GLUT in the plasma membrane are one of the most important cellular nutrient transporters, as glucose plays a central role in cellular metabolism. Besides acting as shuttles in different tissues, a growing body of evidence suggests that GLUT participate in various physiological and metabolic functions in human beings and animals. For instance, GLUT Na+/glucose co-transporter 1 (SGLT1)-mediated glucose uptake increases anti-apoptotic proteins of the small intestine and protects enterocytes from apoptosis and barrier defects( Reference Linda, Flynn and Turner 1 ). Obesity contributes to type 2 diabetes with the down-regulated expressions of GLUT1 and GLUT4 in skeletal muscle( Reference Ciaraldi, Mudaliar and Barzin 2 , Reference Kim, Zisman and Fillmore 3 ). Therefore, the knowledge of understanding the regulation of GLUT contributes to our understanding of whole-body glucose homoeostasis and human metabolic diseases.
In human beings and animals, highly specialised GLUT are expressed in various tissues. In muscle and adipose tissues, GLUT1 and GLUT4 are responsible for the majority of whole-body glucose metabolism( Reference Bryant, Govers and James 4 ). GLUT1 is largely responsible for basal glucose transport, as >40 % of this transporter isoform is present on the cell surface in the absence of insulin( Reference Piper, Hess and James 5 ). However, the majority of GLUT4 is localised in intracellular tubulovesicular structures clustered in the cytoplasm and is translocated to the plasma membrane for its functions( Reference Slot, Geuze and Gigengack 6 , Reference Rodnick, Slot and Studelska 7 ). Different from the system in muscle, SGLT1 and GLUT2 mediate glucose absorption and utilisation in the small intestine. Glucose and, to a lesser extent, galactose are transported from the lumen of the intestine into enterocytes by SGLT1( Reference Ferraris and Diamond 8 , Reference Kojima, Nishimura and Yajima 9 ), whereas fructose is transported by a facilitated-diffusion GLUT5( Reference Wright, Hirayama and Loo 10 – Reference Douard and Ferraris 12 ). After being transported in the cells, glucose and fructose are passively transported from enterocytes into the systemic system by another facilitated-diffusion GLUT such as GLUT2( Reference Ferraris and Diamond 8 , Reference Shirazi-Beechey 13 ). Some studies have shown that different concentrations of dietary carbohydrate and artificial sweeteners up-regulate the intestinal GLUT expression in piglets( Reference Moran, Al-Rammahi and Arora 14 , Reference Moran, Al-Rammahi and Arora 15 ), which indicates that other dietary nutrients can also regulate intestinal GLUT expression.
In addition to providing fuel and essential nutrients, circulating substrates derived from food, including amino acids, have specific direct and indirect functions to activate receptors and signalling pathways, and then regulate whole-body nutritional homoeostasis( Reference Ryan and Seeley 16 ). Recently, some studies have reported that amino acids participate in the regulation of plasma glucose levels( Reference Bernard, Liao and Doerner 17 ). Among all the amino acids, branched-chain amino acids (BCAA) (especially isoleucine) have been shown to strongly enhance glucose consumption and utilisation( Reference Doi, Yamaoka and Fukunaga 18 ). In an in vivo oral glucose tolerance test conducted in normal rats, isoleucine prevented a rise in plasma glucose concentration, and the effect of isoleucine was greater than that of leucine or valine( Reference Doi, Yamaoka and Fukunaga 18 ). In another in vitro experiment, both leucine and isoleucine stimulated glucose uptake in C2C12 myotubes, with the effect of isoleucine greater than that of leucine( Reference Doi, Yamaoka and Fukunaga 18 ). Similarly, Nishitani et al. ( Reference Nishitani, Takehana and Fujitani 19 ) and Doi et al. ( Reference Doi, Yamaoka and Nakayama 20 ) reported that isoleucine stimulated plasma glucose uptake in rats. However, the effect of isoleucine on the expression of GLUT is still unknown. Therefore, this experiment was conducted to determine whether isoleucine could regulate muscular and intestinal GLUT in in vitro and in vivo trials.
