Haemodialysis (HD) is getting remarkably involved in the treatment of patients with end-stage kidney diseases worldwide( Reference Couser, Remuzzi and Mendis 1 ). Recently, the ratio of diabetic patients receiving HD has been exceedingly increased. Carotid intima–media thickness (CIMT) is a severe complication in diabetic patients under HD. This complication is often combined with protein–energy wasting, inflammation and subsequently cardiovascular events (CVE)( Reference Nambi, Pedroza and Kao 2 , Reference Minoguchi, Yokoe and Tazaki 3 ). Diabetes mellitus is associated with an excess inflammatory status and accounts for one of the major cause of end-stage renal disease (ESRD)( Reference Sabatino, Regolisti and Cosola 4 ). The risk of CVE is the largest among diabetic patients compared with non-diabetics with ESRD( Reference Whaley-Connell and Sowers 5 ). It has been reported that patients under HD are susceptible to many risk parameters for cardiac events including insulin resistance, enhanced reactive oxygen species (ROS), reduced antioxidant defence, infection and comorbid conditions such as hypertension, exacerbation of inflammatory cytokines, which in turn influence the health-related quality of life in diabetic patients under HD( Reference Sohrabi, Eftekhari and Eskandari 6 – Reference Yen, Lin and Lin-Tan 8 ).
Recent evidence has demonstrated that Mg supplementation significantly improved CIMT in patients undergoing HD( Reference Mortazavi, Moeinzadeh and Saadatnia 9 ). Inflammatory cytokines also take part in the pathophysiology of diabetic nephropathy( Reference Lim and Tesch 10 , Reference Wu, Sytwu and Lin 11 ). Clinical evidence has reported that enhanced inflammatory markers are common in diabetic HD patients and may result in the progressive atherosclerosis( Reference Almeida, Lourenço and Fonseca 12 ). It has been demonstrated that Mg levels in diabetes HD subjects were significantly lower than those of healthy controls( Reference Silva, Fragoso and Silva 13 ), and lower Mg status is associated with enhanced atherosclerosis of the common carotid artery( Reference Tzanakis, Virvidakis and Tsomi 14 ). Previously studies have shown the beneficial effects of Mg supplementation on metabolic profiles and insulin resistance in patients with type 2 diabetes mellitus (T2DM)( Reference Barbagallo and Dominguez 15 , Reference Barbagallo and Dominguez 16 ) and CIMT in HD subjects( Reference Mortazavi, Moeinzadeh and Saadatnia 9 ). In addition, Mg administration significantly improved glucose homoeostasis outcomes in diabetic subjects( Reference Paolisso, Scheen and D’Onofrio 17 ). A growing body of evidence derived from clinical trials shows that Mg administration improves insulin sensitivity and dyslipidaemia in diabetic and non-diabetic patients( Reference Rodriguez-Moran and Guerrero-Romero 18 – Reference Chacko, Sul and Song 20 ). Unlike, another study failed to find any improvement in glycaemic control and metabolic profiles in response to ingesting Mg supplements( Reference Navarrete-Cortes, Ble-Castillo and Guerrero-Romero 21 ). Mg element is an essential co-factor in the enzymatic process of high-energy phosphate, acts as a Ca ion channel antagonist and secretion of prostacyclin and nitric oxide (NO); therefore, Mg is involved in the metabolic pathways that regulate glucose and lipid profiles( Reference Lopez Martinez, Sanchez Castilla and Garcia de Lorenzo y Mateos 22 – Reference Sontia and Touyz 24 ).
To our knowledge, data from studies investigating the effects of Mg administration on CIMT, glycaemic control, lipid profiles, biomarkers of inflammation and oxidative stress in diabetic HD patients are limited. Therefore, this study was aimed to evaluate the effects of Mg supplementation on CIMT and metabolic profiles in diabetic HD subjects.
