α-Glucosidase inhibitors, which are oral antidiabetic agents, delay carbohydrate absorption from the small intestine by inhibiting intestinal α-glucosidase activity. These drugs (e.g. acarbose, voglibose, miglitol) improve postprandial hyperglycaemia in diabetic patientsReference Inzucchi1, Reference Scheen2. Acarbose, a pseudotetrasaccharide, has been reported to be a competitive inhibitor of sucrase, glucoamylase and isomaltaseReference Schmidt, Frommer, Junge, Muller, Wingender, Truscheit and Schafer3–Reference Goda, Yamada, Hosoya and Moriuchi6. Voglibose, an N-substituted valiolamine derivative, has been reported to have stronger α-glucosidase inhibitory activity against maltase and sucraseReference Horii7.
Miglitol, a 1-deoxynojirimycin derivative, is another drug selected for development as an antihyperglycaemic drugReference Puls, Krause, Muller, Schutt, Sitt and Thomas8. Miglitol is a strong inhibitor of glucoamylase, sucrase and isomaltaseReference Samulitis, Goda, Lee and Koldovsky9, and has been used clinically in Europe and the USAReference Scott and Spencer10, Reference Fehmann11. On the other hand, voglibose is not approved in Europe or the USA but has been widely used in the treatment of patients with diabetes in Asia. Miglitol causes less diarrhoea than acarbose and voglibose because it is almost completely absorbed in the upper region of the small intestine in rats, whereas acarbose and voglibose are not absorbedReference Puls, Krause, Muller, Schutt, Sitt and Thomas8, Reference Ahr, Boberg, Brendel, Krause and Steinke12. Miglitol has recently been reported to reduce the plasma glucose concentration in a dose-dependent manner in normal ratsReference Tsukamoto, Nakayama and Mitsuzono13 and in several animal models of diabetesReference Madar14–Reference Russell, Graham and Dolphin16.
The question of whether or not a reduction in hyperglycaemia is achieved by acute or chronic oral administration of miglitol has not been investigated in Goto-Kakizaki (GK) rats, an animal model of non-obese, non-insulin-dependent diabetes characterised by low insulin secretionReference Goto, Kakizaki and Masaki17, Reference Goto and Kakizaki18. The effects of acarbose and voglibose have, however, already been investigatedReference Koyama, Wada, Mizukami, Sakuraba, Odaka, Ikeda and Yagihashi19–Reference Azuma, Toyofuku and Iesaki23. It has been reported that β-cells in GK rats are defective in their glucose-stimulated insulin secretion, whereas insulin synthesis is not affectedReference Portha, Serradas, Bailbe, Suzuki, Goto and Giroix24–Reference Giroix, Vesco and Portha26. This animal model exhibits functional and structural features of diabetic complications and is therefore considered suitable for studying type 2 diabetesReference Goto, Suzuki, Ono, Sasaki and Toyota27, Reference Suzuki, Goto, Toyota and Shafrir28. It has also been reported that total β-cell mass was decreased in type 2 diabetic patients compared with control subjectsReference Clark, Wells, Buley, Cruickshank, Vanhegan, Matthews, Cooper, Holman and Turner29 and that β-cell loss was enhanced by sucrose-feeding via an increase in the rate of apoptosis in GK ratsReference Koyama, Wada, Sakuraba, Mizukami and Yagihashi30. Furthermore, a reduction in β-cell volume was found to be inhibited by a reduction of hyperglycaemia in GK rats treated with vogliboseReference Koyama, Wada, Mizukami, Sakuraba, Odaka, Ikeda and Yagihashi19. However, the effects of oral administration of miglitol for β-cell reduction as well as glycaemic control in GK rats are still unknown.
In the present study, we examined the acute effects of a single dose of miglitol on blood glucose concentrations and insulin secretion after sucrose-loading, as well as the chronic effects of long-term miglitol treatment in a dietary mixture on glycaemic control and β-cell reduction in GK rats.
Materials and methods
Animals
Diabetic GK male rats were obtained from CLEA Japan Inc., Japan. Non-diabetic Wistar male rats were purchased from Japan SLC (Hamamatsu, Japan). The rats had free access to standard laboratory chow (MF; Oriental Yeast, Tokyo, Japan) and water, and were housed in a room controlled for temperature (22 ± 3°C), humidity (55 ± 15 %) and light (hours of time 07.00–19.00 hours).
