During an eccentric muscle action – such as while lowering a dumbbell – the muscle is being forcibly lengthened while generating force( Reference Allen 1 ). It is well established that exercises that involve unaccustomed and/or repeated eccentric actions may result in muscle damage and immediate decline in muscle strength( Reference Allen 1 – Reference McLeay, Barnes and Mundel 4 ). The complete restoration of muscle function can take from a few to several days after exercise, depending on the extent of the damage( Reference Paulsen, Mikkelsen and Raastad 2 – Reference McLeay, Barnes and Mundel 4 ). It has been assumed that the delayed recovery of muscle strength may be in part associated with further damages elicited by reactive molecules released within the injured muscle( Reference Zerba, Komorowski and Faulkner 5 – Reference Pizza, Peterson and Baas 8 ).
There is growing evidence that supplementation with foods rich in phytochemicals that have antioxidant and/or anti-inflammatory properties may improve the recovery from eccentric exercise-induced muscle damage (EEIMD)( Reference Trombold, Barnes and Critchley 9 – Reference Connolly, McHugh and Padilla-Zakour 12 ). Having several choices of dietary strategies for strength recovery can be quite advantageous for sportspeople. In this sense, the consumption of mate tea (MT), a beverage made from an infusion of roasted leaves of yerba mate (Ilex paraguariensis), might be an interesting option. Yerba mate is a plant originally from South America that represents a good source of phytochemicals with antioxidant and anti-inflammatory properties, such as phenolic compounds and saponins( Reference Bracesco, Sanchez and Contreras 13 ). The consumption of MT or green yerba mate infusion leads to an increase in antioxidant defence in plasma and in immune cells in humans( Reference Matsumoto, Bastos and Mendonça 14 – Reference Klein, Stefanuto and Boaventura 16 ). Acute or regular consumption of yerba mate-based beverages such as MT and green yerba mate infusion has shown improvements related to clinical disorders such as dyslipidaemia, diabetes and obesity( Reference Bracesco, Sanchez and Contreras 13 – Reference Klein, Stefanuto and Boaventura 16 ). On the other hand, information about the benefits of yerba mate regarding exercise is scarce. It was recently demonstrated that acute supplementation with ground yerba mate leaves improved fat oxidation during submaximal aerobic exercises in healthy subjects( Reference Alkhatib 17 ). In an animal study, treatment with yerba mate extract over a 4-week swimming training attenuated lipid peroxidation, as well as increased superoxide dismutase enzyme activity in muscles and the liver( Reference Morgan-Martins, Silva and Licks 18 ). However, the effects of MT intake on the recovery of muscle function after damaging exercise are still unknown.
Therefore, the primary purpose of this study was to investigate whether MT supplementation influences the recovery of muscle strength after a bout of eccentric exercise in physically active subjects. We also assessed the effects of MT on blood oxidative stress biomarkers following exercise.
Methods
Subjects
In all, twelve healthy and physically active male students (25·1 (sd 3·6) years, 75·1 (sd 9·8) kg, 175·8 (sd 5·8) cm) were recruited from the Department of Physical Education of a local University. The volunteers were selected according to the following criteria: non-athletes; not having participated in resistance training programme in the previous 3 months; not been involved in any structured endurance training programme; non-smokers; free of any disease, infection or inflammatory processes; not having a history of upper limb injury; and not using any medication, herbals, ergogenic aids or antioxidant supplements. All the experimental procedures were explained to the subjects before obtaining a signed written agreement. The study was approved by the Ethics Committee on Human Research of the Federal University of Santa Catarina. The trial was registered as RBR-5pj5bb.
Experimental design
This study was a randomised, controlled, cross-over trial. The experimental design is displayed in Fig. 1. The trial comprised two 11-d treatment periods (P1 and P2) with a 17-d washout period between each treatment (Fig. 1). Participants were randomly assigned to initiate P1, where they drank 200 ml of either MT or water (control, CON). The beverages were consumed three times per day (morning, afternoon and night). On the morning of the 8th day, subjects were instructed to drink one of the three daily doses of MT or CON 1 h before exercise tests. After this, all the subjects performed a damaging eccentric exercise protocol with one arm, which was chosen randomly. The maximal isometric force (i.e. isometric strength) of elbow flexor muscles was measured before (PRE) and at 0, 24, 48 and 72 h after exercise. Blood samples were obtained at PRE and at 24, 48 and 72 h after exercise, before the isometric strength test. During the second test period (P2), exercise tests were performed with the contralateral arm. All the participants were instructed to eat a standardised breakfast composed of skimmed milk, sugar, white bread, honey and bananas 2 h before the exercise tests. All the subjects maintained their usual physical activities, except in the 24 h before the eccentric exercise bout, during which they were asked to refrain from intense activities using the upper limbs.