Methods
Trial design and participants
The present study, registered in the Iranian website for clinical trials (http://www.irct.ir, no. IRCT2017090133941N19), was a randomised, double-blind, placebo-controlled clinical trial which was done among fifty-four diabetic HD patients aged 18–80 years who were referred to the Yasrebi Clinic in Kashan, Iran, between December 2017 and June 2018. This intervention was conducted in accordance with the Declaration of Helsinki and informed consent was taken from all subjects. This investigation was approved by the ethics committee of Kashan University of Medical Sciences (KAUMS). Patients with inflammatory and malignant diseases, those taking Mg supplements, antioxidant and/or anti-inflammatory supplements within 3 months before enrolment in the study and subjects taking immunosuppressive agents were not included in the present study. Immunosuppressive agents might have different side effects such as dyslipidaemia, hyperglycaemia, liver and kidney injury among patients, so we excluded the subjects on immunosuppressive agents from the present study( Reference Velickovic-Radovanovic, Mikov and Catic-Djordjevic 25 ).
Study design
First, subjects were matched according to sex, BMI and age. Patients were requested to continue their routine physical activity and not to take any anti-inflammatory and antioxidant medications or supplements that might influence their nutritional status during the 24-week intervention. Consumption of Mg and placebos throughout the study was checked through assessing Mg levels by an enzymatic method. A 3-d food records and physical activity records were completed by all participants at weeks 0, 6, 12, 18 and 24 of the intervention. To obtain macro- and micro-nutrient intake of participants based on 3- d food diaries, Nutritionist IV software (First Databank) modified for Iranian foods was used.
Intervention
Patients were randomly divided into two groups to take either 250 mg/d Mg supplements as magnesium oxide (Twenty First Century Pharmaceutical Company) (n 27) or placebo (Barij Essence Pharmaceutical Company) (n 27) for 24 weeks.
Assessment of anthropometric measures
Body weight and height were quantified in an overnight fasting status using a digital scale (Seca) at baseline and after the 24-week intervention. BMI was calculated by weight and height measurements (weight (kg)/height (m2)).
Assessment of outcomes
CIMT was considered as primary outcome and glycaemic control, lipid profiles and biomarkers of inflammation and oxidative stress were considered as secondary outcomes. Measurement of the CIMT was conducted in patients at the 2- cm distance of the common carotid bifurcation, by the same sonographer, at baseline and after the 24-week intervention using a Doppler ultrasonography device (Samsung Madyson V20) with linear multi-frequencies of 7·5–10 MHz probe. The physician was blinded to any clinical information of the subjects.
A 10 ml fasting blood sample was collected at baseline and after the 24-week intervention at Kashan reference laboratory. Then the samples were stored at –80°C before analysis. Serum insulin and high-sensitivity C-reactive protein (hs-CRP) levels were quantified using an ELISA kit (DiaMetra and LDN) with inter- and intra-assay CV below 7 %. The homoeostasis model of assessment-insulin resistance (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI) were determined according to the standard formula( Reference Pisprasert, Ingram and Lopez-Davila 26 ). HbA1c values in whole blood were assessed by Glycomat kit (BiocodeHycel) using the method of exchange chromatography. Enzymatic kits (Pars Azmun) were used to quantify serum Mg, fasting plasma glucose (FPG), lipid profiles with inter- and intra-assay CV below 5 %. The plasma NO using Griess method( Reference Tatsch, Bochi and Pereira Rda 27 ), total antioxidant capacity (TAC) by the method of ferric reducing antioxidant power developed by Benzie & Strain( Reference Benzie and Strain 28 ), total glutathione (GSH) using the Beutler & Gelbart( Reference Beutler and Gelbart 29 ) method and malondialdehyde (MDA) concentrations by the thiobarbituric acid reactive substances spectrophotometric test( Reference Janero 30 ) were determined with inter- and intra-assay CV below 5 %.