Effects of a single dose of miglitol in Goto-Kakizaki rats
GK rats were randomly assigned to a group based on body weight and the results of oral glucose tolerance testing (2 g/kg body weight). Rats whose blood plasma glucose concentration was less than 300 mg/dl were excluded. Eight rats (10 weeks old) were used in each group and were fasted for 18–20 h before the experiments. The experimental procedures were approved by the Ethical Committee for Animal Research of Mitsubishi Chemical Safety Institute Ltd.
Sucrose-loading test
The rats were administered with a single oral dose of miglitol or voglibose (miglitol 1, 3, 10 mg/kg body weight; voglibose 0·0125, 0·0375, 0·125 mg/kg body weight) dissolved in saline. Miglitol and voglibose provided by Bayer Yakuhin Ltd. (Osaka, Japan) were used in the present study. GK rats administered with only saline served as controls. The drug solutions were administered using a gastric tube in a volume of 5 ml/kg immediately before sucrose-loading (2 g/kg body weight, 5 ml/kg body weight).
Blood for glucose and insulin measurements was obtained from a subclavian vein at 0·25, 0·5, 1, 2 or 4 h after sucrose-loading. Blood glucose was measured by the enzyme electrode method using a blood glucose meter (Horiba, Kyoto, Japan). Plasma insulin was estimated using an insulin ELISA kit (Morinaga Institute of Biological Science, Kanagawa, Japan). Glucose concentrations were calculated as the incremental blood glucose concentrations integrated over a period of 2 h (ΔAUC0–2 h) after sucrose-loading.
Effects of 8-week miglitol administration on Goto-Kakizaki rats
All animals received the high-sucrose and high-fat control diet (Table 1) for 2 weeks before assignment to a study group.
Table 1 Composition of the high sucrose and high fat control diet
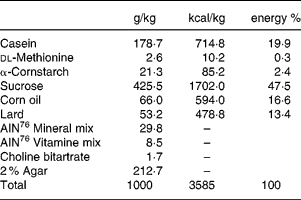
Seven week-old male GK rats were assigned to one of six groups based on body weight and the results of the oral glucose tolerance test. Each group consisted of 5–7 animals. Each experiment consisted of one control Wistar rat group and six GK rat groups as follows: (1) control Wistar rats fed the control diet; (2) control GK rats fed the control diet; (3) miglitol-treated GK rats fed a diet containing 10 mg miglitol/100 g control diet; (4) miglitol-treated GK rats fed a diet containing 20 mg miglitol/100 g control diet; (5) miglitol-treated GK rats fed a diet containing 40 mg miglitol/100 g control diet; (6) voglibose-treated GK rats fed a diet containing 0·1 mg voglibose/100 g control diet; (7) voglibose-treated GK rats fed a diet containing 0·2 mg voglibose/100 g control diet. The rats were fed the control diet with either miglitol or voglibose ad libitum for 8 weeks. Feed mixtures containing miglitol or voglibose were prepared once a week.
The experimental procedures used in the present study conformed to the guidelines of the Animal Usage Committee of the University of Shizuoka.
Parameters
Blood glucose, body weight and food intake were measured after 0, 2, 4, 6 or 8 weeks of feeding. Physical signs were observed on an occasional basis.
HbA1c was assayed by HPLC (Waters, Tokyo, Japan) using a TSKgel Boronate-5PW column system (Tosoh Corporation, Tokyo, Japan). Blood samples were collected for determination of plasma glucose after the rats were fasted overnight. Plasma glucose was measured by the glucose oxidase method using a commercial kit (Wako Pure Chemical Industries, Osaka, Japan).
After treatment for 8 weeks, all animals underwent an oral glucose tolerance test. The rats were orally administered a glucose solution (2 g/kg body weight, 10 ml/kg body weight) after an overnight fast, and their plasma glucose and plasma insulin concentrations were determined using blood samples from the tail vein 0, 15, 30, 60 and 120 min later. The data for GK rats given 0·2 mg voglibose/100 g control diet were excluded from the assessment because it appeared that energy restriction occurred as a result of diarrhoea in these rats.
After the oral glucose tolerance test, the animals were again given the control diet with miglitol or voglibose ad libitum by 23.00 hours. They were then fasted overnight and killed by decapitation. The whole pancreas was excised, weighed following the removal of connective tissue and extraneous fat, and then fixed in 3·7% (v/v) formaldehyde and processed for paraffin embedding.
