Global catches from the feed-grade fisheries that provide fish oil (FO) and fish meal for aquafeed formulations have reached their sustainable limitsReference Pike and Barlow1 and it is likely that within a decade or so there may be insufficient FO to meet the quantities required for current aquaculture growthReference Tacon2. Consequently, there has been considerable interest in introducing sustainable alternatives to fish meal and FO that reduce reliance on marine raw materialsReference Kaushik, Coves, Dutto and Blanc3, Reference Tacon4. A number of recent studies suggest that dietary vegetable oil (VO) inclusion does not result in reduced growth performance or feed conversion in Atlantic salmon (Salmo salar)Reference Bell, Henderson, Tocher and Sargent5, Reference Torstensen, Bell, Sargent, Rosenlund, Henderson, Graff, Lie and Tocher6, rainbow trout (Oncorhynchus mykiss)Reference Caballero, Obach, Rosenlund, Montero, Gisvold and Izquierdo7, gilthead sea bream (Sparus aurata)Reference Izquierdo, Obach, Arantzamendi, Montero, Robaina and Rosenlund8 or European sea bass (Dicentrarchus labrax)Reference Mourente, Good and Bell9. However, at levels above 50 % VO inclusion, significant accumulation of fatty acids derived from VO, especially 18 : 2n-6, and reduction of EPA (20 : 5n-3) and DHA (22 : 6n-3) occurs in fish tissuesReference Bell, Henderson, Tocher and Sargent5, Reference Torstensen, Bell, Sargent, Rosenlund, Henderson, Graff, Lie and Tocher6, Reference Izquierdo, Obach, Arantzamendi, Montero, Robaina and Rosenlund8, Reference Mourente, Good and Bell9.
The nutritional status of an organism, including fish, is known to influence immune functionsReference Blazer10 and the overall resistance of an organism to disease is therefore dependent on their nutritional status. The first review suggesting that fatty acids might be important in immune function was by Meade & MertinReference Meade and Mertin11 and more recent reviews have confirmed the importance of PUFA, of both the n-6 and n-3 series, as modulators of immune functionReference Calder12, Reference Yaqoob13. Fatty acids are incorporated into the plasma membrane from dietary lipids, so that the fatty acid composition of cellular membranes reflects the composition of dietary lipidsReference Clamp, Ladha, Clark, Grimble and Lund14. In fish, dietary fatty acids and tissue fatty acid compositions are closely correlatedReference Sargent, Tocher, Bell, Halver and Hardy15 and changes in the dietary n-3/n-6 ratio can influence the compositions of fish immune cells, including blood leucocytesReference Thompson, Henderson and Tatner16–Reference Bell, Ashton, Secombes, Weitzel, Dick and Sargent18.
Fatty acids have diverse roles in all cells. They are important as a source of energy, as structural components of cell membranes and as signalling molecules. In mammalian studies, dietary fatty acids may be able to modulate the immune system through several mechanisms including reduction of lymphocyte proliferation, cytokine synthesis and phagocytic activity, and also by modification of natural killer cell activityReference De Pablo and De Cienfuegos19. The main event in the modulation of immune function may be associated with changes in the cell membrane due to dietary fatty acid manipulation. It is likely that modulation of the overall immune system occurs as a result of alterations in membrane fluidity, lipid peroxidation, eicosanoid production or regulation of gene expressionReference Calder20.
In the present study, triplicate groups of juvenile European sea bass were fed diets that were based on 60 % substitution of FO with a blend of rapeseed (RO), linseed (LO) and palm oils (PO). The level of 60 % substitution was chosen as this was the maximum level of VO inclusion that could be tolerated in marine fish without loss of growth performanceReference Izquierdo, Obach, Arantzamendi, Montero, Robaina and Rosenlund8, Reference Mourente, Good and Bell9. The oils were blended in two different formulations to achieve a fatty acid composition as similar to anchovy oil as possible, in terms of energy content, and provide a similar balance of SFA, MUFA and PUFA to that found in FO, but without highly unsaturated fatty acids. The fish were fed the diets for 64 weeks starting at an initial weight of approximately 5 g. Growth parameters and the fatty acid compositions of liver and peripheral blood leucocytes (PBL) were monitored after 64 weeks. The impact of the dietary blends on selected aspects of the innate immune response (haematological parameters, serum lysozyme activity and macrophage respiratory burst activity) and histopathology were also assessed in the experimental fish at this time, together with levels of plasma prostaglandin E2 (PGE2).