The questions of subjective global assessment (SGA) questionnaire were also asked by the same person after 12 weeks of the intervention. Then the SGA classifications were converted to numerical equivalents( Reference Nursal, Noyan and Tarim 31 ).
Sample size
We used a randomised clinical trial sample size calculation formula where type I (α) and type II errors (β) were 0·05 and 0·20 (power=80 %), respectively. According to the previous trial( Reference Mortazavi, Moeinzadeh and Saadatnia 9 ), we used 0·13 mm as the sd and 0·105 as the change in mean (d) of CIMT as a primary outcome. Based on the formula, we needed twenty-five participants in each group; after allowing for five dropouts in each group, the final sample size was thirty persons in each group. We used the standard deviation (0·13 mm) of the CIMT from the Mortazavi et al. ( Reference Mortazavi, Moeinzadeh and Saadatnia 9 ) paper with study design similar to ours. For sample size calculation, the minimal clinically important effect size is also required which is determined by the researcher (it should not be derived from the literature)( Reference Mansournia and Altman 32 , Reference Nielsen, Bertelsen and Verhagen 33 ). In addition, we hypothesised that effect size of 0·105 mm of CIMT would result in a significant change in CIMT in diabetic HD patients. In a study by Mortazavi et al. ( Reference Mortazavi, Moeinzadeh and Saadatnia 9 ), Mg supplementation for 6 months to HD patients significantly reduced CIMT by 0·08 mm. Also the standardised effect size equals 0·0105/0·13=0·8 which is considered as a large effect size according to Cohen( Reference Mansournia and Altman 32 ). With sd=0·13 participants in each group, we have at least 80 % power (probability) of detecting a difference equal to or >0·105 (if it really exists) as statistically significant at the 5 % level.
Randomisation
Randomisation assignment was conducted using computer-generated random numbers. Randomisation and allocation were concealed from the researchers and patients until the final analyses were completed. The randomised allocation sequence, enrolling participants and allocating them to interventions were conducted by a trained nutritionist at the dialysis clinic.
Statistical methods
The Kolmogorov–Smirnov test was done to determine the normality of data. To detect the differences in anthropometric measures and dietary intakes between two groups, independent-samples t test was used. Multiple linear regression models were used to assess treatment effects on study outcomes after adjusting for confounding parameters including age and BMI. The effect sizes were presented as the mean differences with 95 % CI. P<0·05 were considered statistically significant. All statistical analyses were done using the Statistical Package for Social Science version 18 (SPSS Inc.).
Results
Three patients in each group withdraw from the trial, due to personal reasons, and finally fifty-four patients (Mg (n 27) and placebo (n 27)) completed the study (Fig. 1). The compliance rate was high; more than 90 % of capsules were taken during the course of the trial in both groups. No side effects were reported following the consumption of Mg supplements in diabetic HD patients throughout the study.
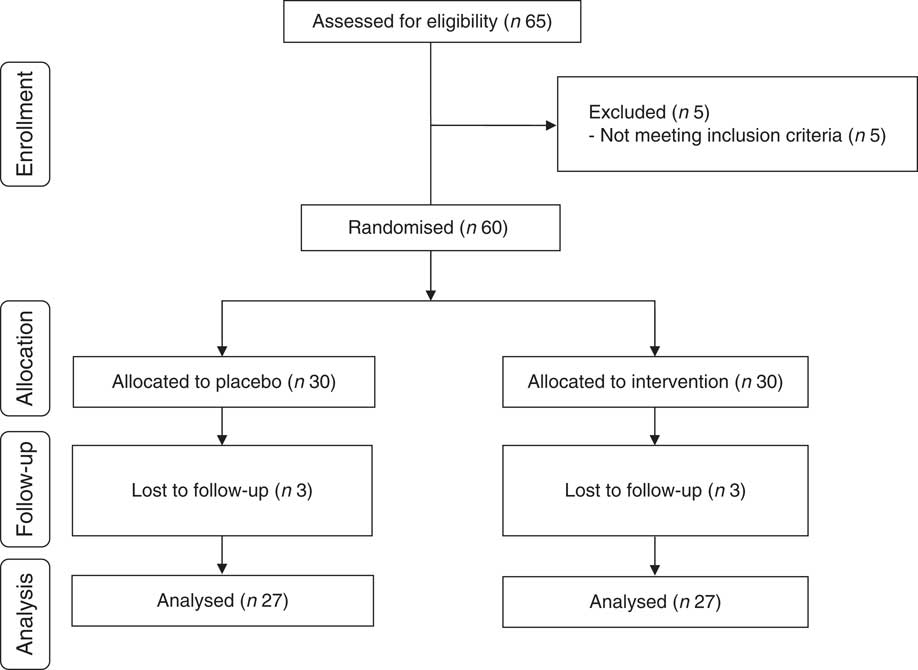
Fig. 1 Summary of patient flow diagram.
