Guanidinoacetic acid (GAA) is the precursor of creatine and is produced via arginine transferring guanidine group to glycine(Reference Wallimann, Tokarska-Schlattner and Schlattner1,Reference Ostojic2) . Creatine is phosphorylated to phosphocreatine which transfers high-energy phosphate group to ADP to regenerate ATP(Reference Wallimann, Tokarska-Schlattner and Schlattner1,Reference Ostojic2) . Recent studies observed that GAA addition increased blood creatine concentration(Reference Speer3,Reference Li, Wang and Wu4) as well as average daily gain (ADG), ruminal total SCFA concentration and apparent total-tract nutrient digestibility in bulls(Reference Li, Wang and Wu4). Others reported that GAA addition increased ADG in pigs(Reference Jayaraman, La and La5) or broiler chickens(Reference Ale Saheb Fosoul, Azarfar and Gheisari6) and improved carcass characteristics and meat quality in pigs(Reference Zhu, Gu and Hu7). Studies in C2C12 cells reported that insulin-like growth factor-1 (IGF-1) mRNA expression increased with creatine addition(Reference Louis, Van Beneden and Dehoux8) and that the phosphorylation levels of protein kinase B (Akt), mammalian target of rapamycin (mTOR) and ribosomal protein S6 kinase (P70S6K) increased with the addition of creatine(Reference Deldicque, Theisen and Bertrand9) or GAA(Reference Wang, Ma and Qiu10). In view of the results, it was speculated that dietary GAA addition could stimulate hepatic gene expression of IGF-1/Akt/mTOR/P70S6K pathway of bulls. The conversion of GAA to creatine requires a methyl group from S-adenosylmethionine and yields homocysteine (Hcy)(Reference Wallimann, Tokarska-Schlattner and Schlattner1,Reference Ostojic2) . Therefore, dietary addition of GAA might cause a deficiency of methyl group and an increase of Hcy in bulls(Reference Speer3). In addition, the decrease in blood folate concentration was observed with supplementing GAA in diets of bulls(Reference Li, Wang and Wu4).
In the methionine cycle, 5-methyltetrahydrofolate provides a methyl group to Hcy to regenerate methionine and the decrease of folate level would cause Hcy level to increase and methylation rate to decrease(Reference Wallimann, Tokarska-Schlattner and Schlattner1,Reference Reed, Nijhout and Sparks11) . Studies observed that blood levels of folate increased and methionine tended to be increased with the addition of folic acid (FA) in cows(Reference Graulet, Matte and Desrochers12) and that blood levels of folate increased and Hcy decreased with dietary coated folic acid (CFA) addition in calves(Reference La, Li and Wang13) or bulls(Reference Wang, Liu and Zhang14). Moreover, addition of CFA increased ADG and hepatic expression levels of IGF-1, phosphoinositide 3-kinase (PI3K), mTOR and P70S6K in calves(Reference La, Li and Wang13).
Based on these researches, it was speculated that supplementing CFA in GAA addition diets could contribute to the conversion of GAA to creatine and increased ADG more compared with CFA or GAA addition alone. To study the interaction between CFA and GAA on growth performance would be not only of basic scientific interest but also has implications for the beef industry. Therefore, the present study investigated the impacts of supplementing GAA or/and CFA on growth performance, nutrient digestibility and mRNA levels of hepatic IGF-1, PI3K, Akt, mTOR and P70S6K in Angus bulls.
Materials and methods
Beef cattle, experimental design and diets
This experiment was carried out from April 2019 through July 2019, at Qixian Wanmu Beef Farm. Beef cattle care and use scheme were approved by Shanxi Agriculture University Animal Care and Use Committee. Fifty-two Angus bulls (359 (sd 9·6) d of age and 430 (sd 8·7) kg of body weight (BW)) were blocked according to BW and then randomly assigned to one of the four groups in a 2 × 2 factorial design. The CFA of 0 (CFA−) or 6 mg/kg dietary DM FA (CFA+) was supplemented in diets with GAA of 0 (GAA−) or 0·6 g/kg DM (GAA+), respectively. The diets of Angus bulls (Table 1) were formulated according to the National Research Council(15). The level of CFA and GAA supplementation was determined based on the results of Wang et al.(Reference Wang, Liu and Guo16) and Li et al.(Reference Li, Wang and Wu4), respectively. The additive of GAA (feed grade, 980 g/kg; Hebei Panheng Technology Co. Ltd) was purchased commercially, and CFA (20 g FA/kg) was made based on Wang et al.(Reference Wang, Liu and Zhang14). The release rate of FA from CFA was measured by using rumen and duodenum cannulated steers and was 0·23 and 0·67 in the rumen and intestine, respectively. This study lasted for 80 d including a 20 d of adaptation period followed a 60 d of sample collection period. Angus bulls were housed in single pens (2·5 m × 3 m) and had free access to diets and water.