Fig. 1 Experimental design. EE, eccentric exercise; ![]() , isometric strength test: before and at 0, 24, 48 and 72 h after EE;
, isometric strength test: before and at 0, 24, 48 and 72 h after EE; ![]() , blood sample: before and at 24, 48 and 72 h after EE.
, blood sample: before and at 24, 48 and 72 h after EE.
Mate tea preparation and intake
Participants received a completely sealed 50-g package of lyophilised instant MT (Leão Alimentos e Bebidas®). Instructions for preparing and drinking MT were given verbally and in writing. MT was prepared by dissolving 1 g (one teaspoon) of instant MT in 200 ml of cold water (5 mg/ml), according to the manufacturer’s instructions. No sugar, sweetener or fruits were allowed to be added to the beverage.
Determination of total phenolics of mate tea
The total phenol content of MT was measured according to the modified Folin–Ciocalteu method as described by Singleton et al. ( Reference Singleton, Orthofer and Lamuela-Raventos 19 ). In brief, 300 μl of MT was added to 1 ml of 95 % ethanol, 5 ml of distilled water and 0·5 ml of 50 % Folin–Ciocalteau reagent. After 5 min, 1 ml of 5 % sodium bicarbonate was added. The mixture was left at room temperature in the absence of light for 1 h. The absorbance of the coloured product was measured at 765 nm, and 3-caffeoylquinic acid was used as the standard. The average inter-assay CV for triplicate preparations was 7·3 %.
The phenolic acids of MT were determined by HPLC (Shimadzu LC-10) as described by Strassmann et al. ( Reference Strassmann, Vieira and Pedrotti 20 ). The instant MT was prepared and filtered through a 0·45-µm micropore membrane, and aliquots were injected into a C18 reverse-phase column (4·6×250 mm, 5-μm; Shim-pack) with a Shim-pack C18 guard column (4·0 mm×10 mm, 5 μm). An isocratic mobile phase consisting of n-butanol–acetic acid–water (97·0:0·28:2·72, v/v/v) was used at a flow rate of 0·8 ml/min. The assay was monitored at 325 nm. The concentrations of phenolic acids were quantified by 5-point calibration curves of standards. Gallic, caffeic, 3-caffeoylquinic and 4,5-dicaffeoylquinic acids were used as standards. The final concentration of phenolic compounds was determined by averaging the results of three consecutive injections.
Determination of total saponins in mate tea
The content of total saponins in MT was measured by a spectrophotometric method after acid hydrolysis of the MT saponins and extraction of sapogenins, as described by Gnoatto et al. ( Reference Gnoatto, Schenkel and Bassani 21 ), and reaction with vanillin and perchloric acid, according to Fan & He( Reference Fan and He 22 ). Ursolic acid, the major triterpenic nucleus of saponins present in I. paraguariensis ( Reference Gnoatto, Schenkel and Bassani 21 ), was used as a standard, and the results are expressed as milligram equivalents of ursolic acid per millilitre. The inter-assay CV of total saponins of the three MT preparations was 8·1 %.
Eccentric exercise
The eccentric exercise protocol was performed on an isokinetic dynamometer (Biodex System-4Pro®; Biodex Medical Inc.). Subjects performed three sets of twenty repetitions of unilateral, maximal, isokinetic eccentric actions of the elbow flexor muscles at an angular velocity of 45°/s, with 2-min rest between sets. The range of motion was from 50 to 170° of full elbow flexion. Subjects were seated with their elbow on a Scott Bench (Troya), and the axis of rotation was aligned with the lateral epicondyle of the humerus. Each repetition lasted 3 s. During the exercise, participants were verbally encouraged to put in maximal effort. Total work was recorded for each set.
Isometric strength test
To evaluate the maximal force generation of the elbow flexors, participants performed three maximum voluntary isometric muscle actions at 90°, each lasting 3 s, with 2 min of rest between sets. Strength was reported as the highest peak torque recorded at each trial. Subjects were verbally encouraged throughout the test. Before the tests, subjects warmed up by actively flexing and extending the elbow joint (10 repetitions) at 120°/s. All the volunteers participated in familiarisation tests on the isokinetic dynamometer before the beginning of the study. Isometric strength was expressed as a percentage of pre-exercise levels. The rate of isometric strength recovery was calculated by subtracting the value of the strength at 24, 48 or 72 h after exercise from the value of the strength immediately after eccentric exercise (0 h).
Blood sampling
Blood samples were collected between 07.00 and 08.00 hours, before any exercise test and after 15 min of rest in a sitting position. The exercise test protocols started at 08.00 hours. Samples were obtained from the contralateral arm that was being tested. The median antecubital vein was punctured using a hypodermic needle (25×7 mm) and blood was collected into Vacutainer® tubes containing either EDTA, heparin or without any anticoagulants. Plasma and serum samples were obtained by centrifugation (1000 g for 10 min, at 4°C). For the analysis of glutathione status, EDTA whole blood (1 ml) was transferred to an Eppendorf tube containing 100 µl of 310 mm-N-ethylmaleimide (NEM310; Sigma-Aldrich), vortex-mixed and frozen at −80°C for later analysis.