Methods
Animals and experimental design
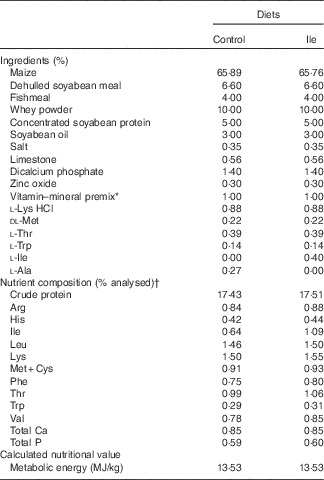
All procedures used in this experiment complied with the animal care protocol that was approved by the China Agricultural University Animal Care and Use Committee (Beijing, China). In all, twelve, 21-d-old, male, Landrace×Large×White piglets were obtained from a commercial pig farm and transported to the Laboratory of Animal Metabolism at China Agricultural University (Beijing, China). Piglets were randomly assigned into two dietary treatments (control treatment and isoleucine supplemented treatment, Table 1) according to their initial body weight (six pigs per treatment). To ensure the same content of N in the two experimental diets, the control diet was supplemented with 0·27 % l-alanine, whereas the isoleucine diet was supplemented with 0·4 % isoleucine, both added at the expense of maize. Pigs had ad libitum access to drinking water and their respective diets. The basal diet was formulated to meet or exceed National Research Council( 21 ) recommendations for all other nutrients and were supplied in mash form.
Table 1 The composition and analysed nutrient content of the experimental diets (% as-fed basis)

* Provided per kg of complete diet: retinyl acetate, 2700 µg; cholecalciferol, 75 µg; DL-α-tocopheryl acetate, 64 mg; vitamin K3, 3 mg; vitamin B12, 12 μg; riboflavin, 5·5 mg; pantothenic acid, 15 mg; niacin, 40 mg; choline chloride, 551 mg; folacin, 0·8 mg; vitamin B1, 1·5 mg; vitamin B6, 3 mg; biotin, 100 μg; Mn, 40 mg; Fe, 100 mg; Zn, 100 mg; Cu, 150 mg; I, 0·3 mg; Se, 0·3 mg.
† Values are the means of a chemical analysis conducted in duplicate.
Piglets were housed in individual metabolic cages (1·25×0·55×0·80 m3) in a temperature-controlled nursery room (29–30°C for the 1st week, 28–29°C for the 2nd week). Pigs were weighed at the beginning and at the end of the experiment. The amount of feed in the feeder was monitored, and an attempt was made to ensure that feed was always available in the feeder. The feed was weighed before being added to the feeder. Any wasted feed or feed refusals were also weighed, and this amount was subtracted from the amount of feed added to the feeder to determine feed disappearance. These values were used to calculate average daily gain (ADG), average daily feed intake (ADFI) and feed:gain ratio (F:G).
Sample collection
Blood samples were collected from all twelve pigs at the end of the experiment by jugular venepuncture before the morning feeding. Blood samples were collected using heparin-free vacutainer tubes (Becton Dickinson Vacutainer Systems). After blood collection, all samples were centrifuged for 15 min at 8000 g at room temperature. The serum was separated and stored at −80°C.
At the end of the 14-d experiment, all twelve pigs of the experiment were euthanised by an intracardiac injection of sodium pentobarbital (72 mg/kg body weight), and intestinal and muscular samples were collected to evaluate protein expressions of GLUT; six newborn piglets were also killed in order to determine the effect of age on the expression of GLUT. About 5 g of muscle sample was removed from the masseter (red), psoas (white) and longissimus lumborum (intermediate) muscles of each piglet and collected into sterile Eppendorf tubes. The small intestine was dissected from the mesentery and immediately placed on ice. The duodenum was considered as the part of the intestine from the pylorus to the ligament of Treitz. A segment 10 cm proximal to the ileocaecal junction was considered as the ileum, and the remainder of the small intestine was considered the jejunum. Segments (3 cm in length) of mid-duodenum, mid-jejunum and mid-ileum (the entire alimentary canal without digesta) were immediately flushed with PBS and collected into sterile Eppendorf tubes for further Western blot analysis. Each tube was immediately frozen in liquid N2 and then stored at −80°C.