Materials and methods
Experimental fish and diets
European sea bass (Dicentrarchus labrax L.), 7·9 (sd 0·5) cm in length and 5·2 (sd 1·0) g in weight, were purchased from MARESA (Huelva, Spain) and transported to the marine aquarium facility at the University of Cádiz, Faculty of Marine and Environmental Sciences, Puerto Real (Cádiz). On arrival the fish were placed in nine 5000-litre rectangular tanks at 600 fish per tank (approximately 0·6 kg/m3), with salinity of 39‰, temperature of 20°C and saturated with oxygen. Following 2 weeks acclimation (July 2002), triplicate groups of fish were fed to satiation, using mechanical belt automatic feeders with three isoenergetic and isonitrogenous experimental diets formulated to provide a constant lipid content of approximately 22 % (Nutreco ARC, Stavanger, Norway). The diets contained approximately 47 % protein, primarily provided by fish meal, and 21·4, 24·1 and 21·5 % lipid for diets of pellet size 2, 3 and 5 mm, respectively. Two experimental diets contained 60 % of three VO, LO, PO and RO, blended to provide a balance of SFA, MUFA and PUFA similar to that found in FO, but without highly unsaturated fatty acids. The control diet contained anchovy oil and the added oil combinations for the three experimental diets were: Diet A: 100 % anchovy oil (control); Diet B: 40 % anchovy oil, 35 % LO, 15 % PO and 10 % RO; Diet C: 40 % anchovy oil, 24 % LO, 12 % PO and 24 % RO. The formulation and proximate compositions of the experimental diets are shown in Table 1, while diet total lipid content and fatty acid compositions are shown in Table 2.
Table 1 Formulation and proximate composition of experimental diets (5 mm pellet size; g/kg feed)
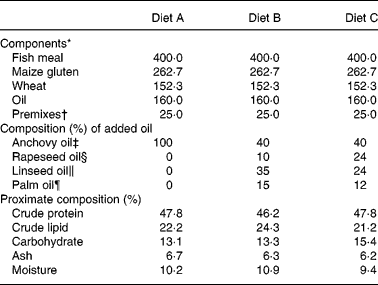
* Fish meal from Scandinavian LT-fish meal (Nordsildmel, Norway); maize gluten from Cargill (Staley, NC, USA); wheat from Statkorn (Oslo, Norway).
† Vitamin and mineral premix added exceed National Research Council (1993) recommendations.
‡ Anchovy oil (Denofa, Fredrikstad, Norway) supplemented with 200 ppm butylated hydroxytoluene.
§ Crude rapeseed oil (Oelmühle, Hamburg, Germany), no antioxidant added.
∥ Crude E.C.C. linseed oil (N.V. Oliefabriek, Lictervelde, Belgium) supplemented with 500 ppm Ronoxan A (Roche, Basel, Switzerland).
¶ Crude palm oil (Denofa, Norway).
Table 2 Total lipid content (% of dry mass) and fatty acid composition (weight % of total fatty acids) of the experimental diets (5 mm pellet size) (Mean values and standard deviations for three determinations)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Standard deviation of 0·0 implies sd < 0·05.
† Includes 20: 0 and 22: 0.
‡ Includes 18 : 1n-11, 20 : 1n-11, 20 : 1n-7 22 : 1n-9 and 24 : 1.
§ Includes 16 : 2, 16 : 3, 16 : 4, 20 : 2n-6, 20 : 3n-6 and 22 : 5n-6.
Sample collection and biometric measurements
After feeding the experimental diets for 64 weeks, thirty fish per tank (i.e. ninety fish per replicate) were sampled for length and live mass, and Fulton's condition factor (K) and specific growth rates were recorded. Ten fish per replicate (i.e. thirty fish per dietary treatment) were sampled and liver (live and dry mass), hepatosomatic index and flesh (live and dry mass) were recorded. Liver samples for fatty acid analyses were dissected from four fish per replicate (i.e. twelve fish per dietary treatment) and immediately frozen in liquid nitrogen and stored at − 80°C until analysed. Blood for eicosanoid analysis (2 ml) was collected in heparinised syringes from six fish per dietary treatment, and centrifuged at 12 000 g for 2 min. The plasma was collected and acidified by the addition of 2 m-formic acid (50 μl/ml) and immediately frozen in liquid nitrogen for eicosanoid analysis. Heparinised blood samples were also used for haematological analyses. Live mass of the liver was determined by blotting the tissue on filter paper before weighing, and dry mass was determined after heating to 60°C for 24 h and cooling under vacuum before weighing. Hepatosomatic index was calculated as liver live mass × 100/fish live mass. Fulton's condition factor (K) = (W/L 3) × 100, where W is the fish weight (g) and L is the total length (cm). Specific growth rate was calculated as % weight gain/dReference Wootten21. Non-specific mortality was measured at the end of the experiment and expressed as a percentage of surviving fish.