Dietary intake
Participants maintained their usual dietary pattern throughout the study, except for the restriction (more than three times a week) of beverages with known antioxidant properties, such as black tea, green tea, wine and fruit juices; 3-d food records (1 weekend day and 2 non-consecutive days during the week) were collected during each of the two experimental periods of the study (i.e. P1 and P2). Nutrient intakes were analysed for energy, macronutrients, vitamins (A, C and E) and minerals (Zn, Cu, Mn and Se) intakes (Avanutri 4.0).
Total phenolics in plasma
Total phenolic compounds in plasma were measured by the Folin–Ciocalteau colorimetric method, according to the methodology described by Serafini et al. ( Reference Serafini, Maiani and Ferro-Luzzi 23 ). In brief, 500 μl of duplicate plasma samples were acidified and, after extraction of complexed phenols with alcoholic sodium hydroxide, proteins were precipitated using 0·75 m-metaphosphoric acid and re-extracted with a mixture of acetone–water (1:1). Next, aliquots (50 μl) of the samples were added to 0·5 ml of Folin–Ciocalteau reagent (50 %). After 5 min, 1 ml of 5 % sodium bicarbonate was added. The mixture was left at room temperature in the dark for 1 h. The absorbance of the coloured product was measured at 765 nm, and chlorogenic acid was used as a standard. The average inter-assay CV for duplicate preparations was 6·8 %.
Glutathione status
GSH was determined in the whole blood by HPLC, according to the procedures described by Giustarini et al. ( Reference Giustarini, Dalle-Donne and Milzani 24 ). Aliquots of the EDTA whole blood treated with NEM310 (see the Blood sampling section) were de-proteinised by the addition of 15 % TCA and then centrifuged at 14 000 g at room temperature for 2 min. The supernatant was analysed by HPLC using a C18 column (TSK-gel ODS-80Ts; Tosoh, 4·6 mm×150 mm×5 μm). The mobile phase was composed of 0·25 % acetic acid and acetonitrile (94:6, v/v) at a flow rate of 1·25 ml/min. Signals were recorded at 265 nm with 400 nm as the reference. GSH content was quantified through calibration GSH curve standards of 5 points and linear regression analysis.
GSSG was determined in the whole blood by spectrophotometry, according to Giustarini et al. ( Reference Giustarini, Dalle-Donne and Milzani 24 ). Aliquots of the EDTA whole blood treated with NEM310 were de-proteinised and centrifuged as described above. Next, the supernatant was extracted with three volumes of dichloromethane. The mixture was vortexed for 5 min and centrifuged at 14 000 g at room temperature for 30 s. The supernatant (20 µl), 925 µl of 200 mm-PBS, 5 µl of 20 mm 5,5′-dithiobis-2-nitrobenzoic acid, 20 µl of 7·5 % TCA with 75·0 mg/ml EDTA and 20 µl of 4·8 mm NADPH were transferred to an assay tube and the absorbance was recorded (Spectrophotometer UV-1800; Shimadzu) at 412 nm for 1 min (blank). Subsequently, 20 µl of 20 IU/ml glutathione reductase was added to the mixture, and the enzyme kinetic reaction was monitored at 412 nm for 1 min. Next, 10 µm-GSSG was added to the tube, and the absorbance was recorded at 412 nm for 1 additional minute. GSSG concentration was calculated by the formula (GSSGb=S×(GSSGc)/St×49·5×2), where S=Δsample−Δblank; St=(Δsample+ΔGSSG)−Δsample; 49·5=dilution factor of sample in the cuvette; and 2=dilution factor due to sample acidification. The results of both GSH and GSSG were normalised per gram of Hb, which was measured by colorimetric end point assay. In brief, an aliquot of whole blood was mixed with Drabkin reagent, and the absorbance was read at 540 nm after 5 min at room temperature.
Lipid hydroperoxides
Lipid hydroperoxides (LOOH) were determined in heparin-plasma using ferrous oxidation-xylenol orange (FOX2), as described by Nourooz-Zadeh et al. ( Reference Nourooz-Zadeh, Tajaddini-Sarmadi and Wolff 25 ). The method is based on the fast oxidation of Fe+2 to Fe+3 in acid medium mediated by lipid peroxides. In the presence of xylenol orange, Fe+3 forms a complex (Fe+3–xylenol orange), which is measured spectrophotometrically at 560 nm. FOX2 reagent, containing 250 mm-H2SO4, 4·4 mm-butylated hydroxytoluene, 1 mm-xylenol orange, and 2·5 mm-iron ammonium sulphate in methanol, was added to aliquots of plasma, in duplicate. In addition,10 µl of 10 mm-triphenylphosphine in methanol was added to two additional microtubes with plasma to reduce LOOH before the addition of FOX2 reagent, thereby generating a blank sample. Subsequently, the mixtures were kept at room temperature for 30 min, the tubes were centrifuged (1000 g , 5 min) and the absorbance was measured (Shimadzu). A standard hydrogen peroxide curve was used to quantify LOOH and the results are expressed in μmol/l equivalent of hydrogen peroxide. The inter-assay CV calculated by the measurement of hydrogen peroxide on different days was 10·2 %. The average CV percentage for duplicate variation was 6·7 %.
