Damage to the intestinal barrier, bacterial translocation (BT) and immunosuppression increase infection risk and mortality( Reference Zheng, Li and Zhang 1 ). Glutamine plays an important role in the regulation of the gut barrier and is fundamental for the proliferation of enterocytes( Reference Rhoads, Argenzio and Chen 2 ).
Glutamine is required for a number of specific biochemical reactions and has a broad range of metabolic functions. This amino acid can function in the formation of nucleic acids, nucleotides and proteins or can produce glucose, glutathione, urea or NO via glutamate. NO is a pluripotent signalling molecule derived from arginine, which is one of the ultimate products of glutamine metabolism( Reference Curi, Lagranha and Doi 3 ). The above-mentioned pathways are integrated and determine metabolic functions.
Glutamine can also exert direct effects on the immune system. The extracellular level of glutamine regulates the proliferation of T cells and the differentiation of B cells into antibodies and secreting cells. It also enhances phagocytosis and superoxide production in neutrophils and macrophages. The latter act by destroying foreign material by exposing it to free radicals and hydrolytic enzymes, by antigen presentation to T lymphocytes (in association with MHC II) and by the activation of lymphocyte subpopulations via cytokine secretion( Reference Carr, Kelman and Wu 4 ).
BT, the passage of bacteria or their products from the intestine to sterile organs, is caused by many conditions such as malnutrition, intestinal obstruction, cancer, septic states and trauma. It is harmful to the health and recovery of patients( Reference Wiest and Rath 5 ). Three mechanisms are involved in BT: reduced intestinal barrier function; modified gut microbiota; inadequate response of the host immune system( Reference Zanoni, Benabou and Greco 6 ). Intestinal permeability changes are associated with higher BT levels and sepsis( Reference Shiomi, Shimizu and Endo 7 ). Local immune response and cytokines modulate intestinal permeability and BT to avoid increased inflammation( Reference Lotz, König and Ménard 8 – Reference Sanjabi, Zenewicz and Kamanaka 10 ). Thus, the host immune response plays a major role in the overall process.
Ding & Li( Reference Ding and Li 11 ) demonstrated that animals pretreated with glutamine displayed reduced BT and damage to the intestinal mucosa after surgical trauma and endotoxaemia compared with untreated animals. In addition, IgA levels were increased in the GLN group (intestinal obstruction group receiving glutamine by oral administration and the specially prepared diet).
NO is responsible for cytotoxic activity that results in oxidative stress and tissue damage; it is an important mediator of the inflammatory response( Reference Wu and Meininger 12 , Reference Barocelli, Ballabeni and Ghizzardi 13 ). NO is an important intracellular and intercellular signalling molecule involved in the regulation of diverse physiological and pathophysiological mechanisms( Reference Aktan 14 ). Basal NO production maintains adequate perfusion, regulates intestinal permeability and minimises mucosal and microvascular barrier dysfunction after intestinal ischaemia( Reference Kubes 15 , Reference Naito, Takagi and Ichikawa 16 ).
Previous studies performed in our laboratory have demonstrated that glutamine reduces BT. These studies have reported positive results using 500 mg/kg per d of glutamine. On the other hand, the concentration of 250 mg/kg per d was inefficient( Reference de Oliveira, Lemos and Diniz 17 , Reference dos Santos, Viana and Generoso 18 ). NO is probably an important metabolite of the glutamine pathway and may be involved in BT. However, the immunological aspects and role of glutamine in BT and intestinal permeability require further investigation. Thus, the goal of the present study was to investigate the effects of NO synthase inhibition after glutamine treatment on BT and intestinal mucosal integrity as well as to assess the local and systemic immune responses to NO synthase inhibition.
Methods
Animals and diets
In the present study, 6-week-old male Swiss mice weighing between 25 and 30 g were used. For each experiment, mice were randomly divided into four groups: (1) the sham group, with no intestinal obstruction and receiving 0·3 ml of saline by oral administration; (2) the IO group, with intestinal obstruction and receiving 0·3 ml of saline by oral administration; (3) the GLN group, with intestinal obstruction and receiving 0·3 ml of glutamine (500 mg/kg per d) (l-glutamine; Ajinomoto do Brazil) by oral administration; (4) the GLN/LN group, with intestinal obstruction and receiving 0·3 ml of glutamine plus NG-nitro-l-arginine methyl ester (l-NAME) (10 mg/kg per d) by oral administration. The mice were orally administered glutamine for 7 d before the induction of intestinal obstruction and were maintained under normal conditions of daylight and darkness in individual cages with free access to water.
