In the past 60 years, due to long-term genetic selection, the growth rate and feed conversion rate of broiler chickens have been significantly improved(Reference Havenstein, Ferket and Grimes1). At the same time, due to the tendency in genetic selection for meat production, the development of the visceral system (especially the cardiovascular and respiratory system) and immune system is retarded relative to the growth rate, which causes hypoimmunity and poor anti-stress ability in broiler chickens(Reference Havenstein, Ferket and Grimes1–Reference Lara and Rostagno3). To make matters worse, there are still a large number of pathogenic sources and stress factors in the intensive farming process of broiler chickens, especially in China. The above problems have put tremendous pressure on nutrition and management of broiler chickens.
With the great improvement in growth performance, the feeding time required to reach market weight is greatly shortened. Taking Arbor Acres broiler chickens as examples, it means that the incubation stage represents more than one-third of a broiler’s life, which has increased the importance of the incubation stage. Interestingly, during the incubation stage, the epigenome, the status of which is directly affected by nutrients and other external environmental factors, undergoes reprogramming involving nutri-epigenetics(Reference Lillycrop and Burdge4,Reference Reik, Dean and Walter5) . Nutri-epigenetics mainly study that the intake of a special nutrient during a specific physiological period regulates gene expression pattern through epigenetic modification to produce a difference in genetic trait at the phenotypic and molecular level of offspring without alterations in DNA sequence(Reference Lyko, Foret and Kucharski6). Epigenetic modification mainly includes four aspects: DNA methylation, histone modification, chromatin allosteric and non-coding RNA. Consequently, nutri-epigenetics may be a potential way to relieve the pressure from hypoimmunity and poor anti-stress ability in broiler chickens.
Vitamin C is a water-soluble vitamin with anti-stress(Reference Khan, Naz and Nikousefat7), antioxidant(Reference Jena, Panda and Patra8) and immune enhancement(Reference Carr and Maggini9) and is involved in active DNA demethylation, histone demethylation and other epigenome regulation as cofactors of related enzymes(Reference Young, Züchner and Wang10). Eggs do not contain detectable vitamin C(Reference Nowaczewski, Kontecka and Krystianiak11), and the embryonic development process is separated from the matrix, leading the embryo to be unavailable to obtain exogenous vitamin C. Studies have shown that broiler chickens could only synthesise vitamin C that met metabolic needs after 2 weeks of post-hatch(Reference Whitehead and Keller12). Newly hatched chicks have insufficient capacity of vitamin C synthesis and need to be supplied vitamin C from feed and drinking water(Reference Whitehead and Keller12). According to the above statement, there may be vitamin C deficiency during embryonic development of broiler chickens. Fortunately, an in ovo feeding (IOF) technology has become a viable method for providing exogenous nutrients into breeding eggs and opens a door for studying vitamin C on nutri-epigenetics in broiler chickens.
According to DNA methylation variation trends during the embryonic development of broiler chickens in a previous study, the genomic DNA methylation patterns could be separated into three stages: from embryonic age 2 (E2) to 4 (rising stage), from E4 to 13 (plateau stage) and from E13 to 19 (rising stage)(Reference Li, Zhu and Zhi13). E0, 11 and 15 were selected as potential injection times for effective nutritional intervention. And, this study was mainly aimed at embryonic age 11 (E11). The hypothesis was tested that IOF of vitamin C at E11 will improve the post-hatch performance, antioxidant capacity and immune status of broiler chickens. Simultaneously, the splenic expression of genes encoding enzymes related to DNA methylation and demethylation was measured to preliminarily explore the potential epigenetic mechanisms. To some extent, this study is beneficial for understanding the nutritional role of vitamin C, supplied during embryonic age, on growth and immunity.
Methods
The use of the animals and the experimental procedures were conducted under the guidelines of the Institutional Animal Care and Use Committee of Northwest A&F University, Shaanxi, China.