Table 1. Ingredient and chemical composition of the basal diet
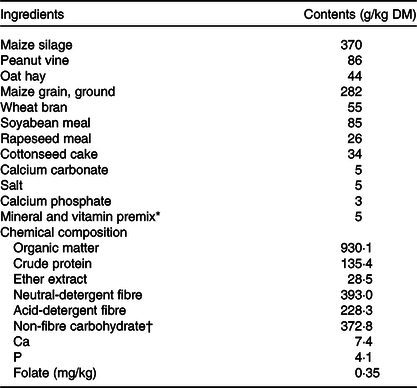
* Contained per kg premix: 1600 mg Cu, 8000 mg Mn, 7500 mg Zn, 1·20 mg iodine, 20 mg Co, 1640 mg vitamin A, 600 mg vitamin D and 200 mg vitamin E.
† Non-fibre carbohydrate, calculated by 1000 − crude protein − neutral-detergent fibre − fat − ash.
Sampling and measurements
All of the thirteen Angus bulls in each group were used to sample collection. On day 1, thirty and sixty of the sample collection period, bulls were weighed before the morning feeding. Feed offered and refused of each bull was recorded daily and sampled every 10 d. Total-tract digestibilities of DM, organic matter (OM), crude protein (CP), neutral-detergent fibre (NDF) and acid-detergent fibre (ADF) were measured by internal acid-insoluble ash method(Reference Van-Keulen and Young17). From day 54 through day 57, about 250 g faecal sample was collected from the rectum at 06.00, 12.00, 18.00 and 24.00 hours daily. Feeds, refusals and faeces sample were stored at −20°C, mixed by bull, dried at 65°C for 48 h and then ground to pass through a 1 mm sieve with a cutter mill (WF-30; Guangzhou Jiuyuan Machinery Equipment Co. Ltd) for chemical analysis.
On days 58 and 59 of the collection period, samples of ruminal fluid for each bull were collected by using an oral stomach tube at 06.40, 12.40, 18.40 and 00.40 hours daily. The first 200 ml of ruminal fluid was discarded, and the following 150 ml was reserved. After measuring pH using a pH meter (PHS-3C Shaoxing Wanli Instrument Co. Ltd), ruminal fluid was strained through four layers of cheesecloth. The filtrate (5 ml) used for measuring SCFA and ammonia N was mixed with 1 ml of 250 g/l meta-phosphoric acid or 1 ml of 20 g/l (w/v) H2SO4, respectively, and then kept at −20°C.
On day 60 of the collection period, blood sample was collected from the coccygeal vessel at 10.00 hours using 10 ml evacuated tubes (Jiangsu Xinkang Medical Equipment Co. Ltd), centrifuged at 2500 g and 4°C for 10 min to separate serum and then stored at −20°C. The liver was biopsied under local anaesthesia by blind percutaneous needle biopsy (14 G × 152 mm; Dispomed Witt oHG) as described by Gross et al.(Reference Gross, Dorland and Schwarz18) and was kept at −80°C until analysis.
Chemical analyses
Samples of feeds offered and refused as well as faeces were measured for DM (method 934.01), ether extract (method 973.18), nitrogen (method 976.05), ADF (method 973.18) and crude ash (method 942.05) on the basis of Association of Official Analytical Chemists (AOAC)(19). The difference between DM and crude ash was used to estimate OM content. The NDF content was determined according to the methods of Van Soest et al.(Reference Van Soest, Robertson and Lewis20) with heat stable α-amylase and sodium sulphite used and expressed inclusive of residual ash. Content of acid-insoluble ash in faeces and feeds was determined according to the procedure described by Van-Keulen and Young(Reference Van-Keulen and Young17). Ruminal SCFA concentration was determined by GC (GC102AF; Shanghai Specialties Ltd) with 2-ethylbutyric acid as an internal standard, and ammonia N was determined by colorimetric spectrophotometer (UV765CRT; Shanghai Jingxue Scientific Instrument Co. Ltd) on the basis of AOAC(19). Serum glucose, total protein, albumin, folate, Hcy, methionine, arginine, creatine and IGF-1 were determined by the Infinite 200 PRO Microplate reader (Tecan Austria GmbH) using double antibody sandwich method. All ELISA kits were specific kits for bovine and provided by Shanghai Meilian Biology Science and Technology Co. Ltd. The concentration of creatine in the liver was measured by reverse-phase HPLC (Ultimate 3000, Thermo Fisher Scientific Inc.) according to the method of Liu et al.(Reference Liu, Li and Li21).