Chemical analyses
Crude protein, Ca and P contents of the experimental diets were analysed according to Association of Official Analytical Chemists( 22 ) procedures. Dietary amino acids except methionine, cystine and tryptophan were determined by ion-exchange chromatography using a Hitachi L-8800 AA Analyzer (Hitachi) after acid hydrolysis with 6 m-HCl (reflux for 24 h at 110°C). Concentrations of methionine and cystine were determined after oxidation with performic acid and subsequent hydrolysis with 6 m-HCl, and the tryptophan content was determined after alkaline hydrolysis at 120°C for 16 h according to Association of Official Analytical Chemists( 22 ) and separation by reversed-phase HPLC (Agilent 1200; Agilent).
C2C12 cell and IPEC-J2 cell culture
As an in vitro model, C2C12 myoblasts (mouse source) (American Type Culture Collection) were used to represent skeletal muscle. Cells were seeded in six-well plates and maintained under subconfluent conditions in Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (cat. no. SH30023·0113; Thermo) with 5 % (vol/vol) fetal bovine serum (FBS, cat. no. 10099-141; Gibco) and antibiotics (100 units penicillin/ml and 100 μg streptomycin/ml). When cells were grown to 90 % confluence, they were induced to differentiate into myotubes using DMEM/F12 medium containing 2 % horse serum (cat. no. 26050-070; Gibco) and antibiotics for 4 d.
The IPEC-J2 cells, originally derived from the jejunal crypts of a neonatal piglet, were used to represent the intestine (Dr. Guoyao Wu, Texas A & M University). Cells were cultured in six-well plates in DMEM/F12 medium supplemented with 5 % (vol/vol) FBS, 5 μg/l insulin-transferrin-sodium selenite (ITS) (cat. no. 0803; Sciencell) and 5 μg/l epidermal growth factor (cat. no. 105-04; Sciencell) until reaching 90 % confluence.
Glucose uptake assay
Glucose uptake was assayed according to the established protocol from a commercial glucose uptake kit (ab136955; Abcam). In brief, after C2C12 cells were differentiated to C2C12 myotubes, they were starved in serum-free DMEM/F12 medium overnight followed by 40 min of incubation in Krebs–Ringer–Phosphate–Hepes buffer. Subsequently, cells were stimulated with 0–10 mm (0, 1, 3, 5, 7 and 10 mm) leucine, isoleucine or valine for 1, 3, 5, 7 or 10 h; 10 mm-2-deoxyglucose was added for an additional 20 min. Cells were washed three times with cold PBS and lysed with extraction buffer, then frozen at −80°C for 10 min and heated at 85°C for 40 min. After cooling on ice for 5 min, the lysates were neutralised by adding neutralisation buffer and centrifuged. The remaining lysate was then diluted with assay buffer. Finally, the colorimetric end-product generation was set up by two amplification steps according to the kit manufacturer’s instructions and then detected at 412 nm using iMark microplate reader (Bio-Rad). In order to test the expressions of the GLUT, the protein samples of IPEC-J2 and C2C12 myotubes cells were harvested after cells were stimulated with 0–10 mm (0, 1, 3, 5, 7 and 10 mm) leucine, isoleucine or valine for 5 h.
Serum glucose and insulin concentration assay
After the frozen serum samples were thawed at 4°C, serum glucose concentration was measured by the glucose oxidase method (Applygen Technologies) following the manufacturer’s instructions. Serum insulin concentrations were measured using a porcine insulin ELISA kit (Huijia Biotech Company) following the instructions of the manufacturer.
