight food items covering the main foods consumed in the study area(Reference Aragie and Genanu43,Reference Selinus44) . Furthermore, the lists of food items were developed based on an extensive interview of the key informants who know the culture and the types of foods consumed in the study area. The FFQ was pretested on forty-five study subjects, and reliability of the FFQ was checked (Cronbach’s α-coefficient = 0·80). The study subjects were asked about their consumption of the food items over the previous 1 year before the study. Sex of the subjects and residency were considered potential effect modifiers. History of raised blood pressure, diabetes and dyslipidaemia were assessed by interviewing adults.
Ethics approval and consent to participate
Ethical clearance was obtained from Institutional Review Board of Jimma University (reference no. JHRPGD/660/2019). Amhara Public Health Institute, North Wollo Zone and Raya Kobo district health office were informed about the study objectives through the letter written from Jimma University IRB office. Written informed consent was taken from all selected subjects. The study subjects were assured that they are free to withdraw their consent and discontinue participation with no need to justification. Privacy and confidentiality of subjects were ensured. The laboratory result was given for adults whose biochemical tests were outside of the normal reference range, and they were referred to the nearby public health facility.
Statistical methods
The data were doubly entered to Epidata 3.1 and exported to SPSS version 25 (SPSS Inc.) for analysis. Categorical variables were described as percentages and compared using Pearson’s χ 2 test. Continuous variables were reported as mean values and standard deviations when normally distributed or medians and interquartile ranges for skewed distribution and compared using independent t test. Household wealth index score was generated from the data using principal component analysis. Then, the principal component analysis scores were grouped into wealth tertile. Ownership of radio, television, motor cycle, electric/kerosene/gas stove, mattress, cart, mill, plough and domestic animals was considered for the wealth index. A dietary pattern was derived by using K-means cluster analysis. Two major dietary patterns were identified as healthy and unhealthy dietary pattern based on the subject’s fruits and vegetable consumption. The healthy dietary pattern indicates high consumption of fruits and vegetables, and the unhealthy dietary pattern indicates low fruit and vegetable consumptions.
Logistic regression analysis was used to evaluate the association between famine exposure in prenatal life and the metabolic syndrome in adults. The estimate (OR) and 95 % CI were reported to describe the associations. Covariates were selected based on evidence from existing literatures and the presence of biologically plausible relationships between the covariates and the outcome variable. Covariates that change (affect) the estimate for the main exposure were included in the regression model. Three sets of regression models were developed. The first set of regression model contained the outcome variable (the metabolic syndrome) and famine exposure status. The second set of regression model was built on the first model and further adjusted for sex and age. The third set of regression model was built on the second model and further adjusted for BMI, physical activity level, cigarette smoking, alcohol drinking, residence, wealth index, occupational status, marital status and interaction terms. Moreover, stratified analysis by sex and residence was performed, and the interaction of famine-exposed groups with sex and residence was performed by likelihood ratio test. All analyses were two-sided, and a P value of 0·05 was used to declare a significant difference.
Results
A total of 447 subjects, 222 (49·7 %) prenatally famine exposed and 225 (50·3 %) non-exposed groups, were enrolled to the study. The mean ages for exposed and non-exposed groups were 35·14 (sd 0·86) and 31·25 (sd 0·65) years, respectively. A total of 135 (60·8 %) of the subjects were females exposed to famine during prenatal life. Of the subjects, 180 (81·2 %) were rural residents and prenatally exposed. Eighty-four (37·8 %) of them cannot read and write among prenatally exposed groups (Table 1).
Table 1. Background characteristics of Ethiopian great famine-exposed and non-exposed groups in North Wollo Zone, Raya Kobo district, Northeast Ethiopia, 2019†
(Mean values and standard deviations; numbers and percentages)

* Statistical significance.
† P value represents independent-samples t tests for continuous variables or χ 2 test for categorical variables.
Magnitude of the metabolic syndrome
The metabolic syndrome among exposed and non-exposed groups was 21·2 and 8·4 %, respectively (Fig. 2). The metabolic syndrome among women and men was 17·1 and 11·6 %, respectively (Fig. 3). It was 16·7 and 14·3 % among rural and urban residents, respectively (Fig. 4).