Incubation, feeding and sample collection
The scheme of experimental design and sample collection is shown in Fig. 1. A total of 240 fertilised eggs from Arbor Acres broiler breeders with an average weight of 63 g (range from 60·5 to 65·5 g) were purchased from the Xianyang Dacheng Poultry Industry Co. Ltd. After disinfection, all eggs were randomly divided into two groups: (1) normal saline group (NS): 0·1 ml normal saline per egg; (2) vitamin C group (VC): 3 mg vitamin C (960-25 G, Sigma-Aldrich Inc.) per egg (dissolved in 0·1 ml normal saline). The injected time was at embryonic age 11, and the injected site was the yolk sac. All treatment solutions were freshly prepared, and all injection procedures were completed in the laboratory at room temperature. Specific incubation and in ovo injection procedures referred to a previous description(Reference Li, Zhi and Liu14). The incubation period was 21 d. At the end of the incubation period, sixty healthy newly hatched chicks (not distinguishing sex) with each individual body weight (BW) close to average BW were selected from each group and randomly allocated to six replicates of ten chicks each. The composition of experimental diet is listed in Table 1. Broiler chickens had free access to water and commercial feed in a temperature-controlled room at the Livestock and Poultry Ecological Breeding Demonstration Base. Feeding management procedures referred to a previous description(Reference Li, Zhi and Liu14). The feeding period was 42 d. On post-hatch day 1 (D1), 21 and 42, one broiler was selected from each replicate with its BW close to average BW. After fasting for 12 h, blood samples were taken from the jugular vein on D1 and the wing vein on D21 and 42, respectively. The plasma samples were obtained after centrifuging at 664 g for 10 min at 4°C and then frozen at –20°C for further analysis. After blood sampling, birds were slaughtered by cervical dislocation and the spleen samples were removed, snap-frozen in liquid N2 and then stored at –80°C for further analysis.

Fig. 1. Scheme of experimental design and sample collection. Breeder eggs generated broiler chicks after a 21 d incubation period and broiler chicks grew into broiler chickens after a 42 d growing period. At embryonic age 11 (E11), 240 breeder eggs were randomly divided into normal saline (NS) and vitamin C (VC) groups and an injection procedure was carried out. After the 21 d incubation period, sixty healthy newly hatched chicks were selected from each group and randomly allocated to six replicates of ten chicks each. On D1, 21 and 42, one broiler per replicate was slaughtered and samples were collected. On D35, one broiler per replicate was selected for blood collection. NS, group in which every egg was injected with 0·1 ml of normal saline into the yolk at E11. VC, group in which every egg was injected with 3 mg vitamin C (dissolved in 0·1 ml of normal saline) into the yolk sac at E11.
Table 1. Composition and nutrient levels of broiler diets (as-fed basis)

DDGS, distillers dried grains with solubles; HMA, methionine hydroxyl analogue; ME, metabolisable energy; CP, crude protein.
* Premix provided the following per kg of diets: vitamin A 5·06 mg, vitamin D 75 μg, vitamin E 38 mg, vitamin K3 3 mg, vitamin B1 3 mg, vitamin B2 10 mg, vitamin B6 5 mg, vitamin B12 0·04 mg, niacinamide 40 mg, calcium pantothenate 16 mg, folic acid 2 mg, biotin 0·3 mg, Fe 66 mg, Cu 15 mg, Mn 95·4 mg, Zn 96·6 mg, iodine 0·38 mg and Se 0·41 mg.
† Nutrient levels were calculated values.
Measurement of hatchability and post-hatch performance
At the end of the incubation period, the number of hatched chicks was counted. The hatchability was calculated as the number of hatched chicks as a percentage of the fertilised eggs. BW (D1, 21 and 42) and feed intake (D1 to 21 and D21 to 42) were recorded for each replicate to calculate average daily feed intake (ADFI), average daily gain (ADG) and feed conversion ratio (FCR).
Biochemical assay of plasma samples
The vitamin C content, total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px), superoxide dismutase and malondialdehyde, the lysozyme activity, and IgA, IgG and IgM contents in plasma were measured using commercially available kits (A009, A015-1, A005, A001-1-1, A003-1, A050-1, E027, E026 and E025, respectively; Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions. Sample quality control and testing parameter settings were strictly in accordance with the product specification.