Distribution of sex, mean age, height, baseline weight and BMI as well as their means after intervention and years of dialysis of study participants were not statistically different between the two groups (Table 1).
Table 1 General characteristics of study participants (Mean values and standard deviations; numbers and percentages)

* Obtained from independent t test.
† Obtained from Pearson’s χ 2 test.
Based on the 3-d dietary records obtained throughout the treatment period, we found no significant change in dietary macro- and micro-nutrient intake (Table 2).
Table 2 Mean dietary intake of study participants at baseline, weeks 6, 12, 18 and 24 of the study (Mean values and standard deviations)
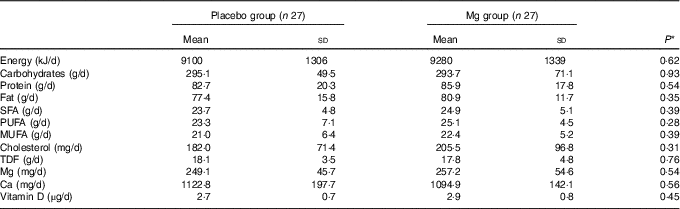
TDF, total dietary fibre.
* Obtained from independent t test.
After the 24-week intervention, Mg supplementation resulted in a significant reduction in mean (β=–0·04 mm; 95 % CI –0·06, –0·02; P<0·001) and maximum levels of left CIMT (β= –0·06 mm; 95 % CI –0·11, –0·009; P=0·02) and mean levels of right CIMT (β=–0·05 mm; 95 % CI –0·08, –0·01; P=0·004) compared with the placebo (Table 3). In addition, taking Mg significantly reduced serum insulin levels (β=–9·42 pmol/l; 95% CI –14·94, –3·90; P=0·001), HOMA-IR (β=–0·56; 95 % CI –0·89, –0·24; P=0·001) and HbA1c (β=–0·74 %; 95 % CI –1·10, –0·39; P<0·001) and significantly increased QUICKI (β=0·008; 95 % CI 0·002, 0·01; P=0·002) compared with placebo. In addition, Mg administration led to a significant reduction in serum total cholesterol (β=–0·30 mmol/l; 95% CI –0·56, –0·04; P=0·02), LDL-cholesterol (β=–0·29 mmol/l; 95% CI –0·52, −0·05; P=0·01), hs-CRP (β=–1·57 mg/l; 95 % CI –2·06, –1·08; P<0·001) and plasma MDA (β=–0·26 µmol/l; 95 % CI –0·53, –0·001; P=0·04), and a significant rise in plasma TAC levels (β=168·91 mmol/l; 95 % CI 113·92, 223·89; P<0·001) compared with the placebo. Mg supplementation did not change maximum levels of right CIMT and other metabolic parameters.