The mice in the sham and IO groups were fed a conventional chow diet and were orally administered a placebo (saline solution). In contrast, the GLN and GLN/LN groups received glutamine and a specially prepared diet to render them isoenergetic and isoproteic (Table 1). The study protocol was approved by the Ethics Committee for Animal Experiments at the Universidade Federal de Minas Gerais and complied with the guidelines for the care and use of laboratory animals recommended by the Institute of Laboratory Animal Resources.
Table 1 Diet composition*
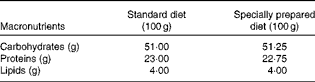
* The standard diet Labina® was given to the simulated group (sham), and intestinal obstruction group (IO). The specially prepared diet, given to the IO+500 mg/kg per d glutamine (GLN) and IO+GLN plus 10 mg/kg per d of NG-nitro-l-arginine methyl ester (l-NAME) (GLN/LN) groups, is a modified diet from Labina® to render them isoenergetic and isoproteic. The diet was prepared according to the macronutrient composition of the standard diet Labina®. Ash, fibre and moisture represent the total volume of the product. Glutamine (500 mg/kg per d) or glutamine plus l-NAME (500 mg/kg per d plus 10 mg/kg per d) was given daily, for 7 d by oral administration, to the GLN or GLN/LN groups, respectively.
Glutamine solution preparation
Glutamine was dissolved in HEPES buffer at a pH between 7·4 and 7·6. A solution was prepared using 10 mmol/l of HEPES, 145 mmol of NaCl and 5 mmol of EDTA. For complete dissolution, the glutamine and buffer were heated at 37°C for 5 min( Reference de Oliveira, Lemos and Diniz 17 ).
Surgical procedure
The mice were anaesthetised intraperitoneally with xylazine (8·7 mg/kg) and ketamine (25·2 mg/kg) solutions. The abdomen was opened through a midline incision and the terminal ileum was isolated and ligated. The mice in the sham group underwent only a laparotomy( Reference de Oliveira, Lemos and Diniz 17 , Reference Salvalaggio, Neto and Tolazzi 19 , Reference Quirino, Correia and Cardoso 20 ). The abdominal wound was closed in two layers.
Escherichia coli radiolabelling
A sample of an E. coli ATCC-10,536 culture grown overnight in trypticase agar was transferred to 10 ml of saline solution. A bacterial culture at 108 colony-forming units/ml was adjusted spectrophotometrically to 31 % transmittance at 580 nm. A 2 ml aliquot of the bacterial suspension was incubated with 1 ml of a stannous chloride solution (580 mm, pH 7·0) at 37°C for 10 min. After incubation, 37·0–55·5 MBq of 99mTc, which was eluted from a 99Molybdenum/99mTechnetium (IPEN) generator as sodium pertechnetate, were added, and the preparation was incubated at 37°C for 10 min. The tubes containing the preparation were then centrifuged at 3000 g for 25 min, and this procedure was repeated three times. During the final procedure, the radioactivity of the supernatant and precipitate was measured using a dose calibrator (Capintec CRC®-15R Dose Calibrator; CAPINTEC, Inc.), and the percentage of 99mTc incorporated into the bacterial cells was determined using the following equation( Reference Diniz, Resende and Nunan 21 ):
 $$\begin{eqnarray} \%\,Labelled\,bacteria = \frac {cpm\,of\,precipitate}{cpm\,of\,precipitate + cpm\,of\,supernatant}\times 100. \end{eqnarray}$$
$$\begin{eqnarray} \%\,Labelled\,bacteria = \frac {cpm\,of\,precipitate}{cpm\,of\,precipitate + cpm\,of\,supernatant}\times 100. \end{eqnarray}$$
Bacterial translocation study
On the eighth day after the above-described treatment, the mice were orally administered 0·1 ml (1·85 MBq) of 99mTc-E. coli at 108 colony-forming units. The surgical procedure was performed on mice 90 min after the administration of 99mTc-E. coli, and 18 h later, the animals were anaesthetised and euthanised. The blood, mesenteric lymph nodes, liver, spleen and lungs were collected, weighed and placed into tubes to determine their radioactivity. The samples were assessed using a counter with a NaI (Tl) crystal (ANSR-Abbott). The results are expressed as counts per min (cpm)/g of tissue( Reference de Oliveira, Lemos and Diniz 17 , Reference dos Santos, Viana and Generoso 18 , Reference Quirino, Correia and Cardoso 20 ).