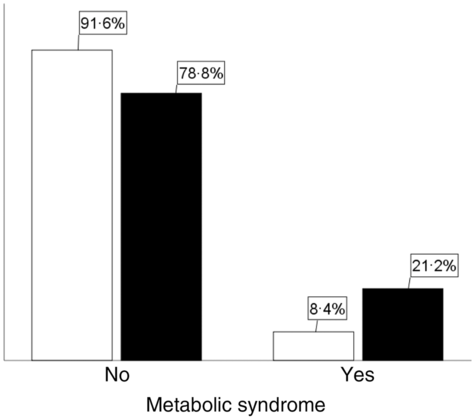
Fig. 2. Magnitude of the metabolic syndrome among famine-exposed and non-exposed adults. Exposure status: ![]() , non-exposed;
, non-exposed; ![]() , prenatal-exposed.
, prenatal-exposed.

Fig. 3. Metabolic syndrome between famine-exposed and non-exposed groups stratified by sex. ![]() , Female;
, Female; ![]() , male.
, male.

Fig. 4. Metabolic syndrome between famine-exposed and non-exposed groups stratified by residence. Residence: ![]() , rural;
, rural; ![]() , urban.
, urban.
Prenatal famine exposure and the metabolic syndrome in adults
On multivariable logistic regression analysis, adults who had prenatal exposure to famine were 2·94 times more likely to have the metabolic syndrome compared with non-exposed groups (adjusted OR 2·94, 95 % CI 1·66, 5·27) after being adjusted for sex, age, BMI, dietary pattern, physical activity, cigarette smoking, alcohol drinking, educational status, marital status, residence, wealth index, occupational status and interaction terms (sex and famine exposure, residency and famine exposure) (Table 2). More specifically, associations between famine exposure and markers of the metabolic syndrome were observed in the present study. We found that famine exposure during prenatal life was significantly associated with waist circumference (2·27 cm, 95 % CI 0·28, 4·26), diastolic blood pressure (2·47 mmHg, 95 % CI 0·84, 4·11), TAG (0·20 mmol/l, 95 % CI 0·10, 0·28) and fasting blood glucose (0·24 mmol/l, 95 % CI 0·04, 0·43) (Table 3). Although there was no statistically significant association between other markers of the metabolic syndrome and prenatal famine exposure, tendencies on the way to positive associations were observed for most of the metabolic markers.
Table 2. Associations between prenatal famine exposure and adulthood metabolic syndrome, North Wollo Zone, Raya Kobo district, Northeast Ethiopia, 2019†
(Adjusted odds ratios (AOR) and 95 % confidence intervals)
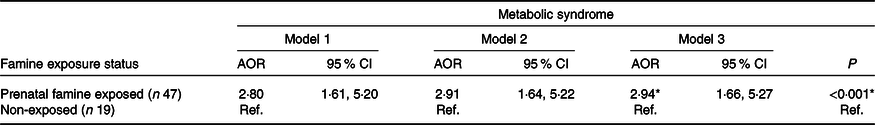
Ref, reference.
* Statistical significance.
† All OR are related to the non-exposed groups. Model 1: metabolic syndrome with famine exposure (unadjusted for any covariate). Model 2: model 1 adjusted for sex and age. Model 3: model 2 further adjusted for BMI, dietary pattern, physical activity, cigarette smoking, alcohol drinking, educational status, marital status, residency, wealth index, occupational status and interaction terms (sex and famine exposure, residency and famine exposure).
Table 3. Associations between prenatal famine exposure and adulthood metabolic risk markers, North Wollo Zone, Raya Kobo district, Northeast Ethiopia, 2019†
(Mean values and standard deviations; mean differences and 95 % confidence intervals; medians and interquartile ranges (IQR))
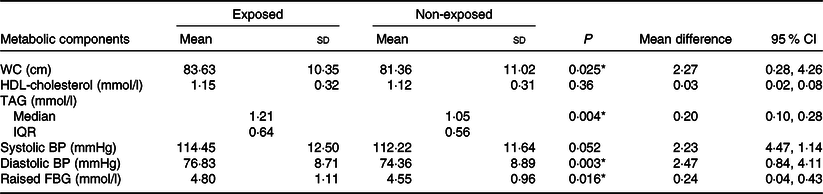
WC, waist circumference; BP, blood pressure; FBG, fasting blood glucose.
† P value represents independent t test for continuous variables.
* Statistical difference.
Further stratified analysis by sex was performed. The highest prevalence of the metabolic syndrome was observed in the female subgroup (adjusted OR 3·60, 95 % CI 1·34, 9·66). Similarly, stratified analysis by residency showed that rural residents have higher risk of the metabolic syndrome (adjusted OR 1·86, 95 % CI 1·65, 2·80; Table 4). However, we observed no significant interaction between famine-exposed group and sex and residency on the risk of the metabolic syndrome (P interaction > 0·05) (data not shown).