Lymphocyte proliferation response
On D35, one broiler with its BW close to average BW was selected from each replicate and blood samples were collected from the wing vein for lymphocyte proliferation response. The proliferative efficiency of lymphocytes was detected by MTT assay(Reference Mosmann15). Specific operation procedures referred to the previous description(Reference Dang, Prasad and De16).
Real-time PCR for mRNA quantification in spleen
RNA extraction from spleen tissue was executed according to the TRIzol Reagent protocol (Invitrogen). The concentration and purity of RNA were verified with the NanoDrop ND-1000 spectrophotometer (Nano-drop Technologies). Aliquots of RNA samples were subjected to electrophoresis with 1·4 % agarose–formaldehyde gels stained with ethidium bromide to verify their integrity. After extraction, cDNA was synthesised by a Primer Script RT Reagent Kit (TaKaRa) following the manufacturer’s instructions and stored at –20°C. The mRNA levels of cytokines (IL-2, IL-4, IL-6, IL-1β, TNF-α and interferon-γ (IFN-γ)), DNA methyltransferases (DNMT1, DNMT3A and DNMT3B) and DNA demethylases (ten-eleven translocation protein 1 (Tet1), Tet2, Tet3, growth arrest and DNA-damage-inducible protein β (Gadd45β), thymine-DNA glycosylase (TDG) and methyl-CpG-binding domain protein 4 (MBD4)) in spleen were determined with an SYBR® Premix Ex Taq Kit on the iCycler IQ5 (Bio-Rad Laboratories). Melting curves and agarose gel electrophoresis were performed to insure a single specific PCR product for each target gene. A reaction system of 25 μl was as follows: 12·5 µl of SYBR® Premix Ex Taq II (2×), 1 µl of forward primer (10 µmol/l), 1 µl of reverse primer (10 µmol/l), 1 µl of cDNA and 9·5 µl of double-distilled water. Protocols were set as follows: 95°C for 10 min; forty cycles of 95°C for 10 s, 60°C for 30 s, 72°C for 30 s; and 72°C for 5 min. The primers for real-time PCR were synthesised by Sangon Biotech and are listed in Table 2. All samples were run in triplicate. After normalling to β-action, the average cycle threshold values were used for quantification by the 2–ΔΔCt method(Reference Livak17).
Table 2. Primer sequence of target genes

F, forward; R, reverse; IFN-γ, interferon-γ; DNMT1, DNA methyltransferase 1; DNMT3A, DNA methyltransferase 3 A; DNMT3B, DNA methyltransferase 3B; Tet1, ten-eleven translocation protein 1; Tet2, ten-eleven translocation protein 2; Tet3, ten-eleven translocation protein 3; Gadd45β, growth arrest and DNA-damage-inducible protein β; TDG, thymine-DNA glycosylase; MBD4, methyl-CpG-binding domain protein 4.
Statistical analysis
The hatchability was analysed using the χ 2 test with SPSS 21.0 (SPSS Inc.). And, all other data were analysed by independent-samples t test with SPSS 21.0. A probability value of P < 0·05 and P < 0·1 was considered to be statistical significance and trends, respectively. According to our previous data(Reference Li, Zhi and Liu14), six replicates per treatment would be sufficient to reflect the influences of IOF of vitamin C on post-hatch performance (BW, ADFI, ADG and FCR) and immune status (stimulation index (SI) for T or B lymphocytes, lysozyme activity and immunoglobulin content in plasma). As expected, in this study, a statistical power of >0·80 and a significance of P < 0·05 were obtained on ADFI (D21–42 and D1–42), ADG (D1–21), FCR (D1–21), SI for T lymphocytes (D35), lysozyme activity (D21), IgA (D1) and IgM (D1 and 21) using mean and standard deviation for analysis on online software (https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html). In order to verify the reliability of the independent-samples t test analysis, the non-parametric Mann–Whitney U test (SPSS 21.0) was also conducted to detect the differences on post-hatch performance, plasma vitamin C content, plasma antioxidant status and immune function parameters (results presented in online Supplementary Table S1–S3). Overall, no major difference was noticed between the parametric test (independent-samples t test) and non-parametric test (Mann–Whitney U test).