Sample size and statistical analysis
To our knowledge, this was the first investigation on the effects of MT supplementation on the recovery of muscle strength after EEIMD. Thus, there is no information available about the sample size necessary to detect significant differences (P<0·05; power of 80 %) between the MT and CON trials for muscle strength (the primary outcome). Previous, similar, cross-over studies have reported improvement in strength recovery in samples of ten to sixteen subjects( Reference Pizza, Peterson and Baas 8 , Reference Trombold, Barnes and Critchley 9 , Reference Connolly, McHugh and Padilla-Zakour 12 ). Therefore, we decided to conduct this cross-over study with a sample size of twelve subjects (assuming 20 % loss). The Shapiro–Wilk test was applied to determine the normality of the data. When necessary, logarithmic transformation of data was used. Data that passed the normality check were analysed using a two-way repeated-measures ANOVA. Tukey’s post hoc test was used for time, treatment and treatment×time analysis data. The Student’s t test was conducted to assess interactions between period and treatment (order effect). For data that did not pass the normality test, Friedman’s test with Tukey’s post hoc test was used for time analysis, and Wilcoxon’s signed-rank test was performed to compare treatments at specific time points. Cohen’s d statistics( Reference Cohen 26 ), using the means and standard deviations( Reference Dunlap, Cortina and Vaslow 27 ), were applied to assess treatment effect size for data that passed the normality test. Effect sizes were assumed as trivial (<0·20), small (between 0·20 and 0·49), medium (between 0·50 and 0·79) or large (>0·80)( Reference Cohen 26 ). For data that did not pass the normality test, effect sizes were examined using Cliff’s δ statistics. The amount of effect sizes was interpreted as trivial (<0·147), small (between 0·147 and 0·33), medium (between 0·33 and 0·474) or strong (>0·474)( Reference Romano, Kromrey and Coraggio 28 ). Retrospective power (1–β) calculation( Reference Faul, Erdfelder, Lang and Buchner 29 ) of effect size for the primary outcome with n 12 was carried out using G*Power® (http://www.gpower.hhu.de/en.html), and the result indicated a power of 0·974. All comparisons were analysed using SigmaPlot 12.0 (Systat Software Inc.). Statistical significance was set at P≤0·05. Data are given as mean values and standard deviations or medians and interquartile ranges (25–75 %). Cohen’s d effect size was calculated using an effect size calculator (http://www.cem.org/effect-size-calculator). Cliff’s δ effect size was computed using Cliff’s Delta Calculator software( Reference MacBeth, Razumiejczyk and Ldesma 30 ).
Results
All twelve participants who were recruited completed both intervention arms and were included in the data analysis. The assessment of the participants’ 3-d dietary records showed that there were no significant differences between the MT and CON trials for energy and the analysed nutrients intakes (Table 1).
Table 1 Dietary intakeFootnote * in the mate tea (MT) and control trials (Mean values and standard deviations)

There was no statistical difference (P>0·05) for any of the variables.
* Averages from 3-d food records.
† As retinol activity equivalents.
Quantitative analysis of mate tea
Total phenols, phenolic compounds and saponin contents in MT are given in Table 2. Considering that the participants had a daily intake of 3 g lyophilised instant mate, the mean intakes of MT phenolic compounds and saponins were 890 and 142 mg/d, respectively. Chlorogenic acids were the major phenolic compounds found in MT followed by gallic acid and caffeic acid.
Table 2 Total phenol, phenolic compounds and saponin contents present in mate tea (MT) (Mean values and standard deviations)
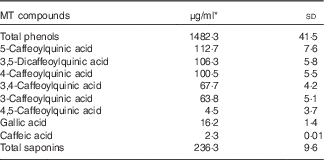
* Mean of triplicate measurements.
Muscle strength
There was no significant difference in the eccentric work performed between treatments (MT, 2454 (sd 149) J v. CON 2498 (sd 495) J; d=−0·12). Immediately after eccentric exercise (0 h), isometric strength was significantly below pre-exercise levels (P<0·001), with no difference between MT and CON (MT 62·3 (sd 10·4) % v. CON 63·2 (sd 11·8) % of PRE; d=−0·08) (Fig. 2). Strength remained significantly reduced from 24 to 72 h after exercise, regardless of the treatment used. Nevertheless, there was a medium effect size (d=0·53) for isometric strength at 24 h after exercise. In addition, from 0 to 24 h after exercise, the pattern of the recovery of strength was distinct between trials. At 24 h, strength was significantly greater than that at 0 h only in the MT trial (Fig. 2; P=0·008). There were no order effects for either isometric strength values (data no shown).