Table 4. Associations between famine exposure in prenatal life and adulthood metabolic syndrome stratified by sex and residency in Raya Kobo district, Northeast Ethiopia, 2019†
(Adjusted odds ratios (AOR) and 95 % confidence intervals)
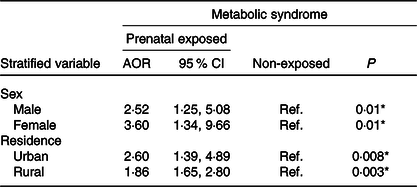
Ref, reference.
* Statistical difference.
† All OR are related to the non-exposed groups. All analyses were adjusted for sex, age, BMI, dietary pattern, physical activity, cigarette smoking, alcohol drinking, dietary pattern, educational status, marital status, residency, wealth index and occupational status.
Role of covariates
Although different models were developed to account for different sets of potential confounders, the association between prenatal famine exposure and adulthood metabolic syndrome remained significant. The highest risk for the metabolic syndrome (adjusted OR 2·80, 95 % CI 1·61; 5·20) was observed after being adjusted for sex and age at outcome assessment (Table 2).
Discussion
The primary aim of this study was to examine the association between prenatal famine exposure and the metabolic syndrome in adults. The focus of this article is therefore to describe the contributions of factors beyond adult lifestyles such as smoking, high-energy and high-fat diets and a lack of physical exercise, which lead to the metabolic syndrome(Reference Bigna and Noubiap11–Reference Yusuf, Hawken and ⓞunpuu13). When examining the association between famine exposure in prenatal life and the metabolic syndrome in adults using multivariable logistic regression models, adults who had prenatal exposure to famine were 2·94 times more likely to develop the metabolic syndrome. This association might have been confounded by other predictors of the metabolic syndrome in adults. To account for this, we adjusted for potential confounders including sex, age, BMI, dietary pattern, physical activity, educational status, marital status, residency, wealth index, occupational status and interaction terms. However, in all of these adjusted models, the observed association remained significant after adjustment for potential confounders. Female sex and rural residence were positively associated with the metabolic syndrome in adults. The finding that prenatal exposure to famine-predicted adult metabolic syndrome indicates the relative significance of adequate fetal nutrition during pregnancy to reduce the magnitude of the metabolic syndrome in adults.
The following mechanisms might explain the association between early life famine exposure and the metabolic syndrome and its markers in adults. Inadequate fetal nutrients ‘building blocks’ for the development of specific tissues may result in structural and physiological alterations of different organs(Reference Fall30,Reference Heijmans, Tobi and Stein45) . It is likely to occur as orchestrated adaptations for short-term survival through reducing fetal demand. These changes could then become permanent alterations in cell number, or clonal selection of particular types of cells, in tissues with finite periods of growth and differentiation; or by altered patterns of gene expression(Reference Waterland and Garza46). Modifiable epigenetic markers on DNA, containing methyl groups and histones, regulate gene expression. These markers enable cells with the same genetic code to have a variety of functional phenotypes. Patterns of DNA methylation are mostly established during prenatal and early postnatal life and are sensitive to the nutritional situation(Reference Burdge, Hanson and Slater-Jefferies47). For instance, hypertension in the offspring through altered methylation and expression of specific genes may be caused by maternal protein restriction during pregnancy. These metabolic abnormalities and altered methylation can be prevented by complementing the maternal diet and methyl-donor nutrients(Reference Lillycrop, Phillips and Jackson48).
The observed association between prenatal famine exposure and the metabolic syndrome in adults was consistent with studies conducted in the Chinese famine-exposed birth cohorts of the year 1959–1961(Reference Wang, Wang and Li23–Reference Zheng, Wang and Ren25). The present study and the Chinese study have used the International Diabetes Federation criterion to define the metabolic syndrome(Reference Alberti, Zimmet and Shaw36). Similarly, study subjects during the Chinese and Ethiopian famine were selected from the settings known for chronic malnutrition(Reference Gráda5). In this study, prenatal famine exposure showed association with metabolic markers such as increased TAG, fasting blood glucose, diastolic blood pressure and waist circumference as compared with the control group in the adjusted model. This finding is consistent with the previous study on the Chinese famine(Reference Li, He and Qi49), Dutch famine(Reference de Rooij, Painter and Phillips50), Ukraine(Reference Lumey, Khalangot and Vaiserman51), Bangladesh(Reference Finer, Iqbal and Lowe26) and Biafran famine in Nigeria(Reference Hult, Tornhammar and Ueda27).