Proximate analysis of diets
Moisture content was determined by thermal drying to constant weight in an oven at 110°C for 24 h. For the total protein content, the micro-Kjeldahl analysis method was followed, using a Digestion system 40-1006 Heating Unit (Foss, Warrington, UK) and a Kjeltec Auto 1030 Analyzer (Foss). To convert total nitrogen to total protein content, as a percentage of dry weight, the factor 6·25 (100/16) was used. Crude fat was determined by acid hydrolysis with a Soxtec System 1047 Hydrolyzing Unit, followed by Soxhlet extraction using a Soxtec System HT6 (Foss). Ash content as a percentage of dry weight was determined by dry ashing in porcelain crucibles in a muffle furnace, at 600°C overnightReference Woyewoda, Shaw, Ke and Burns22.
Lipid analysis
Total lipid in samples was extracted after homogenisation, using an Ultraturrax tissue disrupter (Fisher Scientific, Loughborough, UK), in ten volumes of chloroform–methanol (2:1, v/v) containing 0·01 % butylated hydroxytoluene as antioxidant, basically according to Folch et al. Reference Folch, Lees and Sloane-Stanley23 and essentially as described by ChristieReference Christie24.
Fatty acid methyl esters were prepared from aliquots of total lipids by acid-catalysed transmethylation for 16 h at 50°C, using tricosanoic acid (23 : 0) as internal standardReference Christie24. Fatty acid methyl esters were extracted and purified as described previouslyReference Mourente and Tocher25 and were separated using a Hewlett-Packard 5890A Series II gas chromatograph (Hewlett-Packard, Barcelona, Spain) equipped with a chemically bonded (PEG) Supelcowax-10 fused silica wall coated capillary column (30 m × 0·32 mm i.d.; Supelco Inc., Bellefonte, PA, USA), using an ‘on column’ injection system and flame ionisation detection. Hydrogen was used as the carrier gas with an oven thermal gradient from an initial 50°C to 180°C at 25°C/min and then to a final temperature of 235°C at 3°C/min, with the final temperature maintained for 10 min. Individual fatty acid methyl esters were identified by comparison with known standards and quantified by means of a direct-linked PC and Hewlett-Packard ChemStation software.
Extraction and measurement of prostaglandin E2 concentrations in plasma
The frozen acidified plasma samples were thawed and centrifuged at 12 000 g for 2 min to remove any precipitate. The supernatants were extracted using octadecyl silyl (C18) ‘Sep-Pak’ mini-columns (Millipore) as described in detail by Bell et al. Reference Bell, Tocher, Macdonald and Sargent26. C18 ‘Sep-Pak’ mini columns were pre-washed with 5 ml methanol and 10 ml distilled water, plasma samples were charged on the mini-column, washed with a further 10 ml distilled water and the eicosanoids eluted in 5 ml ethyl acetate. Samples were dried under nitrogen and redissolved in immunoassay buffer. Quantification of PGE was performed using enzyme immunoassay kits, according to the manufacturers protocol (SPI-Bio, Massy, France).
Measurement of cellular immune parameters
Eight fish per dietary treatment were sampled after 64 weeks of feeding the experimental diets. Fish were anaesthetised with a lethal dose of tricaine methanesulphonate (MS-222, Sigma, Poole, UK). Blood samples were collected in heparinised vacuum tubes (vacutainer; Becton Dickinson Vacutainer System, Oxford, UK) from the caudal vein.
Preparation of peripheral blood leucocytes
PBL were isolated from blood from three fish per dietary treatment using the lymphocyte separation medium, Histopaque® (Sigma) and density gradient centrifugation. Blood (1 ml) was diluted with L-15 medium (4 ml) and 3 ml of the diluted blood was layered on to 4 ml Histopaque® and centrifuged at 400 g for 45 min. The leucocyte band was collected using a Pasteur pipette and stored in 1 ml chloroform–methanol (2:1, v/v) at − 20°C until required for lipid extraction. If erythrocyte contamination of PBL was considered to be excessive (>2 %) then the PBL fraction was centrifuged again on 4 ml fresh Histopaque®.