Table 3 Carotid intima–media thickness, metabolic profiles, biomarkers of inflammation and oxidative stress at study baseline and after the 24-week intervention in patients with diabetic haemodialysis that received either magnesium supplements or placebo (Mean values and standard deviations; β-coefficients and 95 % confidence intervals)
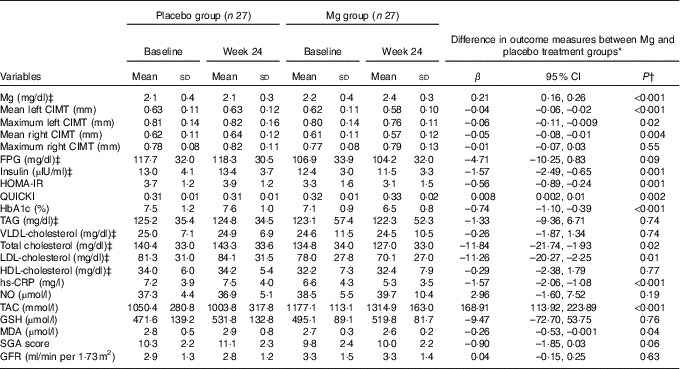
CIMT, carotid intima–media thickness; FPG, fasting plasma glucose; HOMA-IR, homoeostasis model of assessment-insulin resistance; QUICKI, quantitative insulin sensitivity check index; hs-CRP, high-sensitivity C-reactive protein; NO, nitric oxide; TAC, total antioxidant capacity; GSH, total glutathione; MDA, malondialdehyde; SGA, subjective global assessment; GFR, glomerular filtration rate.
* ‘Outcome measures’ refers to the change in values of measures of interest between baseline and week 24. β (difference in the mean outcome measures between treatment groups (Mg group=1 and placebo group=0)).
† Obtained from multiple regression model (adjusted for baseline values of each biochemical variables, age and baseline BMI).
‡ To convert Mg in mg/dl to mg/l, multiply by 10. To convert FPG in mg/dl to mmol/l, multiply by 0·0555. To convert insulin in μIU/ml to pmol/l, multiply by 6. To convert TAG in mg/dl to mmol/l, multiply by 0·0113. To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259.
Discussion
In this study, we investigated the effects of Mg supplementation on CIMT and metabolic profiles in diabetic HD subjects. We found that taking Mg for 24 weeks by diabetic HD patients significantly improved mean and maximum levels of left and mean levels of right CIMT, insulin, HOMA-IR, QUICKI, HbA1c, total cholesterol and LDL-cholesterol, hs-CRP, TAC and MDA levels; however, it did not have any effect on maximum levels of right CIMT and other metabolic profiles. Earlier, hypomagnesaemia was reported in the majority of subjects undergoing HD( Reference Silva, Fragoso and Silva 13 ). Based on this finding, Mg may be an appropriate adjunct therapy for diabetic HD subjects. To our best knowledge, this study for the first time evaluated the effects of Mg supplementation on CIMT and metabolic profiles in diabetic HD patients. According to the inclusion criteria, the age range of study participants was broad (18–80 years old); however, in reality at analysis phase we did not have any subjects younger than 35 years old and the number of participants in the age category of <55 was very low and the majority of study population were older than 55 years old. So age range of study participants was 35–80 years in both groups. Number of participants younger than 55 was 4 in placebo group and 6 in Mg group. All our patients were diabetic and under HD, which can affect the markers of cardio-metabolic risk. However, at the onset of the study, all participants were matched according to sex, BMI and age to decrease potential confounding effects, and we believe that this fact has been considered in our finding interpretation.