Extraction of RNA and quantitative real-time PCR
Based on the instruction of manufacturer, a total RNA isolation kit (Invitrogen) was used to extract total hepatic RNA from the liver biopsies. The quality and content of the isolated RNA were measured by using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies). The ratios of absorbance at 260 and 280 nm of all preparations were approximately 2·0. The integrality of RNA was examined by denaturing agarose gel electrophoresis and ethidium bromide staining. The iScriptTM complementary DNA (cDNA) Synthesis Kit (BioRad Laboratories GmbH) was applied to synthesise cDNA from 500 ng total hepatic RNA from each sample per 10 μl sample reaction according to the manufacturer’s protocol. Reaction conditions were 15 min at 37°C and 5 min at 85°C. Negative control reactions without reverse transcriptase were carried out on each sample to detect possible contamination of genomic DNA or environmental DNA.
The abundance of mRNA for PI3K, Akt1, mTOR, P70S6K, IGF-1 and IGF-1 receptor (IGF-1R) was quantified by quantitative real-time PCR using the iCycler and the iQ-SYBR green detection (BioRad Laboratories). The primer sets used for real-time PCR are listed in Table 2. Subsequent qPCR was performed on a MxPro-Mx3000P multiplex quantitative PCR systems (Stratagene) at a minimum in triplicate. A reaction mixture (20 μl) contained 2 μl cDNA, 10 µl SYBR Premix TaqTM II (TaKaRa Biotechnology Co. Ltd), 0·8 µl PCR Forward Primer (10 μm), 0·8 µl PCR Reverse Primer (10 μm), 0·4 µl ROX Reference Dye II (TaKaRa Biotechnology Co. Ltd) and 6·0 µl dH2O. The PCR was performed under the following cycle conditions: 1 cycle of 95°C for 20 s, 45 cycles of 95°C for 20 s, annealing temperature at 62°C for 20 s and followed by a melting curve analysis(Reference Denman and McSweeney22). At the end of each denaturation and extension step, fluorescence detection was carried out. Relative quantity of mRNA for IGF-1, IGF-1R, PI3K, Akt1, mTOR and P70S6K was made as a percentage of 18S rRNA on the basis of the equation:
where Ct represents the threshold cycle.
Table 2. PCR primers for real-time PCR assay
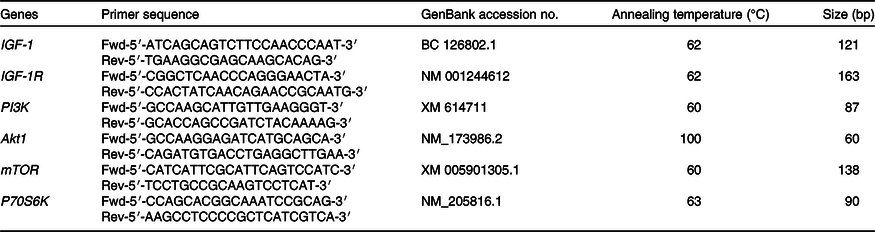
IGF-1, insulin-like growth factor-1; Fwd, forward; Rev, reverse; IGF-1R, insulin-like growth factor-1 receptor; PI3K, phosphoinositide 3-kinase; Akt1, protein kinase B1; mTOR, mammalian target of rapamycin; P70S6K, ribosomal protein S6 kinase.