Results
Hatchability and post-hatch performance
As shown in Table 3, the hatchability of the NS and VC groups was 74·1 and 93·0 %, respectively (χ 2 = 15·018, P < 0·001). IOF of vitamin C significantly increased hatchability. As shown in Table 4, although there was no significant difference on BW (D1), ADFI (D1–21), ADG (D21–42) and FCR (D21–42 and D1–42) between the VC and NS groups (P > 0·05), an increasing trend on BW (D21 and 42) and ADG (D1–42) was found in the VC group (P = 0·051, P = 0·097 and P = 0·096, respectively). Compared with the NS group, ADFI (D21–42 and D1–42) and ADG (D1–21) were significantly higher and FCR (D1–21) was significantly lower in the VC group (P < 0·05).
Table 3. Effect of in ovo feeding of vitamin C at embryonic age 11 (E11) on the hatchability of fertilised eggs
(Mean values and standard errors)

NS, normal saline group; VC, vitamin C group.
a,b Hatchability of fertilised eggs within a column with unlike superscript letters are significantly different (P < 0·05).
Table 4. Effect of in ovo feeding of vitamin C at embryonic age 11 (E11) on the post-hatch performance of broilers*
(Mean values and standard errors)

NS, normal saline group; VC, vitamin C group; BW, body weight; ADFI, average daily feed intake; ADG, average daily gain; FCR, feed conversion ratio.
* On D1, sixty chicks per group; on D21, fifty-four chicks per group; on D42, forty-eight chicks per group. P value is calculated by independent-samples t test (n 6), and a probability value of P < 0·05 and P < 0·1 is considered to be statistical significance and trends, respectively.
Vitamin C content, immune function and antioxidation of plasma
As shown in Table 5, vitamin C content was significantly higher on D1 and exhibited a rising trend on D21 in the VC group (P < 0·05 and P = 0·06, respectively), but no significant difference was observed on D42 between the VC and NS groups (P > 0·05). Compared with the NS group, T-AOC activity was significantly increased on D42 in the VC group (P < 0·05), but T-AOC activity on D21, GSH-Px and superoxide dismutase activities and malondialdehyde content on D21 and 42 had no difference between the VC and NS groups (P > 0·05).
Table 5. Effect of in ovo feeding of vitamin C at embryonic age 11 (E11) on vitamin C content and antioxidant function in plasma of broilers*
(Mean values and standard errors)

NS, normal saline group; VC, vitamin C group; T-AOC, total antioxidant capacity; GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; MDA, malondialdehyde.
* P value is calculated by independent-samples t test (n 6), and a probability value of P < 0·05 and P < 0·1 is considered to be statistical significance and trends, respectively.
On D35, SI for T lymphocytes was significantly higher in the VC group (P < 0·05), but SI for B lymphocytes had no difference between the VC and NS groups (P > 0·05, Table 6). Lysozyme activity (D21) significantly increased in the VC group (P < 0·05) but had no difference between the VC and NS groups on D1 and 42 (P > 0·05). IgA (D1) and IgM (D1 and 21) were significantly higher (P < 0·05), and IgA (D21) had an increasing trend in the VC group (P = 0·091), whereas IgA (D42), IgG (D1, 21 and 42) and IgM (D42) had no difference between the VC and NS groups (P > 0·05).
Table 6. Effect of in ovo feeding of vitamin C at embryonic age 11 (E11) on immune function of broilers*
(Mean values and standard errors)

NS, normal saline group; VC, vitamin C group; SI, stimulation index.
* P value is calculated by independent-samples t test (n 6), and a probability value of P < 0·05 and P < 0·1 is considered to be statistical significance and trends, respectively.