Effects on carotid intima–media thickness
Diabetic HD is associated with some complications, including increased CIMT and other atherosclerotic events( Reference Nambi, Pedroza and Kao 2 , Reference Minoguchi, Yokoe and Tazaki 3 ). The current study supported that Mg for 24 weeks by diabetic HD patients significantly reduced mean and maximum levels of left, and mean levels of right CIMT, but did not affect maximum levels of right CIMT. Previous studies have documented that there is a difference between the left and right CIMT. It was reported that haemodynamic factors including peak velocity, resistivity index and pulsatility index and age, sex, metabolic profiles especially lipid concentrations, blood glucose levels and other risk factors would have different effects on the left and right CIMT( Reference Luo, Yang and Cao 34 ). In a study conducted by Luo et al. ( Reference Luo, Yang and Cao 34 ), left CIMT correlated better with blood biochemical indices, including total cholesterol, LDL-cholesterol and fasting glucose levels. In addition, Hileman et al. ( Reference Hileman, Turner and Funderburg 35 ) demonstrated that decreased markers of oxidative stress, inflammation and monocyte activation resulted in improved CIMT. Earlier, it was documented that Mg may play an active role in the development and regression of atherosclerotic damage in HD patients. It has been reported that atherosclerotic changes in the carotid arteries measured by ultrasonography reflect atherosclerosis of coronary arteries( Reference Fabbian, Cacici and Franceschini 36 ). A recent meta-analysis demonstrated the significant risk of cardiovascular events was associated with CIMT( Reference Lorenz, Markus and Bots 37 ). Data on the effects of Mg supplementation on CIMT in diabetic patients undergoing HD are limited. In a study conducted by Mortazavi et al. ( Reference Mortazavi, Moeinzadeh and Saadatnia 9 ), Mg at a dosage of 440 mg three times per week for 6 months significantly decreased CIMT in HD patients. Turgut et al. ( Reference Turgut, Kanbay and Metin 38 ) also found that Mg supplementation in HD subjects at a dosage of 610 mg/d for 2 months improved CIMT and atherosclerosis. Recent evidence has demonstrated large amounts of Ca deposited in the coronary system of HD patients. In ESRD patients, enhanced P, Ca and parathyroid hormone (PTH) levels are the leading causes in the initiation and the progression of Ca deposition. Mg is considered a PTH antagonist, and evidence indicates that lower Mg status is significantly correlated with CVD and CIMT. A significant impact of Mg administration on CIMT may be explained through reduction in PTH and amelioration endothelial function( Reference Blacher, Guerin and Pannier 39 – Reference Ganesh, Stack and Levin 41 ).
Effects on glycaemic control
HD patients are vulnerable to insulin resistance and oxidative damage( Reference Simental-Mendia, Sahebkar and Rodriguez-Moran 42 , Reference Simental-Mendia, Simental-Mendia and Sahebkar 43 ). We found that consuming Mg supplements for 24 weeks by diabetic HD patients improved insulin, HOMA-IR, QUICKI, HbA1c but did not affect FPG. It has been demonstrated that Mg deficiency, via blockage of insulin metabolism, dependents of kinases and the triggering of the initial phase response are involved in the reduction of insulin sensitivity and then in the defect of glycaemic metabolism( Reference Simental-Mendia, Sahebkar and Rodriguez-Moran 42 ). Data documenting the impact of Mg administration on glycaemic control and lipid parameters in diabetic patients undergoing HD are scarce. Previous studies have demonstrated that Mg consumption is an attractive option for decreasing the risk of developing T2DM and ameliorating glucose metabolism( Reference Kim, Xun and Liu 44 , Reference Rumawas, McKeown and Rogers 45 ). Also, in a meta-analysis conducted by Simental-Mendia et al. ( Reference Simental-Mendia, Sahebkar and Rodriguez-Moran 42 ), Mg supplementation significantly improved HOMA-IR and FPG in both diabetic and non-diabetic subjects. However, another study showed that Mg administration did not have any beneficial effect on improving HbA1c( Reference Gerich 46 ). This finding can be explained by the document showing that HbA1c is an indicator of overall glycaemia over the preceding 4 months; so it is possible that HbA1c may not impact on glycaemic metabolism of short-time duration( Reference Gerich 46 ). Improved glucose homoeostasis parameters are believed to prevent malnutrition and atherosclerosis syndrome, the latter being one of the most important phenomenon in diabetic subjects under HD treatment. The favourable effects of Mg on glycaemic control may be due to the stimulated regulation of ATP-sensitive K channels and voltage-dependent Ca channel, which are implicated in the physiological insulin secretion( Reference Kowluru, Chen and Modrick 47 ).