Haematology
Blood was used immediately for haematological studies. Haematocrit values were obtained using heparinised micro-haematocrit tubes and centrifuging at 12 000 g for 4 min (Microcentrifuge MH2; Sarstedt Ltd, Leicester, UK). Total erythrocyte and total leucocyte counts (including thrombocytes) were made using PBS for dilution and an improved Neubauer haemocytometer (Hawksley, Lancing, UK).
Serum lysozyme activity
An aliquot of blood was allowed to clot at 4°C overnight. Serum was separated by centrifugation at 4000 g for 15 min and stored at − 20°C until analysis. Serum lysozyme activity was assayed by a turbidimetric assay which measures the lytic activity of the sea bass serum against Microccocus lysodeikticus. Reference Anderson and Siwicki27, Reference Rungruangsak-Torrissen, Wergeland, Glette and Waagbø28 A suspension of 190 μl of bacteria (Micrococcus lysodeikticus; Sigma) and 10 μl of serum sample was measured spectrophotometrically at 540 nm in five replicate wells per serum sample after 1 and 5 min at 25°C, using a Dynatech MRX 1·2 ELISA reader (Dynatech Laboratories Ltd, Billingshurst, West Sussex, UK). The bacterial suspension (0·2 mg/ml) was prepared in sodium phosphate buffer (0·04 m, pH 5·8). The results are given as units (U)/ml per min (1 U = the amount of sample causing a decrease in absorbance of 0·001 per min).
Macrophage respiratory burst activity
The reduction of nitroblue tetrazolium salt to formazan by oxygen radicals produced by head kidney macrophages during respiratory burst activity was measured spectrophotometrically as described by Chung & SecombesReference Chung and Secombes29. Isolation and culture of head kidney macrophages were performed as described by SecombesReference Secombes, Stolen, Fletcher, Anderson, Robertson and van Muiswinkel30, however, instead of placing the cell suspensions on Percoll to isolate the macrophages, 200 μl of each kidney suspension were added directly to four replicate wells of a ninety-six-well microtitre plate. Plates were sealed and incubated for 3 h before washing them gently three times to remove non-adherent cells. L-15 (200 μl) containing 10 % foetal bovine serum was added to all wells and cultures were incubated at 18°C for 2–3 d, after which the respiratory burst activity of the macrophages was determined by incubating the cells with 100 μl nitroblue tetrazolium salt (1 mg/ml)–phorbol myristate acetate (1 μg/ml). This was added to three of the four wells and incubated at 18–20°C for 40 min. The assay was developed as described by the authors using a microplate reader as described earlier to read the absorbance at 620 nm. The remaining well was used to determine the numbers of macrophages attached to the plate for individual kidney samplesReference Secombes, Stolen, Fletcher, Anderson, Robertson and van Muiswinkel30. The results were expressed as ‘macrophage activity’ by calculating the mean optical density for each of the triplicate cultures and dividing the mean optical density by the number of cells per well to obtain an optical density per 105 cells and multiplying by 100.
Histological examination of fish tissues
Samples were collected at 64 weeks to identify any effects of dietary treatment on the histology of the heart, liver or intestine. Samples of proximal, mid and distal intestine were collected from six fish from each dietary treatment, in addition to the heart and liver, for histopathological examination. Sections were fixed in 10 % buffered formalin at the time of dissection, embedded in paraffin wax and 5 μm sections were cut and stained with haematoxylin and eosin. Processed sections were examined ‘blind’ to eliminate bias in interpretation. Stained sections of heart were assessed for signs of endocarditis and pericarditis. Liver sections were assessed on fat content, any indication of inflammation in the tissue, the degree of peri-vascular cuffing and finally the presence of single cell necrosis. Intestinal sections were examined on the integrity of the intestinal mucosa, the appearance of the submucosa and lamina propria, and the presence of any inflammatory response.
Statistical analysis
Results are reported as means and standard deviations (n 3) unless otherwise stated. All statistical analyses were performed using the statistical computer package Prism 4·0 (GraphPad Software Inc., San Diego, CA, USA). The significance of treatment effects on biometry and growth rates, liver and leucocyte fatty acid compositions, haematology, serum lysozyme activity and macrophage respiratory burst activity were determined by one-way ANOVA followed by Tukey's multiple comparison test where appropriate. Percentage data and data which were identified as non-homogeneous (Bartlett's test) were subjected to either arcsine, square root or log transformation before analysis. Differences were reported as significant at P < 0·05Reference Zar31. Immune parameter results are reported as means and standard deviations (n 8).