Real-time PCR for mRNA quantification in spleen
On D21, IOF of vitamin C significantly increased the expression of IL-4 and decreased the expression of IL-1β (P < 0·05) but had no influence on the expression of IL-2, IL-6, TNF-α and IFN-γ (P > 0·05, Fig. 2(a)). On D42, the expression of IL-4 was increased, the expression of IFN-γ was decreased in the VC group (P < 0·05), but the expression of IL-2, IL-6, IL-1β and TNF-α had no influence between the VC and NS groups (P > 0·05, Fig. 2(b)).
On D21, the expression of DNMT1 was increased (P < 0·05), the expression of DNMT3A had a rising trend (P = 0·065) and the expression of Tet2, Tet3 and Gadd45β was decreased in the VC group (P < 0·05), whereas the expression of DNMT3B, Tet1, TDG and MBD4 had no change between the VC and NS groups (P > 0·05, Fig. 3(a)). On D42, IOF of vitamin C significantly increased the expression of DNMT3A and decreased the expression of Tet3, MBD4 and TDG (P < 0·05) but had no influence on the expression of DNMT1, DNMT3B, Tet1, Tet2 and Gadd45β (P > 0·05, Fig. 3(b)).
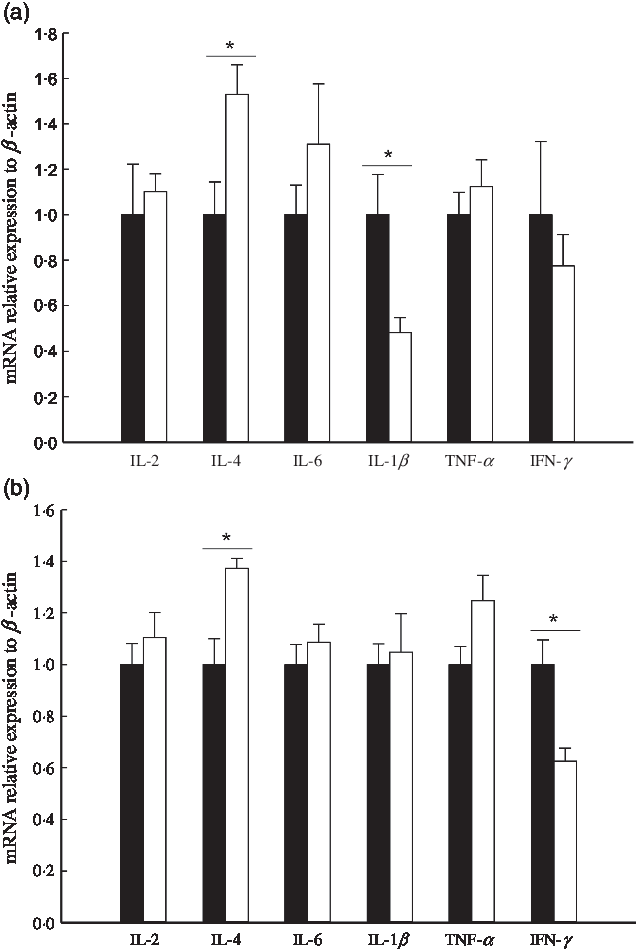
Fig. 2. Effect of in ovo feeding of vitamin C on the mRNA expression of IL-2, IL-4, IL-6, IL-1β, TNF-α and interferon-γ (IFN-γ) on D21 (a) and D42 (b) in spleen of broilers. NS stands for the group in which every egg was injected with 0·1 ml of normal saline into the yolk at embryonic age 11 (E11). VC stands for the group in which every egg was injected with 3 mg vitamin C (dissolved in 0·1 ml of normal saline) into the yolk sac at E11. Values are means with their standard errors. * Mean values are significantly different (P < 0·05, n 6). ![]() , NS;
, NS; ![]() , VC.
, VC.