Fig. 2 Isometric elbow flexion strength (expressed as a percentage of pre-exercise levels) in the mate tea (MT, ![]() ) and control (CON,
) and control (CON, ![]() ) trials. Treatments were consumed at a dosage of 200 ml, three times a day for 11 d. Values are means and standard deviations. Two-way repeated-measures ANOVA and Tukey’s post hoc test. Significant time effect: *** P<0·001. Significantly different from 0 h after exercise: ** P<0·01.
) trials. Treatments were consumed at a dosage of 200 ml, three times a day for 11 d. Values are means and standard deviations. Two-way repeated-measures ANOVA and Tukey’s post hoc test. Significant time effect: *** P<0·001. Significantly different from 0 h after exercise: ** P<0·01.
In order to stress the evidences above – that MT may improve the isometric strength at 24 h after eccentric exercise – we re-analysed the data, taking into account the rate of muscle strength recovery. On the basis of the results, the rate of strength recovery during the 0–24-h interval was higher in MT than in CON (15·3 (sd 9·2) % and 6·7 (sd 6·1) %, respectively; P=0·009) (Fig. 3), with a large effect size (d=1·11). The recovery rates at the 0–48-h and 0–72-h intervals were not different between treatments (P=0·277 and 0·250, respectively), with small effect sizes (d=0·31 and 0·41, respectively).

Fig. 3 Rate of strength recovery of isometric elbow flexion in the mate tea (MT, ![]() ) and control (CON,
) and control (CON, ![]() ) trials. Treatments were consumed at a dosage of 200 ml, three times a day for 11 d. Values are means and standard deviations in the 0–24, 0–48 and 0–72 h periods. Two-way repeated-measures ANOVA and Tukey’s post hoc test. The mean value was significantly different from that of the control trial: ** P<0·01.
) trials. Treatments were consumed at a dosage of 200 ml, three times a day for 11 d. Values are means and standard deviations in the 0–24, 0–48 and 0–72 h periods. Two-way repeated-measures ANOVA and Tukey’s post hoc test. The mean value was significantly different from that of the control trial: ** P<0·01.
Biochemical variables
A significant time (P=0·011) and treatment (P=0·008) effect occurred for the plasma concentration of total phenolic compounds (Fig. 4). In both treatments, total phenolics were unaffected from PRE to 48 h after exercise, and then decreased significantly from 48 to 72 h after exercise (MT, P=0·040 and CON, P=0·016). However, the levels of total phenolics were higher in MT than in CON at all time points (PRE: d=1·12, P=0·005; 24 h: d=0·59, P=0·014; 48 h: d=0·55, P=0·044; and 72 h: d=0·80, P=0·021).

Fig. 4 Plasma concentration of total phenolics in the mate tea (MT, ![]() ) and control (CON,
) and control (CON, ![]() ) trials. Treatments were consumed at a dosage of 200 ml, three times a day for 11 d. Values are means and standard deviations. Two-way repeated-measures ANOVA and Tukey’s post hoc test. Significant time (P<0·05) and treatment (P<0·01) effects. The mean value was significantly different from that of the control trial: * P<0·05, ** P<0·01.
) trials. Treatments were consumed at a dosage of 200 ml, three times a day for 11 d. Values are means and standard deviations. Two-way repeated-measures ANOVA and Tukey’s post hoc test. Significant time (P<0·05) and treatment (P<0·01) effects. The mean value was significantly different from that of the control trial: * P<0·05, ** P<0·01.
The blood concentration of GSH did not change over time in the MT trial (Fig. 5). However, there was a significant treatment effect (P=0·002) for GSH. The levels of GSH were higher in MT than in CON at PRE (d=1·02, P=0·032) and at 48 h (d=0·86, P=0·012) and 72 h (d=1·08, P=0·002) after exercise. In the CON trial, GSH had a non-significant rise from PRE to 24 h after exercise and then decreased significantly at 48 h (P=0·040) and 72 h (P=0·009) after exercise.
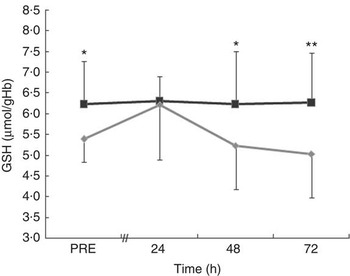
Fig. 5 Blood concentration of GSH in the mate tea (MT, ![]() ) and control (CON,
) and control (CON, ![]() ) trials. Treatments were consumed at a dosage of 200 ml, three times a day for 11 d. Values are means and standard deviations. Two-way repeated-measures ANOVA and Tukey’s post hoc test. Significant treatment (P<0·01) effect. The mean value was significantly different from that of the control trial: * P<0·05, ** P<0·01.