Results
There were no significant differences in the total length of fish between dietary treatments, but fish fed Diet B showed significantly lower values for total live mass and liver mass than fish fed Diets A (control) and C. Fish fed Diets A (control) and B presented significantly lower values for flesh dry mass (%) at the end of the 64-week feeding trial (Table 3).
Table 3 Effect of partial replacement (60 %) of dietary fish oil with vegetable oils (rapeseed, linseed and palm oils) on growth and performance of European sea bass fed experimental diets for 64 weeks* (Mean values and standard deviations)
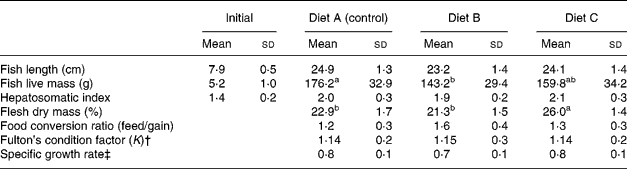
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* See Tables 1 and 2 for details of diets. For fish length and weight, n 90; for liver mass, hepatosomatic index and flesh mass, n 30.
† K = (W/L 3) × 100, where W is the weight (g) and L is the total length (cm).
‡ Specific growth rate = (LnW 1 − LnW 0) × 100/t, where W is the weight (g), L is the length (cm), W 0 is the initial weight (g), W 1 is the final weight (g), t is the time (d).
The total lipid fatty acid compositions of livers from sea bass following 64 weeks of feeding the experimental diets are shown in Table 4. Total SFA (primarily 16 : 0) were identical in all treatments. Total MUFA, primarily oleic acid (OA), were significantly higher in liver total lipids from fish fed Diet C, followed by fish fed Diets B and A (control) due to the higher inclusion of OA in the VO blends. The proportion of linoleic acid (18 : 2n-6) in total lipid from liver was highest in fish fed the VO diets, due to the high inclusion of linoleic acid in RO, LO and PO, and was about 50 % lower in liver of fish fed Diet A (control). However, total n-6 PUFA values were not significantly different for all treatments. In contrast, arachidonic acid (ARA; 20 : 4n-6) was highest in liver of control fish (Diet A) and fish fed Diet B, followed by fish fed Diet C. Total n-6 highly unsaturated fatty acid, primarily ARA, was highest in liver from fish that had been fed Diet A (control) followed by fish from treatments B and C that showed identical values. The percentage of linolenic acid (18 : 3n-3) in liver total lipids was highest in fish fed Diets B and C (which contained the highest proportions of LO and, in consequence, the highest level of linolenic acid), followed by fish fed Diet A (control). Liver total lipid percentages of EPA, DHA and total n-3 PUFA were highest in fish fed Diet A (control) due to the highest content of these fatty acids in FO. The level of total PUFA was not significantly different in liver total lipids from all treatments (Table 4).
Table 4 Total lipid content (% of dry mass) and total lipid fatty acid composition (weight % of total fatty acids) of liver from European sea bass fed the experimental diets for 64 weeks* (Mean values and standard deviations for three determinations)

Inclusion of VO in the diets of sea bass modified the fatty acid composition of their PBL. The fatty acid compositions of PBL from VO-fed fish were different from the corresponding PBL of fish fed FO, with the latter having more MUFA and higher n-6 PUFA. PBL of sea bass maintained on Diets B and C had significantly increased levels of 18: 0, OA, linoleic acid, 20 : 2n-6 and linolenic acid, and significantly reduced amounts of n-3 PUFA, ARA, EPA, DHA and others (Table 5). The overall ratio of n-3/n-6 was significantly reduced in sea bass fed the VO diets.
Table 5 Total lipid fatty acid composition (weight % of total fatty acids) of peripheral blood leucocytes from European sea bass fed the experimental diets for 64 weeks* (Mean values and standard deviations for three determinations)

The effect of partial replacement of dietary FO with VO blends on the concentration of plasma PGE2 in European sea bass after 64 weeks of feeding the diets is shown in Fig. 1(a). The highest values were found in plasma of fish fed the control (FO) diet and Diet C, with significantly lower values seen in fish fed Diet B (46 % less). The source of dietary lipid did not affect any of the haematological parameters measured. No significant differences were found in haematocrit values (Fig. 1(b)) or the total number of leucocytes (Fig. 1(c)) and erythrocytes (Fig. 1(d)) between groups. The production of superoxide anion by head kidney macrophages, measured by the reduction of nitroblue tetrazolium salt, is presented in Fig. 1(e). It appears that following phorbol myristate acetate triggering, the respiratory burst activity was significantly reduced in fish fed the VO-based diets. Whether or not this change affects the innate immune response of the fish needs further investigation. It could be that the respiratory burst event takes place earlier or later than seen with macrophages from fish fed the FO diet and that this activity has not been measured at the optimal time for fish fed the VO diets. No effect of dietary VO was observed on sea bass serum lysozyme activity (Fig. 1(f)). Fish fed the FO diet showed the highest (1452·5 U/ml per min; but not significantly different) value of lysozyme activity in serum compared to 1351·1 U/ml per min found for fish fed Diet B and 1171·1 U/ml per min for Diet C.