Intestinal permeability study
Another set of mice were orally administered 0·1 ml of 18·5 MBq diethylenetriaminepentaacetate (99mTc-DTPA). A 99mTc-DTPA dose with the same radioactivity was reserved and used as a standard to correct for the physical decay of 99mTc. For the measurement of radioactivity, 90 min after the administration of 99mTc-DTPA, sixty mice were divided into four groups of fifteen mice and were separately assessed at different time points (4, 8 and 18 h). The mice underwent the aforementioned surgical procedure. Following the operation, the mice were anaesthetised and euthanised, and 500 μl of blood were collected to measure radioactivity. The data are expressed as the percentage of dose using the following equation( Reference dos Santos, Viana and Generoso 18 , Reference Viana, Santos and Generoso 22 ):
Ileal histological analysis
Samples of the small intestine were collected for histological analysis 18 h after surgery. A 1 cm ring of the distal ileum adjacent to the intestinal obstruction was resected, fixed in a 4 % buffered formalin solution, dehydrated, cleared, embedded in paraffin, cut into 4 to 5 mm-thick sections, stained with haematoxylin and eosin, coded and qualitatively analysed using optical microscopy performed by a single pathologist who was unaware of the experimental conditions for each group( Reference Viana, Santos and Generoso 22 , Reference Generoso, Viana and Santos 23 ). The determination of villus height was done using five villus samples obtained from the images of five fields obtained from the histological sections of at least three mice in each group and represented as the average height of villi.
Serum IL-10 and interferon-γ concentration determination
As has been described previously, 18 h after the intestinal obstruction, blood was collected from the axillary plexus. The blood was centrifuged at 1000 g for 10 min, and serum was separated and stored at − 70°C until the assay was performed. The concentrations of IL-10 and interferon-γ (IFN-γ) were measured with ELISA using commercially available antibodies in accordance with the manufacturer's instructions (BioSource International, Inc.)( Reference Generoso, Viana and Santos 24 ).
Intestinal secretory Ig type A concentration determination
As has been described above, four groups of five mice were treated. The levels of secretory Ig type A (sIgA) were measured in the intestinal fluid as described previously( Reference Martins, Silva and Vieira 25 ). After euthanasia, the small intestines were removed from the mice of each group, and the contents were separated, weighed and suspended in PBS using 500 mg of the intestinal contents per 2 ml of PBS supplemented with an anti-protease cocktail (1 μm-aprotinin, 25 μm-leupeptin, 1 μm-pepstatin and 1 mm-phenylmethanesulfonyl fluoride). After 30 min of centrifugation at 2000 g at 4°C, the supernatant was collected and frozen at − 70°C until use. Ig levels in the intestinal fluid were evaluated with ELISA using goat anti-mouse IgA (Sigma Chemical Company) and horseradish peroxidase-conjugated goat anti-mouse IgA (Sigma). The colour was developed using o-phenylenediamine (Sigma), and the absorbance at 492 nm was determined using an ELISA plate reader (Bio-Rad Laboratories). Ig concentrations were determined using a purified mouse IgA standard (Southern Biotechnology Associates, Inc.)( Reference Generoso, Viana and Santos 23 , Reference Rodrigues, Cara and Fretez 26 ).
Statistical analysis
The results were evaluated using the Kolmogorov–Smirnov test for normality and a box plot for outliers. Data with normal distributions were tested using a one-way ANOVA and the Tukey post hoc test or a two-way ANOVA and the Bonferroni post hoc test, whereas non-parametric data were tested using a Kruskal–Wallis ANOVA and Dunn's test. P values less than 0·05 were considered significant. The analysis was performed using the GraphPad Prism 5 program.
Results
Effects of nitric oxide synthase inhibition on bacterial translocation
The administration of l-NAME with glutamine increased the BT to levels that were significantly higher than those of the sham and GLN groups (P< 0·05; Table 2). Moreover, compared with that in the IO group, BT was higher in the blood and livers of the GLN/LN group and similar to that in the other investigated organs.