) trials. Treatments were consumed at a dosage of 200 ml, three times a day for 11 d. Values are means and standard deviations. Two-way repeated-measures ANOVA and Tukey’s post hoc test. Significant treatment (P<0·01) effect. The mean value was significantly different from that of the control trial: * P<0·05, ** P<0·01.
There were no significant changes over time or differences between conditions in either GSSG levels or GSH:GSSG ratio in the blood (Table 3). However, there was a medium effect size for GSSG at 72 h after exercise (δ=−0·37). The effect size for GSSG was small at PRE (δ=−0·25) and at 24 h after exercise (δ=−0·23) and negligible at 48 h (δ=0·13) after exercise. In addition, effect size was medium for GSH:GSSG ratio at PRE (δ=0·38) and 72 h (δ=0·42) but was small at 24 h (δ=0·28) and trivial at 48 h (δ=0·13) after exercise.
Table 3 Oxidative stress markers’ concentrations in blood at pre-exercise (PRE) and after eccentricFootnote * (Median values and interquartile ranges (IQR))
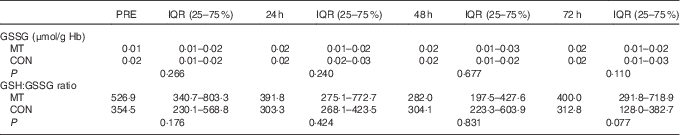
MT, mate tea; CON, control.
* Friedman’s and Wilcoxon’s signed-rank tests.
Plasma LOOH levels did not change over recovery time in either of the treatment trials (Fig. 6). LOOH levels were not significantly different between treatments at all time points after exercise. The effect sizes for LOOH were small at PRE (d=−0·20) and at 24 h (d=0·21) and 48 h (d=−0·45) after exercise and trivial (d=0·14) at 72 h after exercise.
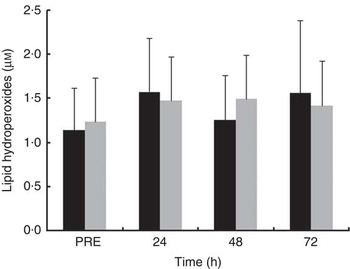
Fig. 6 Plasma concentration of lipid hydroperoxides in the mate tea (MT, ■) and control (CON, ![]() ) trials. Treatments were consumed at a dosage of 200 ml, three times a day for 11 d. Values are means and standard deviations. Two-way repeated-measures ANOVA and Tukey’s post hoc test.
) trials. Treatments were consumed at a dosage of 200 ml, three times a day for 11 d. Values are means and standard deviations. Two-way repeated-measures ANOVA and Tukey’s post hoc test.
Discussion
This study investigated the effect of MT intake, a beverage rich in antioxidant phytochemicals, on muscle strength recovery and oxidative stress markers after eccentric exercise in healthy subjects. Our primary finding was that the consumption of 600 ml of MT (three 200 ml drinks/d) providing 890 mg of phenolic compounds/d improved the rate of recovery of isometric strength by 8·6 % during the 1st day after eccentric exercise. This positive effect of MT on muscle recovery was large and significant (d=1·11; P=0·007) and is in agreement with reports that dietary phytochemical supplementation ameliorated muscle function after EEIMD( Reference McLeay, Barnes and Mundel 4 , Reference Trombold, Barnes and Critchley 9 – Reference Connolly, McHugh and Padilla-Zakour 12 ). In addition, although the measures of recovery were not significantly different between treatments (Fig. 2), MT had a medium effect (d=0·53) on isometric strength at 24 h after exercise. This means that the influence of MT on muscle strength at 24 h after exercise was ‘likely to be perceived’ according to Cohen’s statistics( Reference Cohen 26 ). The intake of MT also favoured the circulating levels of total phenols and GSH before and after eccentric exercise.