Effects on lipid profiles
Patients with HD are susceptible to dyslipidaemia( Reference Simental-Mendia, Simental-Mendia and Sahebkar 43 ). We found that consuming Mg supplements for 24 weeks by diabetic HD patients reduced total cholesterol and LDL-cholesterol levels but did not affect other lipid profiles. Despite the non-pharmacological adjunct therapies, we still face the challenge of the epidemic growth of dyslipidaemia and CVD. Several finding have indirectly evaluated the impact of Mg intake on improving lipid parameters( Reference Jamilian, Samimi and Faraneh 48 , Reference Solati, Ouspid and Hosseini 49 ). We have previously reported that Mg supplementation at a dosage of 250 mg/d for 6 weeks by subjects with gestational diabetes improved few lipid profiles( Reference Jamilian, Samimi and Faraneh 48 ). Solati et al. ( Reference Solati, Ouspid and Hosseini 49 ) showed that Mg supplementation (300 mg/d) for 3 months to patients with T2DM significantly reduced LDL-cholesterol levels. However, in a meta-analysis by Simental-Mendia et al. ( Reference Simental-Mendia, Simental-Mendia and Sahebkar 43 ), Mg administration had no significant effects on lipid profiles in both diabetic and non-diabetic subjects. The beneficial actions of Mg on lipid profiles may be due to increased cholesterol esterification mediated by higher lecithin–cholesterol acyltransferase activity( Reference Gueux, Rayssiguier and Piot 50 , Reference Sales, Santos and Cintra 51 ).
Effects on biomarkers of inflammation and oxidative stress
A significant proportion of subjects with diabetes ultimately develop diabetic nephropathy( Reference Dronavalli, Duka and Bakris 52 ). Dialysis causes some relevant changes in the function of the immune system, monocyte-derived dendritic cells and in the release of various pro-inflammatory parameters and oxidative stress increase( Reference DeAngelis, Reis and Ricklin 53 , Reference Choi, Woo and Kim 54 ). We found that diabetic patients undergoing HD who were supplemented with Mg for 24 weeks had significantly reduced serum hs-CRP and plasma MDA and enhanced TAC levels but did not improve plasma NO and GSH levels, when compared with patients who received placebo. Some studies demonstrated that Mg supplementation had beneficial impacts on oxidative stress and inflammation in patients without diabetic HD. In accordance to these results, Mg supplementation ameliorated inflammation and oxidative stress in the metabolic syndrome( Reference Song, Ridker and Manson 55 ). Also, Mg administration (320 mg/d) among adults for 7 weeks significantly reduced hs-CRP( Reference Nielsen, Johnson and Zeng 56 ). In another study, after Mg intake at a dosage of 250 mg/d for 12 weeks, the median values of hs-CRP were significantly decreased and the plasma TAC levels were enhanced in patients with diabetic foot ulcer( Reference Razzaghi, Pidar and Momen-Heravi 57 ). However, Mg supplementation did not affect metabolic profiles in diabetic subjects with normomagnesaemia( Reference Navarrete-Cortes, Ble-Castillo and Guerrero-Romero 21 ). Anti-inflammatory impacts of Mg intake may be due to the effects of its antagonism to Ca channels, inhibition of N-methyl-d-aspartate receptors and the inactivation of NF-κB( Reference Aneiros, Philipp and Lis 58 , Reference Mazur, Maier and Rock 59 ). As well as, Mg supplementation may enhance TAC levels through decreasing ROS production and increasing glutathione-peroxidase activity( Reference Liu, Guo and Wang 60 , Reference Boujelben, Ghorbel and Vincent 61 ).