Table 2 Biodistribution of 99mTc-Escherichia coli (counts per min/g) (Medians with their 25th and 75th interquartile ranges (IQR))

Sham, simulated group receiving a standard diet; IO, intestinal obstruction group receiving a standard diet; GLN, intestinal obstruction group receiving glutamine by oral administration and a specially prepared diet; GLN/LN, intestinal obstruction group receiving glutamine plus NG-nitro-l-arginine methyl ester (l-NAME) by oral administration and a specially prepared diet; MLN, mesenteric lymph nodes.
a,b,cMedian values with unlike superscript letters were significantly different between the groups (P< 0·05). Simultaneous comparison was done among the different treatment groups (n 7 per group).
Effects of nitric oxide synthase inhibition on intestinal permeability
The uptake of 99mTc-DTPA in the blood of the IO group mice was significantly higher than that of the sham group at each investigated time point and reached a maximum at 18 h (Fig. 1). Glutamine pretreatment (500 mg/kg per d) reduced intestinal permeability to physiological levels (i.e. those of the sham group). NO synthase inhibition by l-NAME did not alter intestinal permeability at the investigated time points.
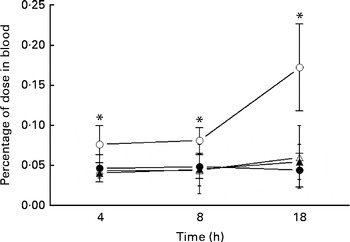
Fig. 1 Intestinal permeability of mice in the sham group receiving a standard diet (●), the IO group with intestinal obstruction receiving a standard diet (○), the GLN group with intestinal obstruction receiving glutamine by oral administration and a specially prepared diet (Δ) and the GLN/LN group with intestinal obstruction receiving glutamine plus NG-nitro-l-arginine methyl ester (l-NAME) by oral administration and a specially prepared diet (▲) (n 5 per group). Values are means, with standard deviations represented by vertical bars. * Mean values were significantly different from those of the other groups (P< 0·05). % dose = ((counts/min (cpm) in blood × 100)/cpm of the administered dose).
Effects of nitric oxide synthase inhibition on the distal ileum mucosal morphology
In the sham group (Fig. 2(A)), normal ileal architecture was observed. These findings are in contrast with the marked histological damage to the ileum produced by intestinal obstruction (Fig. 2(B)). There was intense swelling of the lamina propria and damage to the villi, which were dramatically shortened. Leucocyte infiltration and lymphatic dilation were focally present in the lamina propria. The glutamine-pretreated mice exhibited partially preserved intestinal structures; the continuity of the epithelium and the caliciform cells was preserved, and the lamina propria displayed discrete oedema (Fig. 2(C)). In the NO synthase inhibition group, GLN/LN, the ileal mucosa and the overall structure were preserved, similar to those of the sham group (Fig. 2(D)).
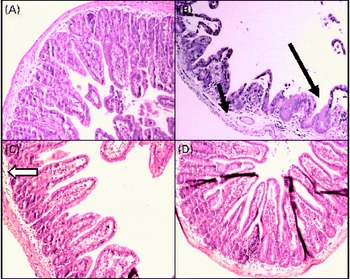
Fig. 2 Alterations in the structure of mucous membrane of the ileum under microscopy in (A) the sham group (simulated group receiving the standard diet) presenting a normal structure of the ileal mucosa; (B) the IO group (intestinal obstruction group receiving a standard diet) with representative aspects of ileal mucosa showing evident damage of ileal architecture, villus swelling and shortening and intense swelling in the lamina propria (![]() ), associated with the infiltration of leucocytes (
), associated with the infiltration of leucocytes (![]() ) and the dilatation of lymphatic ducts, and villi are more sparse and enlarged (
) and the dilatation of lymphatic ducts, and villi are more sparse and enlarged (![]() ) than those in the sham group; (C) the GLN group (intestinal obstruction group receiving glutamine by oral administration and a specially prepared diet) showing evident protection of mucosa structure indicated by the absence of oedema and the preservation of villus structure despite persistence of a few enlarged villi (
) than those in the sham group; (C) the GLN group (intestinal obstruction group receiving glutamine by oral administration and a specially prepared diet) showing evident protection of mucosa structure indicated by the absence of oedema and the preservation of villus structure despite persistence of a few enlarged villi (![]() ); and (D) the GLN/LN group (intestinal obstruction group receiving glutamine plus NG-nitro-l-arginine methyl ester (l-NAME) by oral administration and a specially prepared diet) presenting the preservation of ileal mucosa structural aspects, very similar to the sham group. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
); and (D) the GLN/LN group (intestinal obstruction group receiving glutamine plus NG-nitro-l-arginine methyl ester (l-NAME) by oral administration and a specially prepared diet) presenting the preservation of ileal mucosa structural aspects, very similar to the sham group. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
We determined the average villus height (Fig. 3). The sham, GLN and GLN/LN groups presented similar average villus heights, confirming the results of our qualitative histological analysis. The IO group presented an intense and significant reduction in villus height, indicating the degree of intestinal damage.