Statistical analyses
Feed efficiency was calculated as ADG divided by DM intake (DMI). Data for DMI were averaged for every 30 d, and then DMI, BW, ADG and FCR were analysed by the mixed procedure of SAS (Proc Mixed; SAS, 2002)(23) with a 2 (CFA addition) × 2 (GAA addition) randomised complete block design, the model as follows:
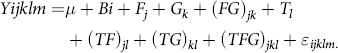 $$\[Yijklm = \mu + Bi + {F_j} + {G_k} + {(FG)_{jk}} + {T_1} + {(TF)_{jl}} + {(TG)_{kl}} + {(TFG)_{jkl}} + {\varepsilon _{ijklm.}}\]$$
$$\[Yijklm = \mu + Bi + {F_j} + {G_k} + {(FG)_{jk}} + {T_1} + {(TF)_{jl}} + {(TG)_{kl}} + {(TFG)_{jkl}} + {\varepsilon _{ijklm.}}\]$$
Other measurements were analysed by using the model:
where Yijklm is the dependent variable, μ is the overall mean, B i is the effects of the i th block, F j is the fixed effects of CFA addition (j = with or without), G k is the fixed effects of GAA addition (k = with or without), (FG) jk is the CFA × GAA interaction, T l is the fixed effect of time, (TF) jl is the Time × CFA interaction, (TG) kl is the Time × GAA interaction, (TFG) jkl is the Time × CFA × GAA interaction and ϵ ijklm is the residual error. Other data for nutrient digestibility, rumen fermentation, blood metabolites and hepatic gene expression were analysed by using the same statistical model as described above, but time and the interaction between time and treatment were removed. Mean separations were done by probability of difference tests (PDIFF in SAS) for influences that were significant at P < 0·05. Significant difference between treatments was declared at P < 0·05.
Results
DM intake, average daily gain and feed efficiency
The BW of 60 d, ADG and feed efficiency increased (P < 0·05) with GAA or CFA addition, but the increased magnitude of these parameters was greater with supplementing CFA in diets without GAA compared with diets with GAA (Table 3). Dietary GAA or CFA inclusion did not affect DMI, and no difference was observed for BW on day 0 or on day 30 among treatments.
Table 3. Effects of guanidinoacetic acid (GAA) and coated folic acid (CFA) addition on DM intake, average daily gain (ADG) and feed efficiency (FE) in Angus bulls (n 13)
(Mean values with their standard errors)

* GAA− = without GAA; GAA+ = 0·6 g/kg of GAA; CFA− = without CFA; CFA+ = 6 mg/kg of folic acid from CFA.
† GAA: GAA effect; CFA: CFA effect; CFA × GAA: interaction between CFA and GAA addition. The P values of time for DM intake, ADG and FE were 0·001, 0·001 and 0·240. The time × CFA, time × GAA and time × CFA × GAA interactions for all the studied variables were not significant (P > 0·05).
Nutrient digestion and rumen fermentation
The CFA × GAA interaction was not significant for apparent nutrient digestibility and rumen fermentation parameters (Table 4). Digestibilities of DM, CP, NDF and ADF were increased (P < 0·05) by GAA or CFA addition. Digestibility of OM increased (P = 0·038) with GAA supplementation, but was unchanged with the addition of CFA. Rumen pH decreased (P < 0·05) and total SCFA concentration increased (P < 0·05) in bulls consuming diets with GAA or CFA inclusion. Dietary inclusion of GAA did not affect SCFA molar percentage, but decreased (P = 0·001) ammonia N concentration. Percentages of acetate, isobutyrate and isovalerate and acetate:propionate ratio increased (P < 0·05), percentage of propionate decreased (P = 0·026), but percentages of butyrate and valerate and ammonia N concentration were unchanged with CFA supplementation.
Table 4. Effects of guanidinoacetic acid (GAA) and coated folic acid (CFA) addition on nutrient digestibility and ruminal fermentation in Angus bulls (n 13)
(Mean values with their standard errors)

* GAA− = without GAA; GAA+ = 0·6 g/kg of GAA; CFA− = without CFA; CFA+ = 6 mg/kg of folic acid from CFA.
† GAA: GAA effect; CFA: CFA effect; CFA × GAA: interaction between CFA and GAA addition.
Blood parameters
Dietary CFA or GAA addition increased blood creatine concentration, but the increased magnitude was greater with supplementing CFA in GAA+ diets than in GAA− diets (Table 5). Blood concentrations of glucose, arginine and methionine were unaltered, but total protein, albumin and IGF-1 increased (P < 0·05) with GAA or CFA addition. Blood folate concentration decreased (P = 0·043) with the addition of GAA, but increased (P = 0·013) with CFA addition. Blood Hcy concentration was not affected by GAA, but decreased (P = 0·012) with dietary CFA inclusion.