Islet pathology and morphometry of β-cells
Serial 4 μm thick paraffin sections were stained with haematoxylin & eosin or azan for islet pathology and immunostained for morphometric analysis of islet β-cells. To identify the areas of β-cells in the islets, immunoperoxidase staining was performed using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA). Briefly, deparaffinised sections were first incubated with guinea-pig anti-insulin antibody (Dako, Carpinteria, CA, USA). The slides were then incubated with biotinylated anti-guinea-pig immunoglobulin antibody. The secondary reaction products were visualised with diamino-benzidine. Nuclei were lightly counterstained with haematoxylin.
Calculation of the volume density of β-cells was performed using an image processor for analytical pathology (IPAP-WIN; Sumika Technoservice Corporation, Osaka, Japan). Areas of blood vessels, fat and connective tissue were excluded from the measurements. The identity of the sample was masked to the examiners during the process of morphometric analysis.
Statistical analysis
All experimental values are expressed as means with their standard errors for each group. Statistical analysis was performed using Dunnett's test, analysis of linear regression and the Jonckheere-Terpstra test using SAS Proprietary Software (SAS Institute, Tokyo, Japan). The levels of body weight, food intake, blood glucose, plasma insulin and HbA1c were compared between control GK rats and rats treated with several concentrations of miglitol or voglibose. The level of volume density of β-cells was compared between control Wister rats and GK rats with/without miglitol or voglibose treatment. A level of P < 0·05 was considered to indicate statistical significance.
Results
Effects of a single dose of miglitol in Goto-Kakizaki rats
Blood glucose concentration
α-Glucosidase inhibitors inhibit the digestion of sucrose in the lumen of the small intestine. To estimate the effect of miglitol on postprandial hyperglycaemia as a result of inhibiting sucrose digestion in the lumen, the blood glucose concentrations after sucrose loading were measured. Blood glucose reached the highest concentration (382 mg/dl) 1 h after sucrose-loading in GK rats in the control group, following which values recovered to the pretreatment level at 4 h (Fig. 1(A)). Blood glucose concentrations were not significantly different between control GK rats and 1 or 3 mg/kg miglitol-treated GK rats. Blood glucose concentrations in the 10 mg/kg miglitol-treated GK rats decreased significantly compared with the control group at 1 h after sucrose-loading (P < 0·05). In voglibose-treated GK rats, blood glucose concentrations were not significantly different from those of the control group at any dose.

Fig. 1 Effects of miglitol (left panel) and voglibose (right panel) on concentrations of blood glucose (A) and plasma insulin (B) in sucrose-loaded Goto-Kakizaki rats. Mean values and their standard errors (n 8). Mean values were significantly different from those of the control group (Dunnett's test): *P < 0·05.
The incremental blood glucose concentration integrated over a period of 2 h (ΔAUC0–2 h values) after the administration of sucrose in the control group was 275 mg/dl per h (Table 2). At doses of 1 and 3 mg/kg, miglitol did not affect the ΔAUC0–2 h of blood glucose. At 10 mg/kg, the ΔAUC0–2 h of blood glucose fell by 45 % compared with the control group (P < 0·05). None of the doses of voglibose affected the ΔAUC0–2 h of blood glucose.
Table 2 Incremental blood glucose concentration in sucrose-loaded Goto-Kakizaki (GK) rats after a single dosage treatment with miglitol or voglibose (Mean values with their standard errors)
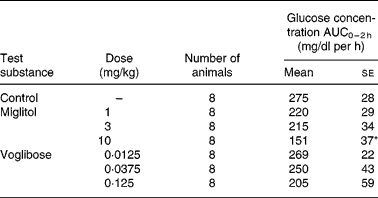
ΔAUC0–2 h, Incremental blood glucose concentration integrated over a period of 2 h in miglitol- or voglibose-treated GK rats.
Mean values were significantly different from those of the control group (Dunnett's test): *P < 0·05.
Plasma insulin concentration
Plasma insulin concentrations were increased at 0.25 h after sucrose-loading in control GK rats and returned to pretreatment levels 0.5 h after sucrose-loading (Fig. 1(B)). Thereafter, plasma insulin concentrations in control GK rats increased again 1 h after sucrose-loading and reached their highest values (1·58 ng/ml) 2 h after sucrose-loading.