Nonetheless, we observed inconsistent results between prenatal exposure to the Ethiopian famine and the risk of the metabolic syndrome in adulthood. It was inconsistent with the Dutch famine study(Reference de Rooij, Painter and Holleman22). These variations could be explained by the heterogeneity of the method in terms of definitions used, the cut-off parameters and characteristics of subjects employed. The Dutch study used the definitions of National Cholesterol Education Program to quantify the metabolic syndrome. Moreover, the Dutch famine was lasted for 6 months (26 November 1944 to April 1945)(Reference de Rooij, Painter and Holleman22) which had short span, compared with the Ethiopian famine which spanned for 2 years(Reference Dercon and Porter2). Furthermore, the Dutch famine study was based on subjects who were previously well nourished, while the situation is different in Ethiopia such that the population has been exposed to marginal nutrition before the famine and this might exacerbate the long-term consequences of maternal undernutrition during pregnancy(Reference Remais, Zeng and Li52). The finding of the present study was also inconsistent with the study of Stanner et al.(Reference Stanner, Bulmer and Andres53) on the Leningrad famine. We speculate that the inconsistent findings may be attributed to the small sample size used in the Leningrad study.
Female sex and rural residency were also positively associated with the metabolic syndrome in adults. However, no interaction was observed between famine, sex and residence. The finding is consistent with that of studies conducted in Dutch(Reference Lumey, Stein and Kahn54,Reference Portrait, van Wingerden and Deeg55) and Chinese(Reference Li, Jaddoe and Qi24,Reference Wang, Zou and Wang56,Reference Wang, Li and Yang57) famine studies. The sex difference may be because females are particularly exposed to hunger in their infancy period due to sex discrimination(Reference Azbite58,Reference Gupta59) . Consequently, famine exposure during infancy period is associated with increased metabolic syndrome in adults(Reference Fall30,Reference Wang, Zou and Wang56) . Furthermore, mortality in male fetus is greater than female, which may be elucidated by the biological delicacy of the male fetus in intra-uterine life(Reference Kraemer60). Likewise, the difference in residence may be attributed to the severity of famine during prenatal life. The rural populations were severely affected by the Ethiopian great famine(Reference Dercon and Porter2,Reference Kidane61) . In addition, the residents of the rural populations in the study setting may be occupied by higher number of females, and the lasting impact of nutritional insult in prenatal life tends to entail more harmful on the rural populations.
The present study finding has implications for the global rise of chronic NCD. It increases understanding of the long-term consequences of prenatal undernutrition. More interestingly, these findings may partly explain the reason behind alarmingly raising chronic NCD in Ethiopia, a country known for the history of chronic food insecurity and yet the fastest growing economy in Africa. Furthermore, these findings may help policymakers to lay context-specific strategies peculiar to famine-exposed regions of the country.
Strength and limitations of the study
The strengths of this study are it showed that designing and conducting a study on past famine exposure are possible, this is the first study to examine the effect of famine during fetal life on adult health and disease in Ethiopia; to control for selection bias, a 1-year transitional (washout) period is considered. However, we acknowledge the following limitations. The findings might partly be affected by survival bias, birth registration was not used to define exposure, severity of exposure at individual level was not considered and postnatal and childhood factors are not considered, all of which could have affected the outcomes.
Conclusion
Famine exposure during fetal life was positively associated with adulthood metabolic syndrome. More specifically, famine exposure during prenatal life was associated with increased metabolic markers such as TAG, fasting blood glucose, diastolic blood pressure and waist circumference. The mechanism through which famine exposure during fetal life influenced adult health and disease might need additional study in a similar context. Moreover, additional researches are needed to examine the association between famine exposure in the postnatal life and adult NCD.
Acknowledgements
The authors would like to acknowledge the Institute of Health, Jimma University for funding the study and Amhara Public Health Institute for doing the lipid analyses. The authors are also grateful to the study participants for their dedicated time and volunteer participation.
The study was funded by Jimma University, Institute of Health. The funder has no role in the design, implementation, analysis and in the interpretation and write up of the findings.
G. A., K. H. A. and M. A. conceived and planned the study. G. A. and H. H. implemented and supervised the field work. G. A., K. H. A. and M. A. did the analysis and interpretation. G. A. drafted the manuscript. M. A., K. H. A. and T. B. critically revised the manuscript. All authors reviewed the manuscript and approved the final version for submission.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520002123