Fig. 1 Effects of feeding diets containing fish oil (Diet A), or two 60 % vegetable oil blends (Diets B and C) on plasma prostaglandin E2 (PGE2) concentration (a); % haematocrit (b); total circulating leucocytes (c); total circulating erythrocytes (d); head kidney macrophage activity (nitroblue tetrazolium salt reduction, measured as absorbance at 620 nm/105 cells × 100) (e); serum lysozyme activity (f). Values are means with their standard errors depicted by vertical bars (n 9). a,b Mean values with unlike letters were significantly different (P < 0·05).
Hearts examined from all three dietary groups showed no signs of pathological change. In livers, fat vacuoles were variable in size in many sections with some very large vacuoles present within some hepatocytes and relatively smaller vacuoles in other hepatocytes. Due to the level of vacuolation in some hepatocytes, there was some distortion of the cellular architecture and occasional breakdown of cells. Again, there were no differences between the three dietary groups. Small foci of inflammation were seen in some sections in all three groups, with a slightly higher incidence in sections from the Diet C group. Peri-vascular cuffing was not a feature in any of the dietary groups examined. With regard to the intestinal sections, mucus levels appeared very similar in all segments and in all dietary groups. Absorptive vacuoles were small and multiple in all sections. In the FO diet these were at relatively low levels in the proximal and mid-segments and higher in the distal segments. In fish fed Diet B or Diet C, vacuolation in the proximal segments appeared to be much more pronounced, interestingly less so than in the mid-sections. Some cellular infiltration was seen in the lamina propria of one fish in the FO diet group (Fig. 2(a)) and two fish on Diet C (Fig. 2(b)). Sloughing of the mucosal membrane was not a feature in any of the sections examined. The major difference seen between these groups was the level of absorptive vacuolation in the proximal segment of fish fed Diet B or Diet C, compared with the FO diet.

Fig. 2 Histopathology of sea bass fed fish oil (Diet A), distal intestine showing slight cellular infiltration in the lamina propria and high levels of absorptive vacuoles ( × 175) (a); 60 % vegetable oil blend (Diet C), distal intestine showing cellular infiltration but no sloughing of the mucosal folds ( × 430) (b).
Discussion
Considerable data have been accumulated on the effects of different dietary lipids on tissue fatty acid compositions of both mammals and fish, although the effects of dietary lipids on fish health and immune function are less well documented. Changes in dietary fatty acid composition have been shown to affect both innateReference Sheldon and Blazer32–Reference Waagbø, Hemre, Holm and Lie35 and adaptive immunityReference Thompson, Henderson and Tatner16, Reference Waagbø, Sandnes, Joergensen, Engstad, Glette and Lie34, Reference Erdal, Evensen, Kaurstad, Lillehaug, Solbakken and Thorud36–Reference Fracolossi and Lovell38, as well as the resistance to infectious diseasesReference Thompson, Henderson and Tatner16, Reference Erdal, Evensen, Kaurstad, Lillehaug, Solbakken and Thorud36–Reference Salte, Thomassen and Wold39. However, the role of n-3 and n-6 fatty acids in fish immune response is unclear, and reports are not conclusive and are often contradictory.
The modulatory process is likely to occur at different cellular levels with the most obvious being a change in cell membrane phospholipid fatty acid composition, affecting the activity of membrane-bound enzymes, receptors and ion channelsReference Theis, Miles, Nebe-von-Caron, Powell, Hurst, Newsholme and Calder40. In addition, eicosanoids, a group of bioactive derivatives of ARA, EPA and dihomo-γ-linolenic acid (20 : 3n-6), which include PG, thromboxanes, leukotrienes and lipoxins, act to regulate the immune responseReference Yaqoob13, Reference Hwang41. Other immune modulatory processes involving fatty acids include changes in intracellular signalling pathwaysReference Khan, Blobe and Hannun42 and direct interactions between fatty acids and nuclear transcription factors in cells of the immune system, such as PPAR, that act to regulate immune cell functionReference Calder20.