In GK rats treated with 1 mg/kg miglitol, there was no effect on plasma insulin concentration compared with the control group. The plasma insulin concentration at 0·25 h after sucrose-loading in the 3 and 10 mg/kg miglitol groups decreased by 44 % and 22 %, respectively, compared with the control group, but the differences were not significant. Voglibose at doses of 0·0125 and 0·0375 mg/kg did not affect the plasma insulin concentration. Plasma insulin concentration 1 h after sucrose-loading in the 0·125 mg/kg voglibose-treated groups decreased by 35 % compared with the control group, but was not significantly different from that of the control group.
Effects of 8-week miglitol administration in Goto-Kakizaki rats
Body weight and food intake
No differences in body weight were seen between the miglitol-treated and control GK rats (Table 3). Food intake in the miglitol-treated GK rats was decreased for 1 week after treatment but then returned to values similar to those of control Wistar and control GK rats (Table 4). Physical signs in the miglitol-treated GK rats were normal throughout the treatment period.
Table 3 Body weight (g) of miglitol- and voglibose-treated Goto-Kakizaki (GK) rats (Means values with their standard errors)
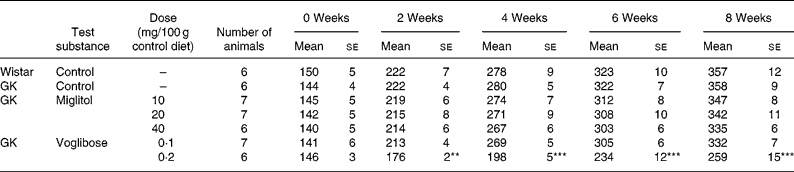
Mean values were significantly different from those of control GK rats (Dunnett's test): **P < 0·01, ***P < 0·001.
Table 4 Food intake (g) of miglitol- or voglibose-treated Goto-Kakizaki (GK) rats (Mean values with their standard errors)
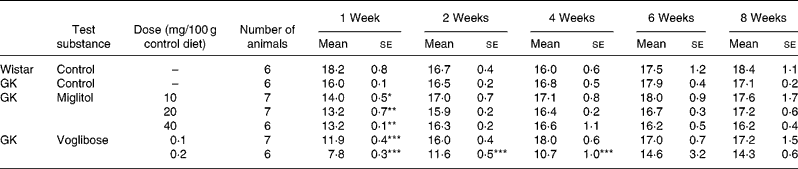
Mean values were significantly different from those of control GK rats (Dunnett's test): *P < 0·05, **P < 0·01, ***P < 0·001.
In GK rats fed the diet containing 0·1 mg voglibose/100 g control diet, no difference in body weight was seen compared with control GK rats, even though food intake was decreased 1 week after the treatment before returning to values similar to those of control GK rats. On the other hand, diarrhoea was observed 1 day after treatment in GK rats administered the diet containing 0·2 mg voglibose/100 g control diet. The body weight of these latter rats was significantly decreased compared with control GK rats throughout the experimental period (P < 0·01, P < 0·001, P < 0·001 and P < 0·001 for weeks 2, 4, 6 and 8 respectively), whereas food intake was significantly reduced at each time point until 4 weeks (P < 0·001, P < 0·001 and P < 0·001, respectively).
HbA 1c. HbA1c concentrations were not significantly different between the control GK rats and the miglitol-treated GK rats (control, 6·90% (se 0·24); 10 mg miglitol/100 g control diet, 6·49% (se 0·11); 20 mg miglitol/100 g control diet, 6·30% (se 0·18), 40 mg miglitol/100 g control diet, 6·45% (se 0·19)). The HbA1c ratio of 8 weeks to 0 week (ΔHbA1c value) in miglitol-treated GK rats decreased in a dose-dependent manner (Fig. 2). In GK rats treated with 40 mg miglitol/100 g control diet, ΔHbA1c was significantly decreased compared with control GK rats (P < 0·01), although the value in GK rats fed the diet containing 0·1 mg voglibose/100 g control diet was similar to that in control GK rats (HbA1c value, 6·68% (se 0·22); Fig. 2). In rats fed the diet containing 0·2 mg voglibose/100 g control diet, the HbA1c value after 8 weeks of treatment (HbA1c value, 4·89% (se 0·27)) decreased to a value similar to the level (HbA1c value, 5·07% (se 0·12)).