Table 5. Effects of guanidinoacetic acid (GAA) and coated folic acid (CFA) addition on blood metabolites in Angus bulls (n 13)
(Mean values with their standard errors)
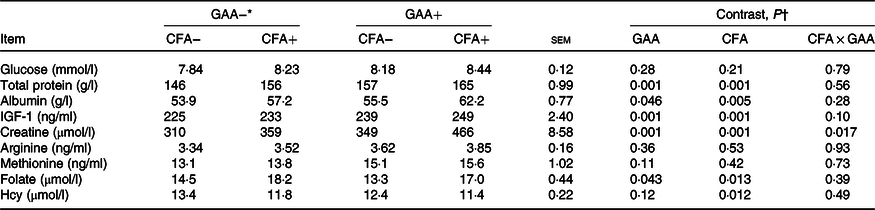
IGF-1, insulin-like growth factor-1; Hcy, homocysteine.
* GAA− = without GAA; GAA+ = 0·6 g/kg of GAA; CFA− = without CFA; CFA+ = 6 mg/kg of folic acid from CFA.
† GAA: GAA effect; CFA: CFA effect; CFA × GAA: interaction between CFA and GAA addition.
Hepatic gene expression and creatine content
Hepatic creatine concentration increased with CFA or GAA addition, and greater increase was observed for supplementing CFA in GAA− diets than in GAA+ diets (Table 6). Hepatic mRNA levels of IGF-1, PI3K, Akt1, mTOR and P70S6K increased (P < 0·05) with the supplementation of GAA or CFA. Expression of IGF-1R was unaffected by GAA, but increased (P = 0·037) with CFA supplementation.
Table 6. Effects of guanidinoacetic acid (GAA) and coated folic acid (CFA) addition on hepatic gene expression and creatine content in Angus bulls (n 13)
(Mean values with their standard errors)
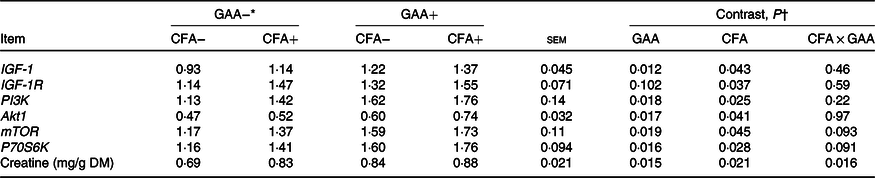
IGF-1, insulin-like growth factor-1; IGF-1R, insulin-like growth factor-1 receptor; Akt1, protein kinase B1; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; P70S6K, ribosomal protein S6 kinase.
* GAA− = without GAA; GAA+ = 0·6 g/kg of GAA; CFA− = without CFA; CFA+ = 6 mg/kg of folic acid from CFA.
† GAA: GAA effect; CFA: CFA effect; CFA × GAA: interaction between CFA and GAA addition.
Discussion
The limited response of DMI was not in line with the findings of Li et al.(Reference Li, Wang and Wu4), where dietary addition of 0·6 g/kg DM GAA increased DMI of bulls. The increment in ADG was probably associated with the increase in total-tract nutrient digestibility with GAA supplementation. The increase in creatine concentration of blood and liver indicated that dietary GAA addition increased the supply of creatine to bulls(Reference Tossenberger, Rademacher and Németh24) and was also a reason for the increase of ADG. Creatine is converted from GAA and serves as an energy reserve of the cell and tissue(Reference Wallimann, Tokarska-Schlattner and Schlattner1,Reference Ostojic2) . Wang et al. demonstrated that GAA supplementation increased intracellular creatine content and resulted in an increase in myoblast differentiation and skeletal muscle growth in C2C12 cells and mice(Reference Wang, Ma and Qiu10). Studies in pigs reported that the increased BW gain with GAA addition was due to the formation of creatine, which caused muscles protein retention increase(Reference Jayaraman, La and La5,Reference Young, Bertram and Theil25) . Likewise, Li et al. reported that ADG increased and feed conversion ratio decreased with supplementing GAA in bulls(Reference Li, Wang and Wu4). The increment in total-tract digestibilities of DM and OM was likely due to an enhancement of rumen nutrient degradation, as reflected by the increase in total SCFA concentration with GAA supplementation. Speer noted that apparent digestibilities of DM and OM were unchanged with abomasal infusion of GAA at 7·5 or 15 g/d in steers(Reference Speer3). Similarly, Li et al. observed increased apparent digestibilities of DM, OM, CP, NDF and ADF with supplementing GAA in diets of bulls(Reference Li, Wang and Wu4). In agreement with Li et al.