Fish tissues and cell membranes, including phagocytic cells (macrophages, neutrophils), contain relatively high concentrations of n-3 PUFA, and their compositions can be altered by changes in dietary lipidReference Bell, Ashton, Secombes, Weitzel, Dick and Sargent18. Specific macrophage functions may also be altered by lipids, mainly due to changes in membrane fluidity. If fluidity is altered by fatty acid composition, then potentially several aspects of phagocyte function may be affected including phagocytosis and eicosanoid production. Calder et al. Reference Calder, Bond, Harvey, Gordon and Newsholme43 reported that unsaturated fatty acid incorporation is associated with an increase in the phagocytosis of zymosan particles.
Reduction in head kidney macrophage respiratory burst activity has been observed in sea bass and gilthead sea bream fed VOReference Mourente, Good and Bell9, Reference Montero, Kalinowski, Obach, Robaina, Tort, Caballero and Izquierdo44. Sea bass fed 60 % RO, LO and olive oil had significantly reduced phagocytic capacity of head kidney macrophage to engulf yeast particlesReference Mourente, Good and Bell9 while Montero et al. Reference Montero, Kalinowski, Obach, Robaina, Tort, Caballero and Izquierdo44 found reduced macrophage activity in sea bream fed 60 % RO. In addition, Sheldon & BlazerReference Sheldon and Blazer32 found that channel catfish macrophage killing activity was positively correlated to the dietary content of n-3 PUFA. They found phagocytosis of live Edwardsiella ictaluri by catfish head kidney macrophages was not significantly affected by feeding soyabean oil compared to fish fed menhaden oil or beef tallow. However, feeding soyabean oil significantly reduced the ability of macrophages to kill engulfed bacteria compared to macrophages from those fed menhaden oil. Macrophages from the latter group also had a significantly higher killing index than macrophages from fish fed soyabean oil. Waagbø et al. Reference Waagbø, Sandnes, Joergensen, Engstad, Glette and Lie34 showed that Atlantic salmon fed diets rich in n-3 PUFA significantly reduced the bacterial killing ability of macrophages at 12°C but not at 18°C, indicating that temperature, perhaps related to membrane fluidity, also influences the activity of macrophages. In contrast, Thompson et al. Reference Thompson, Henderson and Tatner16 found no differences in phagocytosis and bactericidal activities of head kidney macrophages from Atlantic salmon fed diets enriched with either n-3 or n-6 PUFA.
In the present study, the concentration of circulating PGE2 in plasma of sea bass fed the 60 % VO blend (Diet B) was significantly lower than in fish fed FO. In addition, the fish fed 60 % VO (Diets B and C) also showed significantly reduced respiratory burst activity which coincided with a reduction in plasma PGE2 levels. Since the production of PGE2 was reduced in fish fed VO diets, it may be that the activity or expression of the cyclo-oxygenase enzymes is inhibited by dietary lipid. It is also possible that feeding VO for a long period may reduce the levels of ARA in plasma membranes and, thereby, compromise immune function. In support of the present study, a number of studies also showed a reduction in production of PGE2 and leukotriene B4 by stimulated head kidney macrophages from salmon fed a diet containing LO compared to those fed diets containing sunflower oil or FOReference Bell, Dick, McVicar, Sargent and Thompson17, Reference Bell, Ashton, Secombes, Weitzel, Dick and Sargent18, Reference Bell, Dick and Sargent45, Reference Bell, Farndale, Dick and Sargent46. However, no differences in serum lysozyme activity were found in the present study, which was also reported in other studies with fish fed VOReference Bell, Ashton, Secombes, Weitzel, Dick and Sargent18, Reference Montero, Kalinowski, Obach, Robaina, Tort, Caballero and Izquierdo44.
Montero et al. Reference Montero, Kalinowski, Obach, Robaina, Tort, Caballero and Izquierdo44 found that seabream fed a FO diet had higher numbers of circulating erythrocytes compared to fish fed 60 % LO or soyabean oil diets, which may be related to a higher oxygen requirement due to higher peroxisomal β-oxidation activity induced by the VO dietsReference Waagbø, Sandnes, Lie and Nilsen37. Leray et al. Reference Leray, Nonnotte and Nonnotte47 found that the fatty acid composition of erythrocyte membrane phospholipids from trout can be profoundly altered by dietary oils. Trout fed highly saturated coconut oil showed increased n-9 fatty acids in their phospholipids, and, consequently, their erythrocytes had a more shrunken appearance than fish fed FO. Perhaps the high levels of saturates in the diets caused reduced haematocrit levels, linked to a shrunken erythrocyte morphology causing a lower packed erythrocyte volume.