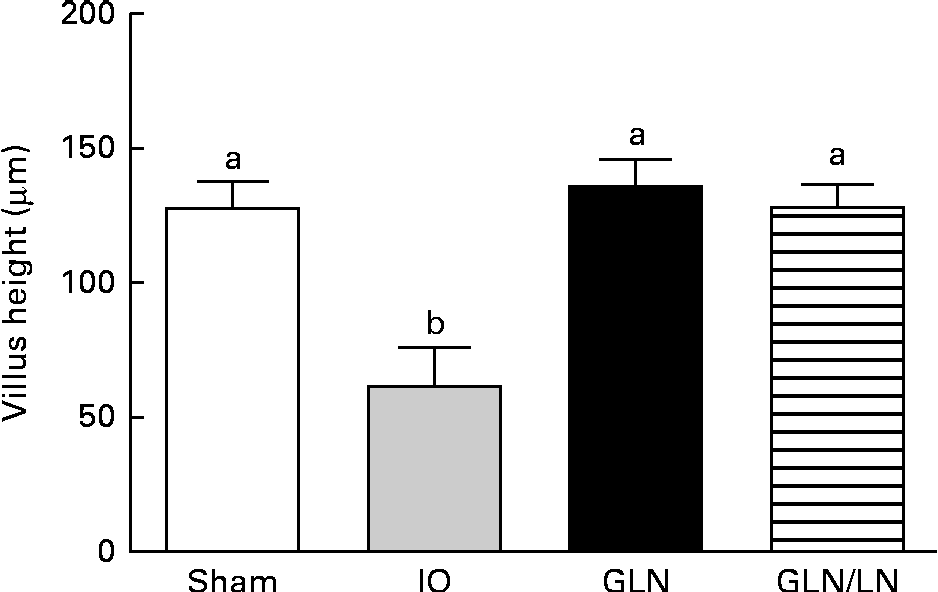
Fig. 3 Determination of the villus height in the sham group receiving a standard diet, the IO group with intestinal obstruction receiving a standard diet, the GLN group with intestinal obstruction receiving glutamine by oral administration and the GLN/LN group with intestinal obstruction receiving glutamine plus NG-nitro-l-arginine methyl ester (l-NAME) by oral administration. The last two groups received a specially prepared diet. Values are means, with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different between the groups (P< 0·05).
Effects of nitric oxide synthase inhibition on the local immune response
Similar sIgA levels were observed in the sham and IO groups (P>0·05) (Fig. 4). In contrast, the glutamine-pretreated mice had higher levels of sIgA (P< 0·05). NO synthase inhibition caused by l-NAME treatment did not change the IgA levels compared with those of the GLN group (P>0·05).