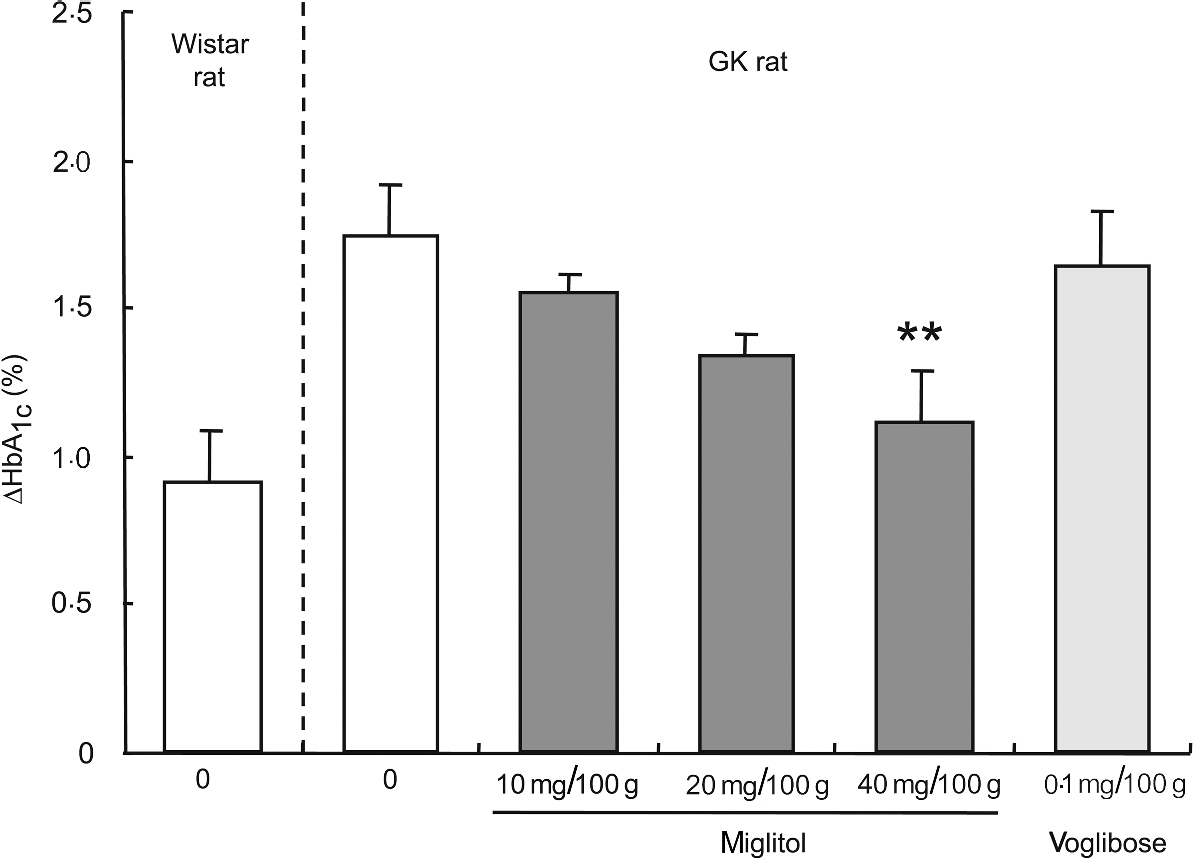
Fig. 2 ΔHbA1c concentrations for 8 weeks in miglitol- and voglibose-treated Goto-Kakizaki (GK) rats. Mean values and their standard errors (n 5–7). Mean values were significantly different from those of control GK rats (Dunnett's test): **P < 0·01.
An analysis of linear regression between the dosage of miglitol and the ΔHbA1c value showed clearly that treatment of the GK rats with miglitol reduced the ΔHbA1c value in a dose-dependent manner (P = 0·0007, R 2 = 0·386). Statistical treatment by Jonckheere-Terpstra analysis, which is well known for showing drug effects in a dose-dependent manner, indicated a significant tendency of HbA1c to decrease with an increase in miglitol concentration (P = 0·0007).
Oral glucose tolerance test
In the oral glucose tolerance test, blood glucose concentrations after glucose-loading were markedly elevated in GK rats, including control GK rats. In miglitol-treated GK rats, the reduction in the blood glucose concentration tended to occur earlier than that in control GK rats (Fig. 3(A)). The ΔAUC0–2 h of plasma glucose in miglitol-treated GK rats was not significantly different from that of control GK rats (Fig. 3(C)). In GK rats treated with 0·1 mg voglibose/100 g control diet, the plasma glucose concentration and ΔAUC0–2 h value of blood glucose were similar to those of control GK rats (Fig. 3(B), (C)).