Fig. 3. Effect of in ovo feeding of vitamin C on the mRNA expression of gene-related DNA methylation (DNA methyltransferase 1 (DNMT1), DNA methyltransferase 3A (DNMT3A) and DNA methyltransferase 3B (DNMT3B)) and DNA demethylation (ten-eleven translocation protein 1 (Tet1), ten-eleven translocation protein 2 (Tet2), ten-eleven translocation protein 3 (Tet3), growth arrest and DNA-damage-inducible protein β (Gadd45β), thymine-DNA glycosylase (TDG) and methyl-CpG-binding domain protein 4 (MBD4)) on D21 (a) and D42 (b) in spleen of broilers. NS stands for the group in which every egg was injected with 0·1 ml of normal saline into the yolk at embryonic age 11 (E11). VC stands for the group in which every egg was injected with 3 mg vitamin C (dissolved in 0·1 ml of normal saline) into the yolk sac at E11. Values are means with their standard errors. * Mean values are significantly different (P < 0·05, n 6). ![]() , NS;
, NS; ![]() , VC.
, VC.
Discussion
Newborn breeding eggs do not contain a detectable dose of vitamin C. Although vitamin C could be synthesised with the embryonic development, it was insufficiently synthesised under artificial incubation conditions(Reference Nowaczewski, Kontecka and Krystianiak11). In the present study, IOF of vitamin C significantly increased vitamin C content in the plasma of newly hatched chicks, suggesting that vitamin C via in ovo injection had already been absorbed by the broiler’s embryo and the nutritional intervention of vitamin C had been successfully realised. During late incubation, a sharp increase in metabolic heat is a main cause of dead embryos leading to reduced hatchability(Reference Tullett18). Vitamin C that combated heat stress in late incubation to decrease embryonic mortality and improve hatchability has been reported(Reference Nowaczewski, Kontecka and Krystianiak11), which was supported by the hatchability results in this study.
Bees develop into queen bees or worker bees depending on whether they can eat royal jelly, which is a typical example of nutri-epigenetics on post-hatch performance(Reference Lyko, Foret and Kucharski6) and provides the possibility for nutritional intervention on post-hatch performance by nutri-epigenetics. Some research showed that IOF of 6 mg vitamin C per egg at E18 had no significant effect on the post-hatch performance from D1 to 10 in broiler chickens, but IOF of 3 mg vitamin C significantly increased ADG and ADFI(Reference Hajati, Hassanabadi and Golian19,Reference Khaligh, Hassanabadi and Nassiri-Moghaddam20) . The above results suggested that the effect of IOF of vitamin C on the post-hatch performance of broiler chickens was related to the injection dose of vitamin C and 3 mg vitamin C was better, which was similar on the hatchability(Reference Ipek, Sahan and Yilmaz21,Reference Elibol, Turkoglu and Akan22) . In this study, the result was found that IOF of vitamin C improved ADG from D1 to 21 and D1 to 42 and ADFI from D21 to 42 and D1 to 42, which is consistent with Hajati’s results. However, FCR, in this study, is different from Hajati’s results. In the previous study, the results showed that IOF of vitamin C at E15 had no significant effect on the post-hatch performance(Reference Zhu, Li and Sun23). Armed with these results, it is speculated that injection time may influence the effect of vitamin C treatment on FCR. DNA methylation variation trends in previous study also prompted that there were differences in the potential of nutritional interventions at different embryonic times(Reference Li, Zhu and Zhi13). Based on these results, the injection dose and time jointly affected the injection effects and IOF of 3 mg vitamin C at E11 had a favourable effect on post-hatch performance.
Poor anti-stress ability is a defect in modern broiler chickens. The plasma antioxidant system is an important part of a broiler’s antioxidant system, which plays an important role in enhancing anti-stress ability(Reference Ahmad, Rasheed and Gupta24). And T-AOC, GSH-Px and superoxide dismutase activities and malondialdehyde content are usually used as indicators for measuring plasma antioxidant capacity(Reference Yang, Li and Duan25,Reference Zhang, Elliott and Durojaye26) . To the best of our knowledge, there have been few studies on the effect of IOF of vitamin C on plasma antioxidant capacity of broilers. The only available research results showed that IOF of vitamin C increased GSH-Px and T-AOC activities and decreased malondialdehyde content in plasma of chicks on D1(Reference El-Senousey, Chen and Wang27). However, plasma antioxidant capacity was merely tested on D21 and 42 in this study, and only T-AOC was elevated on D42 in the VC group. Despite the lack of plasma antioxidant data on D1 in this study, it was reasonably speculated that the improvement of plasma antioxidant capacity on D1 was better than that on D21 and 42. In addition, T-AOC in plasma reflects the amount of plasma antioxidants. IOF of vitamin C significantly elevated plasma vitamin C content on D1 and merely had an increasing trend on D21 and had no influence on D42. This is may be a reason for the limited improvement of plasma antioxidant capacity on D21 and 42 in the VC group.