Western blot analysis
Relative membrane protein levels of GLUT1, GLUT4, SGLT-1, GLUT2 and GLUT5 were determined by the Western blot technique. To obtain the membrane protein, IPEC-J2 cells and C2C12 cells and frozen intestinal and muscular samples were lysed according to the protocol of the Mem-PER Eukaryotic Membrane Protein Extraction Reagent Kit (Thermo), plus a protease inhibitor cocktail purchased from Apply Gene. Protein concentrations were determined using a BCA Protein Assay Kit (Pierce). Equal amounts of proteins (30 μg) were electrophoresed on SDS polyacrylamide gels. Pre-stained protein markers (Fermentas) were analysed in each gel. Proteins were electrotransferred to a polyvinylidene difluoride membrane (Millipore) and blocked with 5 % non-fat dry milk overnight at 4°C. Transfer efficiency was assessed by gel staining with Coomassie Blue. Samples were incubated with corresponding primary antibodies (1:500 dilution for 2 h at 25°C or overnight at 4°C) against GLUT1 (Bioss), GLUT2 (Bioss), GLUT4 (Bioss), GLUT5 (LifeSpan Bioscience), SGLT1 (Millipore) and Na+/K+ATPase (Millipore). The primary antibodies have been validated for use in swine by the manufacturer. After being washed with Tris-Tween-20 buffer (pH 7·4), membranes were incubated with a secondary antibody (GLUT1, GLUT2, GLUT4, GLUT5 and SGLT1: horseradish peroxidase-conjugated goat anti-rabbit IgG; Na+/K+ATPase: horseradish peroxidase-conjugated rabbit anti-mouse IgG) (Zhongshan Golden Bridge) at 1:7000 dilution for 1 h at room temperature. Band densities were detected with the Western Blot Luminence Reagent (Santa Cruz Biotechnology) and quantified using AlphaImager 2200 (Alpha Innotech).
Statistical analysis
Statistical analyses were performed using SAS version 9.2 (SAS Institute). Data from growth performance, serum insulin and glucose concentrations in animal trials were analysed by the T-TEST procedure of SAS. Data from glucose uptake and protein expression of GLUT were analysed using the general linear model (GLM) procedure of SAS. Means were separated by Student–Newman–Keuls multiple range test. Differences at P<0·05 were considered significant.
Results
Piglet performance
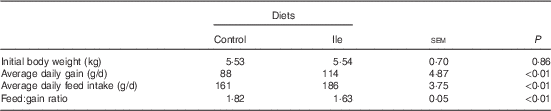
The performance of pigs is presented in Table 2. Pig ADG and ADFI in the isoleucine treatment were greater than those in the control treatment (P<0·05). The F:G was poorer for pigs in the control treatment compared with pigs in the isoleucine treatment (P<0·05).
Table 2 Performance of post-weaning pigs fed low-protein diets supplemented with alanine or supplemented with isoleucine for 14 d (Mean values with their standard errors of six pens of six pigs per diet)
Serum glucose and insulin concentrations
There was little evidence of treatment differences in serum glucose or insulin concentrations in piglets supplemented with isoleucine compared with the control diet (Fig. 1, P>0·05).

Fig. 1 Effect of control or isoleucine diets on serum glucose and insulin concentrations (n 6). Means followed by no letter do not differ significantly (P>0·05). ![]() , Control diet;
, Control diet; ![]() , isoleucine diet.
, isoleucine diet.
Protein expressions of GLUT in muscle
The results of the Western blot analysis indicated the presence of GLUT1 and GLUT4 in red (Fig. 2(A)), white (Fig. 2(B)) and intermediate muscle (Fig. 2(C)). The protein abundance of GLUT1 in red muscle was greater in piglets fed the isoleucine diet compared with piglets fed the control diet (P<0·05), but not in white muscle and intermediate muscle (P>0·05). The protein abundance of GLUT4 in red muscle, white muscle and intermediate muscle was greater in pigs fed the isoleucine diet compared with those fed the control diet (P<0·05). Compared with newborn piglets, protein abundance of GLUT4 in red and intermediate muscle was higher in 35-d-old piglets (P<0·05).

Fig. 2 Effect of control or isoleucine diets on GLUT1 and GLUT4 protein abundance in red muscle (A), white muscle (B) and intermediate muscle (C) obtained from piglets of each group (n 6). GLUT1 and GLUT4 protein abundance in newborn piglets (n 6) is also listed in the figure (effect of age on muscular GLUT can be analysed by comparing the newborn (0 d) and control (35 d) groups). Na+/K+ATPase was used as an internal standard to normalise the signal. a,b,c Means followed by like letter do not differ significantly (P>0·05).