The lipid composition of monocytes, macrophages, lymphocytes and polymorphonuclear cells reflect the fatty acid composition of dietary lipids in mammalsReference Meade and Mertin11, Reference Johnston and Marshall48. Studies by Waagbbø et al. Reference Waagbø, Hemre, Holm and Lie35, Farndale et al. Reference Farndale, Bell, Bruce, Bromage, Oyen, Zanuy and Sargent49 and Montero et al. Reference Montero, Kalinowski, Obach, Robaina, Tort, Caballero and Izquierdo44 reported that dietary oil determines the fatty acid profile of macrophages and immune cells in cod, sea bass and sea bream. Montero et al. Reference Montero, Kalinowski, Obach, Robaina, Tort, Caballero and Izquierdo44 reported selective incorporation of certain fatty acids into head kidney macrophages of sea bream. DHA was found to be preferentially incorporated and retained in this cell type. Generally, fish fed with a VO-containing diet had increased levels of oleic acid, linoleic acid, linolenic acid and total n-6 PUFA in both their liver and their PBL, and decreased levels of EPA, DHA, total n-3 PUFA and a lower n-3/n-6 ratio than fish fed a FO diet. Fish fed a FO diet showed the highest n-3 highly unsaturated fatty acids in immune cells in the present study and in previous studiesReference Waagbø, Hemre, Holm and Lie35, Reference Montero, Kalinowski, Obach, Robaina, Tort, Caballero and Izquierdo44, Reference Farndale, Bell, Bruce, Bromage, Oyen, Zanuy and Sargent49. Evidence suggests that changing the fatty acid composition of immune cells can influence immune function by changing the physiology of the cell membrane but perhaps more importantly by influencing the production of modulatory PG and leukotrienesReference Calder20. The production of eicosanoids is influenced, in part, by the availability of precursor fatty acids and, in particular, the EPA/ARA ratio. In a previous study with Atlantic salmon fed a single VO, 3-fold differences in the EPA/ARA ratio of immune cells were recordedReference Bell, Ashton, Secombes, Weitzel, Dick and Sargent18, while in the present study with VO blends the difference in the EPA/ARA ratio, between the three dietary treatments, was only 13 %. Perhaps the minor changes to the EPA/ARA ratio in the present study can partly explain the lack of effect observed in innate immune function.
The overall histological appearance of sea bass sampled from all of the dietary treatments was normal with very few differences observed between the groups. The only difference was in the levels of absorptive vacuoles present in the proximal intestine. Sea bass fed the VO diets showed elevated numbers of absorptive vacuoles compared to fish fed the FO diet. The presence of increased absorptive vacuoles tends to suggest an ‘active’ mucosa, however, with increased mucosal vacuolation this could, in turn, leave the intestinal mucosal membrane more vulnerable to sloughing and breakdown. However, the vacuolisation was minor and was still regarded as being within normal ranges for Atlantic salmon.
Results of the present study suggest that potential exists for replacing FO with a blend of VO in the feeds of farmed sea bass without compromising growth, non-specific immune function and overall histological appearance. It is important to establish that alternative dietary lipids to FO are not only supplied in the correct quantities and balance for optimal growth and feed conversion, but can maintain optimal immune function and not increase susceptibility to infectious pathogens. The present study suggests that normal immune function can be more successfully attained if dietary FO is replaced by a blend of VO that provides a more physiologically balanced fatty acid composition, in comparison to replacement with a single VOReference Mourente, Good and Bell9, Reference Bell, Dick, McVicar, Sargent and Thompson17, Reference Bell, Ashton, Secombes, Weitzel, Dick and Sargent18, Reference Montero, Kalinowski, Obach, Robaina, Tort, Caballero and Izquierdo44.
Acknowledgements
This work was supported by contract no. QLRT-2000-30 058 of the project ‘Researching alternatives to fish oils in aquaculture, RAFOA’ of the European Commission, Directorate General Fisheries as part of the Fifth Framework Programme ‘Quality of Life & Management of Living Resources’. Joanne Good was supported in her doctoral studies by Ewos Innovation AS, Norway.