Immune cells (B and T lymphocytes) and immunologically active substances (lysozyme and immunoglobulins) in plasma are important components of the body’s immune system(Reference Abbas, Lichtman, Pillai, Abbas, Lichtman and Pillai28). Lymphocyte SI reflect the ability of lymphocytes to proliferate and differentiate when they are stimulated by mitogens or antigens and to some extent reflect the immune function of lymphocytes(Reference Crawford, Adams and Richardson29). Lysozyme and immunoglobulins play an important role in humoral immunity. In this study, T lymphocyte SI on D35 was increased in the VC group, reflecting that IOF of vitamin C enhanced the immune response of T lymphocytes, and IgA (D1 and 21), IgM (D1 and 21) and lysozyme activity (D21) were increased in the VC group, suggesting that IOF of vitamin C enhanced humoral immunity in plasma. A research had proved that dietary vitamin C supplementation increased IgM contents in the serum of piglets(Reference Lauridsen and Jensen30). In addition, Li et al. found that dietary vitamin C supplementation increased IgA and IgM contents in the serum of laying ducks under cold stress(Reference Li, Song and Fan31). Before and after the chicks shelled, environmental changes can also cause stress in chicks. IOF of vitamin C increased IgA and IgM contents on D1, which is consistent with the study of Li et al. (Reference Li, Song and Fan31). As for lysozyme activities, much literature has shown that vitamin C could enhance serum lysozyme activity(Reference Peng, Shi and Gao32,Reference Cecchini, Terova and Caricato33) .
The spleen is the largest lymphoid organ and plays a pivotal role in cellular and humoral immunity in poultry. Compared with mammals, birds lack lymphatic vessels and lymph nodes, putting the spleen in a more prominent role in the immune system. Cytokines are immunomodulatory proteins or small peptides, and fluctuations at their mRNA expression levels can reflect the immune status of the spleen(Reference Alkhalifa34,Reference Guo, Shi and Yan35) . Cytokines can be divided into pro- and anti-inflammatory cytokines based on their role in inflammatory responses(Reference Carrillovico, Lardone and Naji36,Reference You, Luo and Zhang37) . IL-1β, IL-6, TNF-α and IFN-γ belong to pro-inflammatory cytokines, and IL-2 and IL-4 belong to anti-inflammatory cytokines. In this study, it was found that IOF of vitamin C significantly increased the expression of IL-4 on D21 and 42 and decreased the expression of IL-1β on D21 and IFN-γ on D42. Low expression of pro-inflammatory cytokines and high expression of anti-inflammatory cytokines together indirectly reflected that the spleen was at a lower immune stress status. This decreasing tendency of immune stress means positive utilisation of energy and may be beneficial for improving the post-hatch performance of broilers eventually(Reference Liu, Qin and Wang38). At the same time, it was speculated that increased plasma antioxidant and immune function may reduce the spleen’s stimulus intensity from antigens and oxygen free radicals during the spleen’s filtration process, also leading to the fluctuations at mRNA expression of the cytokines.