This study had few limitations. In the present study, we could not evaluate the effects of Mg intake on gene expression of insulin and inflammation signalling pathway and the role of polymorphisms in gene candidates in diabetic HD subjects. In addition, further studies are needed with bigger sample size to confirm our findings. Also, we were not able to assess the levels of calcification of the right and left carotid arteries, PTH and vitamin D at baseline and after the 24-week Mg supplementation, due to the lack of enough funding. In the present study, we were not able to determine whether Mg supplementation improves HD state. However, Mg intake might play an indirect role in HD state due to its effect on improved glycaemic control and other metabolic profiles. In the present study, despite a significant reduction in insulin concentrations, HbA1c levels and HOMA-IR, we did not find any significant effect on FPG following Mg supplementation. Consistent with our findings, in a study conducted by ELDerawi et al. ( Reference ELDerawi, Naser and Taleb 62 ), Mg supplementation (250 mg/d of elemental Mg) for 3 months to patients with T2DM significantly reduced insulin, HbA1c levels and HOMA-IR but did not affect fasting glucose. Moreover, Yokota et al. ( Reference Yokota, Kato and Lister 63 ) reported a significant reduction in insulin levels and HOMA-IR but not in fasting glucose and HbA1c after Mg supplementation for 30 d in patients with T2DM. In addition, a 3-month oral administration of magnesium oxide significantly decreased HbA1c and HOMA-IR but did not affect fasting glucose in pre-diabetic and obese patients with stage 2 and 3 chronic kidney disease( Reference Toprak, Kurt and Sari 64 ). There are also controversial findings; Guerrero-Romero et al. ( Reference Guerrero-Romero, Tamez-Perez and Gonzalez-Gonzalez 19 ) demonstrated that the intake of 50 ml magnesium chloride for 16 weeks significantly improved HOMA-IR, fasting glucose and HbA1c in patients with T2DM. The absence of beneficial effects of Mg supplementation on FPG may be ascribed to the failure in effectively increasing intracellular Mg. Higher doses of Mg or longer duration of supplementation might be required to let intracellular Mg increase. It is also important to note that serum Mg levels do not thoroughly reflect dietary or supplemental Mg intake. Although serum Mg levels are dependent on dietary intake, intestinal absorption, and kidney function, urinary Mg excretion and intracellular Mg levels are better indicators than serum Mg concentrations and are more sensitive to oral supplementation than serum Mg concentrations. Overall, there are different parameters including participants’ characteristics like kidney function, higher doses of Mg or longer intervention which can provide appropriate intracellular levels of Mg necessary for lowering FPG. However, we were not able to assess intracellular Mg concentrations in the present study. Perhaps if the dose or duration of Mg had been increased, the results of FPG would have been significant. Nonetheless, we believe that further studies with more prolonged use of Mg in doses that are higher than usual are required to establish its routine or selective administration in diabetic patients under HD to control their FPG levels.
Conclusions
Overall, we found that taking Mg for 24 weeks by diabetic HD patients significantly improved mean and maximum levels of left and mean levels of right CIMT, insulin, HOMA-IR, QUICKI, HbA1c, total cholesterol and LDL-cholesterol, hs-CRP, TAC and MDA levels; however, it did not have any effect on maximum levels of right CIMT and other metabolic profiles.
Acknowledgements
The authors would like to thank the staff of Akhavan Clinic (Kashan, Iran) for their assistance in this project.
The present study was supported by a grant from the Vice-chancellor for Research, KAUMS, and Iran.
Z. A. contributed in conception, design, statistical analysis and manuscript drafting. H. R. T., M. Z., A. S., F. B., A. G., N. M., M. E., M. B. and M. A. M. contributed in data collection and manuscript drafting.
The authors declare that there are no conflicts of interest.