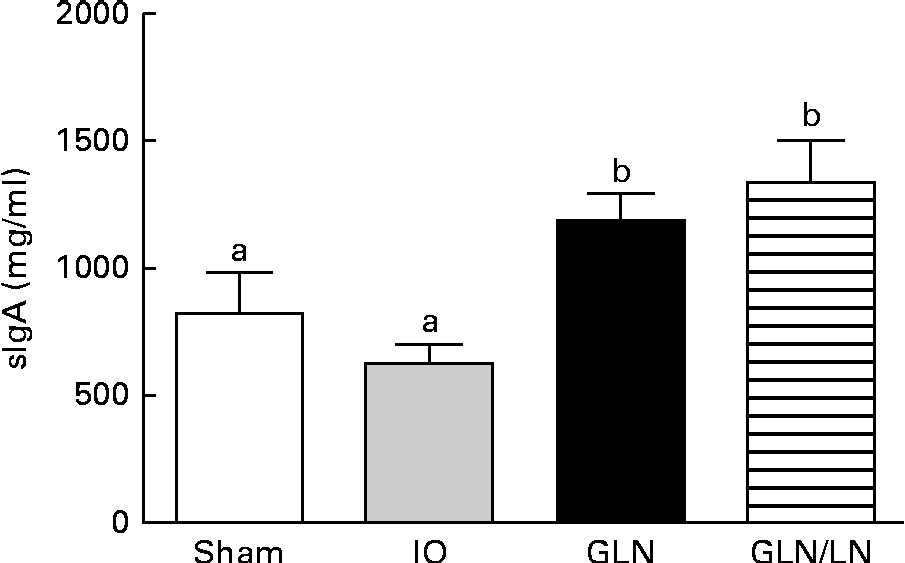
Fig. 4 Secretory IgA (sIgA) levels in the sham group receiving a standard diet, the IO group with intestinal obstruction receiving a standard diet, the GLN group with intestinal obstruction receiving glutamine by oral administration and a specially prepared diet and the GLN/LN group with intestinal obstruction receiving glutamine plus NG-nitro-l-arginine methyl ester (l-NAME) by oral administration and a specially prepared diet (n 5 per group). Values are means, with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different between the groups (P< 0·05).
Effects of nitric oxide synthase inhibition on the systemic immune response
The mice in the IO group had higher levels of IFN-γ than those in the sham group (P< 0·05), but IL-10 levels were similar between the two groups (P>0·05). In contrast, the GLN group had higher IFN-γ and IL-10 levels than the sham and IO groups (Fig. 5). The mice in the GLN/LN group had IFN-γ levels similar to those of the IO group (P>0·05), but their IL-10 levels were as high as those of the GLN group. The ratios of the inflammatory to anti-inflammatory cytokines were 3·82, 3·24, 1·29 and 0·61 in the IO, sham, GLN and GLN/LN groups, respectively.
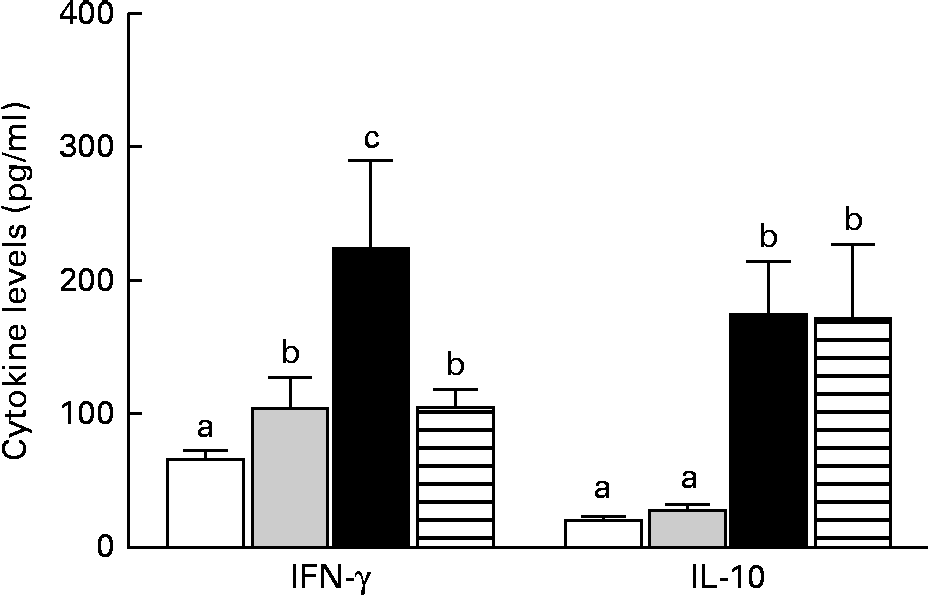
Fig. 5 Serum levels of interferon-γ (IFN-γ) and IL-10 in the sham group receiving a standard diet (□), the IO group with intestinal obstruction receiving a standard diet (![]() ), the GLN group with intestinal obstruction receiving glutamine by oral administration (■) and the GLN/LN group with intestinal obstruction and receiving glutamine plus NG-nitro-l-arginine methyl ester (l-NAME) by oral administration (
), the GLN group with intestinal obstruction receiving glutamine by oral administration (■) and the GLN/LN group with intestinal obstruction and receiving glutamine plus NG-nitro-l-arginine methyl ester (l-NAME) by oral administration (![]() ). The last two groups received a specially prepared diet (n 5 per group). Values are means, with standard deviations represented by vertical bars. a,b,cMean values with unlike letters were significantly different between the groups (P< 0·05).