There is growing evidence pointing out that vitamin C could influence the genome activity by regulating epigenetic processes. Vitamin C, serving as a cofactor for DNA demethylases, could participate in DNA demethylation and cause widespread DNA demethylation(Reference Young, Züchner and Wang10,Reference Chung, Brena and Kolle39) . In this study, E11 was selected as the injection time based on the DNA methylation variation trends during the embryonic development of broiler chickens(Reference Li, Zhu and Zhi13). Therefore, the expression of enzyme-related DNA methylation and demethylation was tested in the spleen. The results were found that IOF of vitamin C promoted the expression of enzyme-related DNA methylation (DNMT1 and DNMT3A) and reduced the expression of enzyme-related DNA demethylation (Tet 2, Tet 3, Gadd45β, MBD4 and TDG). The results suggested that the DNA methylation level may have an increasing trend in the spleen. In other words, it was opposite to the role of vitamin C in demethylation(Reference Paker40). During the late part of incubation, raising the incubator temperature could enhance the tolerance of broilers under heat stress(Reference Zaboli, Rahimi and Shariatmadari41). This change called epigenetic temperature adaptation based on that ‘set point’ or ‘response threshold’ of the thermoregulation system can be altered through influencing gene expression patterns by epigentics, thereby establishing an adaptability to a particular temperature environment(Reference Piestun, Halevy and Yahav42). Therefore, it was speculated that IOF of vitamin C provided a high vitamin C concentration environment for the embryos. After the embryos adapted to the environment, there was no vitamin C supply in the whole growing period and the DNA methylation level of spleen could not be maintained. The plasma vitamin C content was in favour of the above speculation.
Obviously, there are some limitations in this study. Whether the fluctuating expression of pro- and anti-inflammatory cytokines is related to DNA methylation change remains to be further studied. In addition to participating in DNA demethylation, vitamin C is also involved in histone demethylation(Reference Young, Züchner and Wang10). However, this study undetected the expression of enzymes related to histone demethylation.
The chicken (Gallus gallus) is an important model animal widely used in human embryology and immunology research(Reference Burt and Pourquie43,Reference Li, Li and Hu44) . Humans cannot synthesise vitamin C due to the lack of glucose lactone oxidase(Reference Flett, Campbell and Phillips45). Breeding eggs do not contain a detectable dose of vitamin C, and embryonic development is completely separated from the mother. Fortunately, IOF technology has opened a door for studying vitamin C on embryology and immunology. To some extent, this study is beneficial for understanding the nutritional role of vitamin C, supplied during embryonic age, on growth and immunity in humans.
In summary, IOF of vitamin C at E11 could improve post-hatch performance and, to some extent, the antioxidant capacity of broiler chickens. The increase of plasma vitamin C content on D1 demonstrates that vitamin C via in ovo injection could be absorbed by the embryos of broilers. IOF of vitamin C at E11 also improves immune status via increasing T lymphocyte SI, lysozyme activity and the content of IgA and IgM in plasma, up-regulating the splenic expression of anti-inflammatory cytokine IL-2 and down-regulating the splenic expression of pro-inflammatory cytokine IL-1β and IFN-γ. The increasing expression of enzyme-related DNA methylation (DNMT1 and DNMT3A) and the decreasing expression of enzyme-related DNA demethylation (Tet 2, Tet 3, Gadd45β, MBD4 and TDG) indicated that the level of DNA methylation may increase in spleen in the VC group. However, whether the fluctuating expression of pro- and anti-inflammatory cytokines is related to DNA methylation change remains to be further studied.
Acknowledgements
We acknowledge the members of the Innovative Research Team of Animal Nutrition & Healthy Feeding of Northwest A&F University for providing valuable assistance in sample collection and analysis.
This work was supported by the National Key R&D Plan of China from People’s Republic of China Ministry of Science and Technology (grant numbers 2017YFD0500500 and 2017YFD0502200) and the Program for Shaanxi Science & Technology from Shaanxi Provincial Science and Technology Department (grant numbers 2018ZDCXL-NY-0201, 2018ZDXM-NY-051 and 2017TSCXL-NY-04-04). None of these funders had a role in the design, analysis or writing of this article.
Y. Z. and S. L. designed the study and finished data collection and statistical analysis. Y. D., Z. R. and X. Y. reviewed the manuscript. X. Y. is the corresponding author. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S000711452000210X