The mechanisms whereby MT influenced the recovery from EEIMD were not directly examined in the present study. However, antioxidant and/or anti-inflammatory properties of yerba mate might be involved. For example, MT intake has improved gene expression and activity of antioxidant enzymes in leucocytes( Reference Matsumoto, Bastos and Mendonça 14 , Reference Fernandes, Machado and Becker 15 ). MT has also reduced cytokine expression in macrophages( Reference Borges, Vinolo and Nakajima 31 ) as well as inhibited neutrophil and macrophage infiltration into injured tissue( Reference Lanzetti, Bezerra and Romana-Souza 32 ). After EEIDM, phagocytes progressively accumulate in the muscle and initiate inflammatory events that precede tissue repair, including releasing reactive species that can help degrade cellular debris( Reference Paulsen, Mikkelsen and Raastad 2 , Reference MacIntyre, Reid and Lyster 33 – Reference Brickson, Hollander and Corr 36 ). It has been assumed that reactive species released by phagocytes could occasionally act on intact structures, leading to additional damage and delayed recovery of muscle strength( Reference Faulkner, Brooks and Opiteck 3 , Reference Zerba, Komorowski and Faulkner 5 – Reference Pizza, Peterson and Baas 8 , Reference MacIntyre, Reid and Lyster 33 ). Neutrophils are the first phagocytes to invade the muscle, typically peaking in concentration between 6 and 24 h after damage and then quickly decreasing in numbers( Reference Pizza, Peterson and Baas 8 , Reference Fielding, Manfredi and Ding 34 – Reference Brickson, Hollander and Corr 36 ). Phagocytic macrophage concentrations are elevated at 24 h and peak about 48 h after injury( Reference Lapointe, Frenette and Côté 6 , Reference Tidball and Villalta 35 , Reference Brickson, Hollander and Corr 36 ). In this study, the pattern of strength recovery during the first 24 h after exercise was improved in MT compared with CON (Fig. 2 and 3). Thus, one might speculate that the effect of MT on strength recovery was in part associated with the modulation of neutrophil activities in the muscles throughout the 24 h after EEIMD. The minor effectiveness of MT on recovery beyond 24 h after exercise would be in line with the decline in neutrophil numbers within the muscle after peaking in concentration( Reference Tidball and Villalta 35 ). However, significant amounts of neutrophils in damaged muscle have been found 5 d after eccentric exercise( Reference Fielding, Manfredi and Ding 34 ). Thus, another explanation might be that, following the 1st day after exercise, the improvements on recovery promoted by MT would have been overwhelmed by an increment in reactive species generation from other sources (e.g. activated macrophages)( Reference Tidball and Villalta 35 , Reference Brickson, Hollander and Corr 36 ) besides neutrophils.
The attenuation in MT effects on strength recovery beyond 24 h after exercise (Fig. 2) disagrees with the reports that supplementation with polyphenols-rich dietary sources improved muscle strength from 24 to 96 h after eccentric exercise( Reference McLeay, Barnes and Mundel 4 , Reference Trombold, Barnes and Critchley 9 – Reference Connolly, McHugh and Padilla-Zakour 12 ). However, this limited influence of MT on recovery might be due to the extent of muscle damage( Reference Paulsen, Mikkelsen and Raastad 2 ). After EEIMD, strength recovery can be halted for a few or several days depending on the injury severity( Reference Paulsen, Mikkelsen and Raastad 2 , Reference MacIntyre, Reid and Lyster 33 ). In the present study, strength deficits were over 20 % from 48 to 72 h after exercise in both treatments (Fig. 2). These results agree with the reports that supplementation with vegetable and anthocyanins-rich fruit powder concentrates for 4 weeks did not affect the losses of >30 % in isometric strength over the 24–72 h after eccentric exercise( Reference Goldfarb, Garten and Cho 37 ). Conversely, the consumption of cherry juice – rich in anthocyanins – for 8 d( Reference Connolly, McHugh and Padilla-Zakour 12 ) or pomegranate juice – rich in ellagitannins – for 9 d( Reference Trombold, Barnes and Critchley 9 ) improved isometric strength recovery through the 24–96-h period after eccentric exercise. In these studies( Reference Trombold, Barnes and Critchley 9 , Reference Connolly, McHugh and Padilla-Zakour 12 ), strength deficit at 72 h after exercise was about 16 % with placebo, thus suggesting a lesser extent of muscle damage( Reference Paulsen, Mikkelsen and Raastad 2 ) compared with those seen in the present study as well as in the study by Goldfarb et al.( Reference Goldfarb, Garten and Cho 37 ). It is noteworthy that the degree of EEIMD depends on several factors including the exercise protocol used( Reference Paulsen, Mikkelsen and Raastad 2 ), which could in part explain the discrepancies between the studies regarding the degree of damage, and thus treatment effectiveness.
Significant changes in blood GSH, GSSG and/or GSSG:GSH ratio over 4 d after eccentric exercise have been reported by several studies( Reference Paschalis, Nikolaidis and Fatouros 38 , Reference Nikolaidis, Paschalis and Giakas 39 ), whereas others have found just a transitory( Reference Goldfarb, Garten and Cho 37 ) or no change in blood glutathione status( Reference Lee, Goldfarb and Rescino 40 ). In the present study, glutathione status seems to have been influenced by both the treatment and the exercise (Fig. 5). MT prevented the decrease in blood GSH from 24 to 72 h after exercise. In addition, MT had a significant influence on GSH before and at 48 and 72 h after exercise, as indicated by the large effect sizes (d=1·02, 0·86 and 1·08, respectively). Conversely, neither GSSG nor GSH:GSSG ratio was affected in both trials (Table 3), which could be partially explained by the large variability (approximately 50–80 %) found in both blood markers at all time points. However, there was a medium effect size for GSSG levels at 72 h (δ=0·32) that was expressed as a reduction of 21 % in the median of GSSG with MT. In addition, the medium effect sizes for GSH:GSSG ratio before (δ=0·38) and at 72 h (δ=0·42) after exercise corresponded to 50 and 90 % increases, respectively, in the median of GSH:GSSG ratio in the MT trial. Overall, these results suggest that MT intake favoured blood glutathione status before and during the recovery period after EEIMD.