Fig. 3 Effects of miglitol (A) and voglibose (B) on plasma glucose concentration in glucose-loaded Goto-Kakizaki (GK) rats. Mean values and their standard errors (n 5–7). (C) Incremental blood glucose concentration integrated over a period of 2 h (ΔAUC0–2 h) in glucose-loaded GK rats treated with miglitol or voglibose. Mean values and their standard errors (n 5–7).
Islet structure and morphometry
The weight of the pancreas in the control GK rats was greater than that of control Wistar rats by 42 %. With the exception of GK rats treated with 0·2 mg voglibose/100 g control diet, pancreas weight in GK rats treated with miglitol or voglibose was similar to that of control GK rats (Table 5).
Table 5 Pancreas weight and islet morphometry of miglitol- and voglibose-treated Goto-Kakizaki (GK) rats (Mean values with their standard errors)
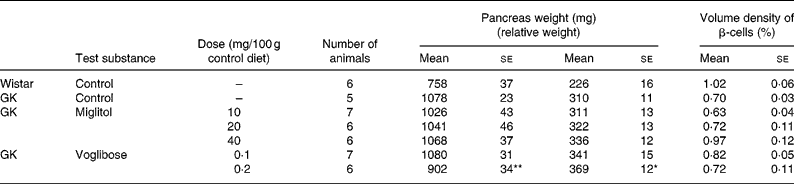
Mean values were significantly different from those of control GK rats (Dunnett's test): *P < 0·05, **P < 0·01.
Although the differences in β-cell volume density between control GK rats and miglitol-treated GK rats or voglibose-treated GK rats were not statistically significant, the reduction in β-cell volume density was slightly inhibited in GK rats treated with 40 mg miglitol/100 g control diet compared with control GK rats (Tabel 5). The volume density of β-cells in control GK rats tended to be reduced by 69 % compared with that in control Wistar rats, but this was not significant (P = 0·0556). On the other hand, the β-cell volume density of rats treated with 40 mg miglitol/100 g control diet was almost equal to that of control Wistar rats (P = 0·9949).
The islets of 15-week-old control GK rats showed irregular contours, fibrosis and loss of β-cells (Fig. 4), while those of age-matched control Wistar rats had a normal appearance. The islets of GK rats treated with the 40 mg miglitol/100 g control diet for 8 weeks showed good islet shape, and those of GK rats treated with 0·1 mg voglibose/100 g control diet were similar to those of control GK rats.

Fig. 4 Islet structures and distribution of immunoreactive insulin-positive cells (β-cells) in miglitol- or voglibose-treated Goto-Kakizaki (GK) rats. Section from a control Wistar rat showing a normal structure: the islet is round in shape, with a clearly defined contour, and the β-cells (insulin-positive cells) are stained brown. Control GK rat and GK rat treated with 0·1 mg voglibose/100 g control diet, showing an irregular islet shape, a rough distribution of the β-cells and strands of fibrous tissue traversing the islet. GK rat treated with 40 mg miglitol/100 g control diet, showing good preservation of the islet. Scale bar = 100 μm. (A) Haematoxylin and eosin staining; (B) azan staining; (C) immunostaining for insulin.
Discussion
In the present study, we planed two separate experiments to explore the effects of acute and chronic oral administration of miglitol on GK rats, an animal model of non-obese, type 2 diabetes.
First, we examined the concentrations of blood glucose and plasma insulin after an oral administration of sucrose with or without miglitol in GK rats. At a dose of 10 mg miglitol/kg body weight, the ΔAUC0–2 h of blood glucose after sucrose-loading decreased by 45 % (P < 0·05; see Table 2). Our results clearly showed that blood glucose concentrations after sucrose-loading were decreased by a single dose of miglitol. For voglibose at a dose of 0·125 mg/kg body weight, the ΔAUC0–2 h of blood glucose after sucrose-loading decreased by 25 % compared with control GK rats, but the difference was not significant. Odaka et al. reported that blood glucose concentrations in 0·1 mg/kg voglibose-treated GK rats decreased by more than 50 % compared with control GK ratsReference Odaka, Sano, Amano and Ikeda31. Such discordant results may be attributable to differences between the colonies or ages of GK rats used for these studies because the glucose tolerances of the experimental animals, that is, fasting blood glucose or peak value of increasing blood glucose after sucrose-loading, were different between the present experiment and theirs. In GK rats, the reduction in β-cells becomes more pronounced and the frequency of diabetic syndrome, which is characterised by hyperglycaemia and impaired glucose tolerance, tends to rise with increasing ageReference Koyama, Wada, Sakuraba, Mizukami and Yagihashi30. Therefore, the age of the animals used in an experiment would appear to be very important.