Protein expressions of GLUT in the small intestine
The results of the Western blot analysis of SGLT1, GLUT2 and GLUT5 from the duodenum, jejunum and ileum are shown in Fig. 3(A)–(C), respectively. The protein abundance of SGLT1 was up-regulated in the duodenum, jejunum and ileum of piglets in the isoleucine treatment compared with piglets in the control treatment (P<0·05). The protein abundance of GLUT2 was greater in the duodenum and jejunum of piglets in the isoleucine treatment compared with piglets in the control treatment (P<0·05). However, supplementation of isoleucine in the diet reduced the expression of GLUT5 in the duodenum, jejunum and ileum of piglets (P<0·05). Compared with newborn piglets, the protein abundance of GLUT2 in the ileum and GLUT5 in the duodenum, jejunum and ileum was higher in 35-d-old piglets (P<0·05).

Fig. 3 Effect of control or isoleucine diets on Na+/glucose co-transporter 1 (SGLT1), GLUT2 and GLUT5 protein abundance in the duodenum (A), jejunum (B) and ileum (C) (n 6). SGLT1, GLUT2 and GLUT5 protein abundance in newborn piglets (n 6) is also listed in the figure (effect of age on intestinal GLUT can be analysed by comparing the newborn (0 d) and control (35 d) groups). Na+/K+ATPase was used as an internal standard to normalise the signal. a,b Means followed by like letter do not differ significantly (P>0·05).
The effect of isoleucine, leucine and valine on glucose uptake in C2C12 cells and IPEC-J2 cells
Cultures of C2C12 cells were treated with various concentrations of leucine, isoleucine or valine (0, 1, 3, 5, 7 or 10 mm) for 1 (Fig. 4(a)), 3 (Fig. 4(b)), 5 (Fig. 4(c)), 7 (Fig. 4(d)) or 10 h (Fig. 4(e)). Cultures of C2C12 cells at both 7 and 10 mm-isoleucine for 3, 5, 7 and 10 h increased (P<0·05) the cellular 2-deoxyglucose uptake, and similar results were observed in C2C12 cells treated with 5 mm-isoleucine for both 3 and 5 h (P<0·05). The 10 mm-leucine treatment for 3, 5 and 7 h and 7 mm-leucine treatment for 5 and 7 h also increased the 2-deoxyglucose uptake (P<0·05). However, valine supplementation had little effect on cellular 2-deoxyglucose uptake at any time point and any concentration (P>0·05). There was little evidence of differences in 2-deoxyglucose uptake in IPEC J-2 cells treated with any concentration of leucine, isoleucine or valine (0, 1, 3, 5, 7 or 10 mm) for 1, 3, 5, 7 or 10 h (data not shown).

Fig. 4 Glucose uptake was analysed after 1 (a), 3 (b), 5 (c), 7 (d) and 10 h (e) treated with 0, 1, 3, 5, 7 or 10 mm-isoleucine (![]() ), leucine (
), leucine (![]() ) or valine (
) or valine (![]() ) in C2C12 cells. Values are means (n 6), with their standard errors. * Significantly different between the isoleucine group and the control group. † Significantly different between the leucine group and the control group.
) in C2C12 cells. Values are means (n 6), with their standard errors. * Significantly different between the isoleucine group and the control group. † Significantly different between the leucine group and the control group.
Protein expressions of GLUT in C2C12 cells and IPECJ-2 cells after treatment with isoleucine, leucine and valine
Results of the Western blot analysis indicated the presence of GLUT1 and GLUT4 in C2C12 Cells (Fig. 5). Protein abundance of GLUT1 and GLUT4 increased with 5, 7 and 10 mm-isoleucine supplementation after 5 h, compared with the control (Fig. 5(A), P<0·05). High concentrations of supplemented leucine (7 mm and 10 mm) also increased (Fig. 5(B), P<0·05) the protein expression of GLUT4. Valine supplementation had little effect on the protein expression of GLUT1 and GLUT4 at any time point and any concentration (Fig. 5(C), P>0·05). In additional, isoleucine, leucine and valine supplementation had little effect on the protein expressions of SGLT1, GLUT2 and GLUT5 in IPEC-J2 cells (data not shown).