(Reference Li, Wang and Wu4), the present study observed an increase in rumen total SCFA concentration, indicating that dietary GAA supplementation stimulated microbial growth and nutrient degradation. Li et al. reported dietary supplementation with 0·6 g/kg DM GAA increased rumen total bacteria and fungi population in bulls(Reference Li, Wang and Wu4). The increase in ammonia N was probably due to an increment of feed protein degradation. The increased nitrogen in diet due to GAA supplementation was only 0·21 g/kg DM. Moreover, Li et al. observed rumen protease activity and primary proteolytic bacteria population increased with GAA addition in bulls(Reference Li, Wang and Wu4). Serum glucose concentration was unchanged and was consistent with the unaltered rumen concentration of propionate (19·3 and 20·4 mm for GAA− and GAA+, respectively). The similar change of creatine concentration in blood and in the liver was in accordance with Tossenberger et al.(Reference Tossenberger, Rademacher and Németh24), who reported change in blood creatine level reflected the changes in the liver and muscle tissues when supplementing GAA in diets of broilers. Likewise, Others reported increased blood creatine concentration with GAA addition in steers(Reference Speer3,Reference Li, Wang and Wu4) . The process of converting GAA to creatine requires a methyl group from S-adenosylmethionine and yields Hcy(Reference Wallimann, Tokarska-Schlattner and Schlattner1). Hcy can accept a methyl group from 5-methyltetrahydrofolate to regenerate methionine(Reference Wallimann, Tokarska-Schlattner and Schlattner1,Reference Reed, Nijhout and Sparks11) . The reduction in blood folate concentration implied that the requirement of FA might be increased due to GAA supplementation. The regulation of FA on the one-carbon unit cycle should be the reason of the unaffected blood concentrations of methionine and Hcy with GAA addition. Similarly, Speer reported that post-ruminal infusion GAA at 15 g/d did not affect blood concentrations of arginine and methionine in steers(Reference Speer3). The change in blood IGF-1 concentration was in accordance with that in hepatic IGF-1 expression and was probably associated with the increase in hepatic creatine concentration with GAA supplementation. Studies found that creatine supplementation increased IGF-1 mRNA expression in C2C12 cells(Reference Louis, Van Beneden and Dehoux8) and human skeletal muscle(Reference Deldicque, Louis and Theisen26). The binding of IGF-1 to IGF-1R can activate PI3K and then result in the phosphorylation and activation of Akt(Reference Nave, Ouwens and Withers27). The target downstream genes of Akt, such as mTOR and P70S6K, are key regulators of protein synthesis metabolism(Reference Nave, Ouwens and Withers27). Therefore, the increment in expressions of PI3K, Akt1, mTOR and P70S6K might be associated with the stimulatory impact of GAA addition on IGF-1 mRNA expression. Studies in C2C12 cells demonstrated that phosphorylation levels of Akt, mTOR and P70S6K increased with GAA(Reference Wang, Ma and Qiu10) or creatine addition(Reference Deldicque, Theisen and Bertrand9). These data together with the observed increase in ADG suggested that dietary addition of GAA probably could promote protein synthesis metabolism in bulls.