). The last two groups received a specially prepared diet (n 5 per group). Values are means, with standard deviations represented by vertical bars. a,b,cMean values with unlike letters were significantly different between the groups (P< 0·05).
Discussion
The present study assessed the influence of NO synthase inhibition on the glutamine pathway and associated processes, such as the immunological response and intestinal permeability, in a murine model of BT. We had previously demonstrated that pre-injury glutamine administration reduced BT to physiological levels( Reference dos Santos, Viana and Generoso 18 ); however, the mechanism was not investigated. It is possible that among the multiple functions of glutamine, glutamate generation via glutaminase might be a metabolic pathway of NO production( Reference van de Poll, Ligthart-Melis and Boelens 27 ). The present results demonstrated that the non-selective NO synthase inhibition caused by l-NAME administration (GLN/LN group) increased BT to all the investigated organs compared with that in the sham and GLN groups and only to the blood and liver compared with that in the IO group. These data can be explained by the potent bactericidal action of NO against micro-organisms, including the majority of the intestinal microbiota( Reference Han, Fink and Delude 28 ). Another mechanism that may be associated with BT is intestinal barrier integrity. However, one interesting finding of the present study was that NO synthase inhibition did not alter intestinal permeability. The reduction in intestinal permeability caused by glutamine was probably mediated by another mechanism, such as an increase in heat shock protein (HSP) production. HSP are highly conserved proteins that are found in all cells and are fundamental to the responses of cells and tissues to stress and injury. HSP induction prevented any endotoxin-induced intestinal mucosal atrophy or increases in intestinal permeability( Reference Wang and Hasselgren 29 ). Wischmeyer et al. ( Reference Wischmeyer, Musch and Madonna 30 ) and Musch et al. ( Reference Musch, Hayden and Sugi 31 ) showed that glutamine enhanced HSP-70 expression in intestinal epithelial cells, which correlated with improved cell survival following oxidant stress, and that the glutamine protection was abrogated when HSP-70 expression was inhibited. Other aspects could be considered such as the role of glutathione as an antioxidant agent protecting the intestinal mucosa against oxidative stress and inhibiting apoptosis( Reference Perricone, Carolis and Perricone 32 – Reference Ban and Kozar 34 ) as well as the regulatory action of protein metabolism. The latter acts mainly on tight junctions having a protector effect on the intestinal barrier( Reference Yeh, Hsu and Yeh 35 ).
Immunological responses are also an important part in the BT process. In the present study, IFN-γ levels were higher in the IO group than in the sham group (P>0·05). In addition, the levels of this cytokine were higher in the GLN group than in the other groups (P< 0·05). These results are similar to the observations of Yeh et al. ( Reference Yeh, Hsu and Yeh 35 ) and Horio et al. ( Reference Horio, Osawa and Takagaki 36 ), who demonstrated that glutamine enhanced IFN-γ levels in an animal model of septic shock and in active macrophages. Pro-inflammatory mediators enhance cell-mediated immunity, and their role in host defence is well known, although excessive, uncontrolled inflammation produces a variety of pathological conditions. This process is controlled by a balance between pro-inflammatory and regulatory responses( Reference Dinarello 37 , Reference Rakoff-Nahoum, Paglino and Eslami-Varzaneh 38 ). In contrast, NO synthase inhibition modified the impact of glutamine by generating levels of IFN-γ similar to those observed in the IO group (P>0·05). The lower IFN-γ levels were probably responsible for the increased BT observed in the GLN/LN group because the protective effect of IFN-γ on BT may be related to NO production, up-regulated phagocyte levels and, subsequently, increased microbe ingestion( Reference Naderi beni, Fattahi and Mirshafiey 39 ). Furthermore, inducible NO is essential for killing certain bacteria and is potentially important for regulating the proliferation and differentiation of T cells( Reference Kubes 15 ). Under normal circumstances, the translocating bacteria are phagocytosed before reaching the mesenteric lymph nodes. The assumption is that if the host is immunocompromised, then the normal defence mechanisms fail and bacteria can translocate to and survive at distant extraintestinal sites, which then leads to increased inflammation( Reference MacFie 40 ).