Fig. 5 The protein abundance of GLUT1 and GLUT4 was analysed after 5 h of treatment with isoleucine (A), leucine (B) or valine (C) in C2C12 cells (n 6). Cells were treated with 0, 1, 3, 5, 7 or 10 mm-isoleucine for 5 h. The protein abundance of GLUT1 and GLUT4 was detected by Western blot. Na+/K+ATPase was used as an internal standard to normalisation. a,b Means followed by like or no letter did not differ significantly (P>0·05).
Discussion
Reducing dietary protein levels with supplemental amino acids is a useful strategy to control the severity of diarrhoea and decrease the manure and urea N contents in weanling piglets without affecting pig growth performance( Reference Opapeju, Krause and Payne 23 ). However, such a strategy could result in an unbalanced supply of dietary amino acids. In the previous study, we demonstrated that the supplementation of BCAA in low-protein diets is necessary for maintaining growth and gut health of weaning pigs when reducing the dietary protein level from 21 to 17 %( Reference Zhang, Qiao and Ren 24 ). However, the physiological and nutritional function of each BCAA (isoleucine, leucine or valine) needs to be clarified. Isoleucine is an essential amino acid, which means that it is not synthesised in animals, and it is important for pig productivity( Reference Stokes, Gunness and Dwyer 25 ). It has been demonstrated that, in addition to the first four limiting amino acids (lysine, methionine, threonine and tryptophan), supplementation of isoleucine in a low-protein diet is necessary for pigs to obtain the performance of those fed a normal protein diet( Reference Zhang, Qiao and Ren 24 , Reference Figueroa, Lewis and Miller 26 , Reference Lordelo, Gaspar and Le Bellego 27 ). Similarly, in this study, we found that the performance of piglets fed the isoleucine-supplemented diet was higher than piglets in the control treatment.
Glucose, a fundamental source of energy and carbon for most eukaryotic cells, has wide effects on cellular function( Reference Gaster, Franch and Staehr 28 ). As one of the crucial body energy stores, glucose is replenished from the diet after being digested and absorbed through the gastrointestinal tract, which then is broadly distributed among the various tissues of the body( Reference Santos, Benite‐Ribeiro and Queiroz 29 ). These absorption and distribution processes are conducted with the involvement of a family of transport proteins, which act as shuttles to move sugar across the cell surface.
In recent years, scientists have studied the role of amino acids in glucose metabolism, but results have been inconsistent. Some studies have shown that an amino acid mixture increased glucose uptake in the presence of insulin in rodents( Reference Bernard, Liao and Doerner 17 ). However, others found that an increase in plasma amino acids after administration of an amino acid mixture induced insulin resistance in skeletal muscle by inhibiting glucose transport and glucose phosphorylation in humans( Reference Tessari, Inchiostro and Biolo 30 , Reference Krebs, Krssak and Bernroider 31 ). Difference in results from different studies could be caused by different combinations and concentrations of amino acids in the mixtures. However, in studies with individual amino acids, both isoleucine and leucine have been demonstrated to stimulate glucose uptake( Reference Doi, Yamaoka and Fukunaga 18 – Reference Doi, Yamaoka and Nakayama 20 ).
Leucine is reported to increase glucose uptake by up-regulating translocation of GLUT4 and GLUT1 in muscle( Reference Nishitani, Takehana and Fujitani 19 ). There are two possible mechanisms for leucine to regulate muscular GLUT. First, leucine has been shown to stimulate insulin secretion, and translocation of GLUT1 and GLUT4 could be up-regulated by increased levels of insulin( Reference Li, Najafi and Daikhin 32 – Reference Koivisto, Martinez-Valdez and Bilan 34 ). Second, leucine up-regulated glucose uptake directly in isolated skeletal muscle in a rapamycin-resistant manner, and this action was blocked by LY-294002 (a specific inhibitor of phosphoinositide 3-kinases; PI3K) and GF-109203X (a specific inhibitor of protein kinase C; PKC)( Reference Doi, Yamaoka and Fukunaga 18 ), suggesting that leucine stimulated glucose uptake in skeletal muscle through the PI3K and PKC signalling pathways. Both the signalling pathways have been demonstrated to participate in the translocation of GLUT4( Reference Chen, Bandyopadhyay and Sajan 35 ). Similarly, in this experiment, when C2C12 cells were treated with 7 and 10 mm-leucine for 5 h, the expression of GLUT4 GLUT and 2-deoxyglucose uptake was increased, but not the expression of GLUT1.