Among the phenolic compounds present in yerba mate infusions, there are many phenolic acids – mostly chlorogenic acids – and some flavonoids such as rutin, quercertin and kaempferol( Reference Bastos, Saldanha and Catharino 41 , Reference Filip, López and Giberti 42 ). These phytochemicals are highly effective in inhibiting the lipoperoxidation chain( Reference Rice-Evans, Miller and Paganga 43 ). The large (d=1·02) and significant effect of MT on the rest levels of plasma total phenolics after an 11-d treatment period (Fig. 4) agrees with the benefits provided by yerba mate in previous short-term studies( Reference Matsumoto, Bastos and Mendonça 14 , Reference Fernandes, Machado and Becker 15 ). In addition, total phenolics were significantly higher with MT at all time points after exercise, showing medium effects at 24 h (d=0·59) and 48 h (d=0·55) and a large effect (d=0·80) at 72 h after exercise. Interestingly, in both treatments, total phenolics decreased significantly from 48 to 72 h after exercise, suggesting that phenolic compounds were useful during the recovery from EEIMD. It is noteworthy that, in the CON trial, the decline in plasma total phenolics from 48 to 72 h (Fig. 4) paralleled the decrease in blood GSH over the same time period (Fig. 5). Altogether, these results may suggest that the decrease in blood GSH availability led to a greater reliance on dietary antioxidants, notably lipid radical scavengers( Reference Rice-Evans, Miller and Paganga 43 ). On the other hand, one could propose that MT phenolics had GSH-sparing actions, mainly after the 2nd day after exercise.
Plasma LOOH levels did not change in either treatment at all time points (Fig. 6). In the study of Goldfarb et al.( Reference Goldfarb, Garten and Cho 37 ), LOOH levels were unaffected over the 72 h after exercise, but plasma malondialdehyde (MDA) increased from 24 to 72 h after exercise in the placebo trial. Conversely, Childs et al. ( Reference Childs, Jacobs and Kaminski 44 ) found significant rise in LOOH but not in MDA in plasma from 48 to 96 h after eccentric exercise. These discrepancies concerning blood oxidative stress markers after eccentric exercise have been imputed to a number of factors such as exercise protocol, time point in blood sampling and the oxidative stress marker examined( Reference Paschalis, Nikolaidis and Fatouros 38 ).
The effects of MT on the biomarkers assessed in this study may have been limited by the small sample size. The lack of a placebo treatment may also have influenced the results, but unfortunately no suitable inert material compatible with the colour and flavour of the lyophilised MT was available. Nevertheless, in addition to have favoured blood antioxidant status, the bioactive properties of yerba mate may also have played some relevant role in the muscle after EEIMD, as suggested by the faster rate of strength recovery during the 1st day after eccentric exercise with mate intake (Fig. 1 and 2).
In conclusion, the consumption of MT increased blood antioxidant levels and improved the rate of strength recovery from 0 to 24 h after exercise. However, MT intake did not affect the strength recovery measures made from 24 to 72 h after eccentric exercise. Having a faster recovery rate of muscle function over the 1st day after eccentric exercise might be particularly important for subjects who need to perform subsequent exercises and/or occupational activities that will involve the previously damaged muscles. Thus, MT intake may be an interesting option for physically active subjects. Further studies should corroborate our findings in larger samples as well as investigate cellular mechanisms underlying the potential effects of MT on muscle recovery from EEIMD.
Acknowledgements
The authors thank Leao Junior Co. for providing the lyophilised instant MT.
The authors declare that Leão Alimentos e Bebidas partially supported this study by providing the lyophilised instant MT.
V. P. P.: conception and design of the study; collection, analysis and interpretation of the data; F. D.: design of the exercise protocol, supervision of the exercise data collection and critical revision of the manuscript; A. C. T.: design of the exercise protocol and collection of exercise data; C. d. Q. C.: biochemical analysis; H. S. B.: biochemical analysis; B. M. d. M.: collection of exercise data; R. L. S.: collection of exercise data; M. V. d. O.: biochemical analysis; E. d. O. P.: biochemical analysis; E. A. N.: supervision of the biochemical analysis and critical revision of the manuscript; E. L. d. S.: supervision of the biochemical analysis, interpretation of data, critical revision of the manuscript and approval of the final version of the manuscript.
None of the authors has any conflict of interest to declare.