The response of growth performance was in accordance with the results of previous studies in calves(Reference La, Li and Wang13) or bulls(Reference Wang, Liu and Zhang14), where unaltered DMI and increased ADG were observed with CFA supplementation. The increase of ADG was likely due to an increment in nutrient digestion and protein metabolism efficiency. The observed increase in blood concentrations of total protein, albumin and IGF-1 as well as hepatic gene expression of mTOR signalling pathway indicated that protein metabolism might be improved due to CFA addition. Likewise, other research reported that performance of cows was improved and was due to an increase in protein metabolism efficiency with dietary FA inclusion(Reference Graulet, Matte and Desrochers12) or rumen-protected B vitamin inclusion(Reference Sacadura, Robinson and Evans28). The change in DM digestibility was consistent with that in rumen total SCFA concentration, indicating that the FA requirement of microbes could not be met from their synthesis and the basal diets. The increase in total-tract NDF and ADF digestibility and rumen acetate percentage suggested that dietary CFA provision stimulated growth of rumen cellulolytic microbes. FA provides one-carbon groups for the synthesis of purine, thymidylate and methionine and plays a crucial role in cell growth and protein synthesis(Reference Brosnan, MacMillan and Stevens29). Early in vitro studies reported that FA was essential for the strains of Ruminococcus (Reference Bryant and Robinson30) and that FA stimulated rumen microbes to digest cellulose(Reference Macleod and Murray31). Recent studies observed that total-tract nutrient digestibility, rumen total SCFA concentration and cellulolytic bacteria count increased with CFA addition in steers(Reference Wang, Liu and Guo16) or in bulls(Reference Wang, Liu and Zhang14). The increment in rumen percentages of isobutyrate and isovalerate suggested that CP degradation was enhanced, and this was the reason for the increase of acetate percentage and digestibility of NDF and ADF with CFA supplementation. Rumen isobutyrate and isovalerate are built up from the deamination and decarboxylation reaction of valine and leucine and can be used as carbon skeletons by micro-organisms to synthesise branched-chain fatty acids, branched-chain amino acids and aldehydes(Reference Andries, Buysse and Debrabander32). Previous studies observed that supplementation with CFA increased rumen CP digestibility in steers(Reference Wang, Liu and Guo16) and that rumen isoacids had the potential to stimulate cellulolytic bacteria growth, acetate production and cellulose digestion in cows(Reference Wang, Liu and Guo33). Similarly, other studies observed increased percentages of isobutyrate and isovalerate with CFA addition in calves(Reference La, Li and Wang13) or bulls(Reference Wang, Liu and Zhang14). The increase in creatine concentration of blood and the liver suggested that addition of CFA promoted creatine synthesis, and this should result from the regulation of FA on the one-carbon units cycle, as shown by the decrease of blood Hcy concentration. The increase in hepatic mRNA expressions of IGF-1, IGF-1R, PI3K, Akt, mTOR and P70S6K with CFA addition was in line with the findings of La et al.(Reference La, Li and Wang13). Hwang et al. reported that FA promoted the differentiation of C2C12 cells through activation of the Akt/mTOR/P70S6K pathway(Reference Hwang, Kang and Sung34). These results suggested that the observed increase in ADG might be associated with the regulation of FA to the expression of genes in the IGF-1/PI3K/mTOR pathway.
The significant CFA × GAA interaction was observed on ADG, feed efficiency as well as blood and hepatic creatine concentration. Blood creatine concentration was higher for the combined addition of GAA and CFA than for supplementation with GAA or CFA alone, indicating that FA could promote the conversion of GAA to creatine. However, no more increase in ADG, feed efficiency and hepatic creatine concentration was observed for the combined addition of GAA and CFA compared with GAA or CFA supplementation alone, suggesting that the response of growth performance to creatine might be dose-dependent. Studies in vitro demonstrated that creatine transporter expression of muscle cells decreased with increasing creatine addition(Reference Guerrero Ontiveros and Wallimann35,Reference Loike, Zalutsky and Kaback36) .
Conclusion
Addition of GAA or CFA increased daily gain and nutrient digestibility of bulls. The creatine supply could be improved with the addition of GAA or CFA in bulls. Both GAA and CFA stimulated hepatic gene expression in the IGF-1/PI3K/mTOR pathway. Even though bulls receiving the combined addition of GAA and CFA had higher blood creatine concentration compared with those consuming GAA or CFA alone, no difference in performance was observed when CFA was added in diets without or with GAA.
Acknowledgements
The authors thank the staff of Shanxi Agriculture University beef cattle unit for care of the animals. All authors read and approved the manuscript.
This work was supported by a grant from College Students Innovation and Entrepreneurship Training Program of the Ministry of Education of the People’s Republic of China (201910113006), Key Research and Development project of Shanxi Province (201903D221001) and Animal Husbandry ‘1331 project’ Key Discipline Construction program of Shanxi Province.
C. W. and Q. L. designed the experiment. Y.-J. L., J.-Z. C., D.-H. W., M.-J. W., C. Z. and Z.-Z. W. conducted the experiment. Y.-J. L., J. Z., G. G. and W.-J. H. collected and analysed the data. Y.-J. L. wrote the manuscript. C. W. and Q. L. revised the manuscript.
The authors declare that no conflicts of interest exist.