Unlike leucine, isoleucine stimulated glucose uptake in the skeletal muscle in vivo and lowered blood glucose in rats without increasing plasma insulin( Reference Doi, Yamaoka and Fukunaga 18 ). Until now, most studies have only demonstrated that isoleucine increases glucose uptake in skeletal muscle by unknown mechanisms( Reference Doi, Yamaoka and Fukunaga 18 – Reference Doi, Yamaoka and Nakayama 20 ). In our study, the muscle GLUT such as GLUT1 (in red muscle) and GLUT4 (in red muscle, white muscle and intermediate muscle) were up-regulated when pigs were fed isoleucine in the diet. Moreover, isoleucine enhanced the protein expressions of GLUT1 and CLUT4 in C2C12 cells with an increase in 2-deoxyglucose uptake from the cultural medium. In vivo and in vitro trials both indicated that muscular glucose uptake in pigs fed low-protein diets without supplementation of isoleucine could partly be inhibited by restricting expressions of GLUT1 and GLUT4. In this experiment, serum glucose and insulin levels were detected, but neither of them was significantly different between different treatments. The time when blood samples were collected could contribute to these results. A more optimal sampling procedure would be to measure pig blood glucose levels continuously after feed intake with vascular catheterisation, rather than collecting blood after overnight fasting. Although the present study demonstrated that isoleucine significantly increased the expressions of GLUT4 and GLUT1 in the muscle of piglets, the mechanism of isoleucine regulating muscular GLUT remains unclear and requires further clarification in future studies.
Besides having the above-mentioned functions in muscle, isoleucine also plays a vital role in the intestine. In addition to glutamine, glutamate and aspartate, BCAA are considered as fuels for small intestinal mucosa and are responsible for providing energy to the intestine for functions such as active nutrient transport and high rates of intracellular protein turnover( Reference Wu 36 , Reference Wu 37 ). Therefore, in a low-protein diet, non-essential amino acids such as glutamine, glutamate and aspartate might be insufficient to meet the energy requirements of the intestine, and the supplementation of isoleucine in the low-protein diet might regulate the expressions of GLUT.
In this experiment, we detected the expressions of intestinal sugar transporter systems (SGLT1, GLUT2 and GLUT5) in the duodenum, jejunum and ileum of piglets, and our objective was to determine whether isoleucine could regulate intestinal monosaccharide absorption in pigs. The SGLT1 transporter is the rate-limiting step for absorption of dietary glucose from the lumen of the intestine into the body, and GLUT2 passively transported glucose and fructose from enterocytes into the systemic system( Reference Ferraris and Diamond 8 , Reference Moran, Al-Rammahi and Arora 15 ). Regulation of glucose transport by the diet might involve increased transcription of SGLT1 and GLUT2 mainly in the crypt cells( Reference Ferraris 38 , Reference Dyer, Hosie and Shirazi-Beechey 39 ). It has been demonstrated that monosaccharide-up-regulated expressions of SGLT1 and GLUT2 in the small intestine is via the luminal sweet taste receptor subunit T1R3 and the taste G protein gustduci( Reference Margolskee, Dyer and Kokrashvili 40 – Reference Mace, Lister and Morgan 42 ). In this study, we demonstrated that supplementation of isoleucine in a low-protein diet significantly up-regulated the expressions of SGLT1 and GLUT2 in the duodenum, jejunum and ileum of piglets. However, unlike dietary sugars, isoleucine could not regulate SGLT1 and GLUT2 via the signalling pathways mentioned above, and more research needs to be carried out to find the underlying mechanism.