To control this exacerbated systemic inflammation, the production of anti-inflammatory cytokines, particularly IL-10, contributes to the down-regulation of pro-inflammatory cytokines. The present results showed that blood IL-10 concentrations were similar in the sham and IO groups (P>0·05) and elevated in the GLN and GLN/LN groups, which were similar to each other. However, when the cytokine ratio (which is important due to their opposing relationships) was considered instead of the absolute values, the IFN-γ:IL-10 ratio was the highest in the IO group (3·82), followed by the sham (3·24), GLN (1·29) and GLN/LN (0·61) groups. These results show that glutamine increased IFN-γ levels while further increasing IL-10 levels, which probably contributed to the beneficial effects of this immunonutrient on BT and intestinal permeability. Similar results were reported by Boelens et al. ( Reference Boelens, Houdijk and Fonk 41 ) and Oliveira et al. ( Reference Oliveira, Oliveira and Santos 42 ), who showed increases in IFN-γ (a Th1 cytokine), IL-4 (a Th2 cytokine) and IL-10 levels after glutamine treatment in trauma patients and septic animals, respectively. Wang et al. ( Reference Wang, Fang and Hasselgren 43 ) showed that sepsis increased mucosal permeability, which was then reduced by the heat shock response, and that increases in IL-10 levels may be involved in the protective effects of the HSP. However, the lowest IFN:IL-10 ratio in the present study was observed in the GLN/LN group and was caused by the decreased levels of IFN-γ in this group. This observation may lead to the conclusion that inflammation is necessary to counter BT. IL-10 induction and NO synthase inhibition presumably have a suppressive effect on the pro-inflammatory response, which profoundly affects the bactericidal activity in phagocytic cells and facilitates the intracellular survival of pathogens( Reference Naderi beni, Fattahi and Mirshafiey 39 , Reference Redpath, Ghazal and Gascoigne 44 ). In addition, IL-10 is responsible for humoral immunity and, consequently, the levels of IgA, which acts by binding to the bacteria or pathogens. Thus, this activity of IgA prevents bacterial internalisation, which is the first step of BT( Reference Kudsk 45 ). In the present study, sIgA levels were enhanced in the GLN group than in the sham and IO groups (P< 0·05). Similar results have been obtained by Kudsk et al. ( Reference Kudsk, Wu and Fukatsu 46 ), Fukatsu et al. ( Reference Fukatsu, Kudsk and Zarzaur 47 ) and Jiang et al. ( Reference Jiang, Ren and Chen 48 ). As an unexpected result, the sIgA levels of the GLN/LN group were similar to those of the GLN group; however, elevated BT was only observed in the former group. The absence of NO probably contributed to the increased BT through other pathways that are independent of sIgA. This result reinforces the notion that IFN-γ and NO play crucial roles in host defence mechanisms via their antimicrobial activity( Reference Akaike, Okamoto and Zaki 49 ). Moreover, the intestinal barrier function can be affected without the disruption of tight junctions; however, the transepithelial resistance is reduced, which increases the flow of small molecules, but not of large molecules, such as DTPA( Reference Abreu, Palladino and Arnold 50 , Reference Arrieta, Bistritz and Meddings 51 ).
In conclusion, the present results indicate that the effects of glutamine on BT involve NO activity, which interferes with the pro-inflammatory systemic immunological response, although the protection of intestinal permeability by glutamine is independent of NO. Further studies are necessary to determine which NO isoform is triggered by glutamine and to assess the cytokine response in the intestinal lamina propria.
Acknowledgements
The authors are grateful to the following sources for providing support: Pró-Reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq); CNPq (the National Council of Technological and Scientific Development); CAPES (the Coordination for the Improvement of Higher Education Personnel); FAPEMIG (the Minas Gerais State Foundation for Research Support). R. G. C. S. designed and conducted the research, analysed the data and helped draft the manuscript. I. E. P. Q., M. L. V. and S. V. G. helped conduct the research. J. R. N. and F. S. M. assisted in the sIgA concentration determination. R. M. E. A. helped conduct the histological analysis. J. A. N.-M. provided assistance with the cytokine ELISA. M. I. T. D. C. and V. N. C. supervised the research and helped draft the manuscript. All the authors read and approved the final manuscript. The authors declare that they have no conflicts of interest.