Next, we investigated the effects on glycaemic status when GK rats were fed a diet containing miglitol for 8 weeks. During the 8-week experimental period, the ΔHbA1c in miglitol-treated GK rats decreased in a dose-dependent manner and was significantly decreased in rats treated with 40 mg miglitol/100 g control diet compared with control GK rats, although ΔHbA1c in GK rats treated with 0·1 mg voglibose/100 g control diet was similar to that of control GK rats (see Fig. 2). This result indicates that chronic blood glucose concentrations in GK rats were controlled by miglitol.
It should be noted that ΔHbA1c was markedly reduced by treatment with the 0·2 mg voglibose/100 g control diet. Treatment with the latter diet induced diarrhoea and reduced food intake in GK rats (see Table 4). The high-sucrose and high-fat diet used in the present study may have accelerated the induction of gastrointestinal symptoms such as diarrhoea, which appeared only in rats treated with 0·2 mg voglibose/100 g control diet. Thus, carbohydrate malabsorption occurs following a slight increase in the dose of a non-absorbable α-glucosidase inhibitor. Undigested carbohydrates enter the lower region of the small intestine, resulting in gastrointestinal disorders such as soft stools or diarrhoea. This suggests that the dosage range of voglibose for which gastrointestinal symptoms do not develop but for which the pharmacological action is seen is very narrow (0·1–0·2 mg voglibose/100 g control diet). Miglitol did not produce diarrhoea even though it was administered at up to 100 mg/100 g diet in a toxicity study in normal Wistar rats for 12 monthsReference Bomhard, Kuhlmann and Puls32. Due to this attribute, miglitol exhibits a more potent inhibitory action on postprandial hyperglycaemia than other α-glucosidase inhibitors, and without any adverse effects.
Finally, we examined the effects of miglitol on histological changes in the islets of GK rats. Histological changes in the islets of GK rats that have been reported include progressive fibrosis and the disappearance of β-cells with increasing ageReference Goto, Suzuki, Ono, Sasaki and Toyota27, Reference Guenifi, Abdel-Halim, Hoog, Falkmer and Ostenson33. The mechanism for the progressive loss of β-cells in GK rats is apoptotic cell deathReference Ihara, Toyokuni, Uchida, Odaka, Tanaka, Ikeda, Hiai, Seino and Yamada22, Reference Koyama, Wada, Sakuraba, Mizukami and Yagihashi30, Reference Miralles and Portha34. Furthermore, recent reports in which clinical information was better characterised have concluded that β-cell mass decreased in type 2 diabetesReference Clark, Wells, Buley, Cruickshank, Vanhegan, Matthews, Cooper, Holman and Turner29, Reference Miralles and Portha34, Reference Butler, Janson, Soeller and Butler35. In the present study, histological examination of the pancreas revealed that miglitol tended to inhibit the loss of β-cells as well as lessen irregular contouring and fibrosis of the islets in GK rats (see Fig. 4). Thus, the controlled blood glucose concentrations may be attributable to preservation of the islet β-cell mass.
The STOP-NIDDM trial has shown that decreasing postprandial blood glucose concentrations, as a consequence of acarbose treatment, could reduce the risk of diabetes in patients with impaired glucose toleranceReference Chiasson, Josse, Gomis, Hanefeld, Karasik and Laakso36. A reduced demand for endogenous insulin by α-glycosidase inhibitors, including miglitol, lessens the burden on a pancreas with decreased secretory capacity, which will potentially enable the preservation of pancreatic function over a long period in patients with type 2 diabetes.
In conclusion, miglitol ameliorates the hyperglycaemic state and impaired function of the pancreatic islets, and tends to inhibit the development of histological changes in the islets of GK rats.
Acknowledgements
This work was supported by COE Program in the 21st Century from the Ministry of Education, Science, Sports and Culture of Japan.