As one part of the intestinal epithelial sugar transporter system, the protein expression of GLUT5 was also evaluated in this study. The GLUT5 transporter, which primarily participates in transportation of fructose from the luminal membrane into enterocytes, was down-regulated in the duodenum, jejunum and ileum of piglets in the isoleucine treatment. Regulation of GLUT5 by the diet seems to involve de novo synthesis of GLUT5 mRNA and protein, leading to increases in fructose transport a few hours after consumption of diets containing fructose( Reference Ferraris 38 ). Unlike humans, who might consume about 16–24 g fructose from fruits and honey each day( Reference Douard and Ferraris 12 ), the amount of fructose in the diets of piglets is low. Thus, for energy conservation, the small intestine might tend to express more SGLT1 and GLUT2 with less GLUT5 when pigs are fed a low-protein diet. However, more experiments are needed to determine whether leucine and valine have similar functions in regulating intestinal GLUT.
In the IPCEJ-2 cells experiment, small differences in glucose uptake were found when adding extra isoleucine, leucine or valine to the culture medium (data not shown). Moreover, small differences in SGLT1, GLUT2 and GLUT5 expressions were observed in IPEC-J2 cells, when these cells were treated with the BCAA (data not shown). This result indicates that IPEC-J2 cells cultured in the plate were not equivalent to the in vivo situation. Some studies also found that the primary energy source for epithelial cells was not from glucose but from glutamine, which could be another reason why little change in uptake of glucose from the glucose medium was observed.
Ageing-induced reduction in GLUT expression is one of the vital risk factors for insulin resistance, obesity and development of type 2 diabetes. In human skeletal muscle, GLUT1 is only ubiquitously expressed during gestation, whereas its expression significantly decreases around birth and finally becomes undetectable within the 1st year of life( Reference Gaster, Franch and Staehr 28 ). Similarly, in rat skeletal muscle, a decrease in GLUT1 immunoreactivity within the fibre was observed between animals of 10, 22 and 42 weeks, whereas the presence of GLUT1 was almost undetectable in adults (aged 22 and 42 weeks)( Reference Santos, Benite‐Ribeiro and Queiroz 29 ). These results are inconsistent with the results of our study. Expression of GLUT4 in red and intermediate muscle of 35-d-old piglets was significantly higher compared with newborn piglets. Further, no increase or decrease in GLUT1 expression was found in different types of muscle between 35-d-old and newborn piglets. Similarly, the protein abundance of GLUT2 in the ileum and GLUT5 in the duodenum, jejunum and ileum was higher in 35-d-old piglets when compared with newborn piglets. Various factors could lead to the differences in this study compared with other reports in the literature. First, the former studies mainly focused on human and rat, but this trial was conducted on piglets. Species differentiation could be one of the factors that could explain the different results. Second, some studies mentioned above used immunohistochemistry methods to discriminate between muscle fibre-derived GLUT-1 and GLUT-1 from other sources, that is, erythrocytes and perineurial sheaths. However, in this study, Western blot was used, which does not differentiate GLUT-1 source as precisely as immunohistochemistry.
In conclusion, isoleucine up-regulated the protein expressions of GLUT1 (in red muscle), GLUT4 (in red muscle, white muscle and intermediate muscle), SGLT-1 (in the duodenum, jejunum and ileum) and GLUT2 (in duodenum and jejunum), but down-regulated the protein expression of GLUT5 (in the duodenum, jejunum and ileum) when pigs were fed a low-protein diet. In C2C12 cells, supplemented isoleucine in the medium also significantly increased the cellular glucose uptake from the medium via the enhancement of protein expressions of GLUT4 and GLUT1 to a greater extent than supplemented leucine. Compared with newborn piglets, 35-d-old piglets had comparatively higher GLUT4, GLUT2 and GLUT5 expressions. These findings have important implications, suggesting that isoleucine could potentially increase muscle growth and intestinal development and health by enhancing the local glucose uptake of animals and human beings.
Acknowledgements
This study was supported by the National Key Basic Research Program (S. Q., grant no. 2012CB124704; P. H., grant no. 2013CB127305); and the 111 Project (D. L., grant no. B16044). National Key Basic Research Program and 111 Project had no role in the design, analysis or writing of this article.
S. Z., S. Q., X. Z. designed the study; Q. Y. and S. Z. conducted the study; M. R. and P. H. assisted with sample analysis; S. Z. and M. R. analysed the data; and S. Z. wrote the paper.
The authors declare that there are no conflicts of interest.