Flavonoids, as a major component of grape and grape products, have been reported to have several favourable effects on human health(Reference Hertog, Feskens and Hollman1). Grape seed extracts (GSE) contain a high concentration of flavonoids, in particular proanthocyanidins, potent antioxidants with many cardiovascular benefits(Reference Corder, Mullen and Khan2,Reference Sánchez-Moreno, Cao and Ou3) . Proanthocyanidins are also known as condensed tannins existing in GSE as monomeric (catechin and epicatechin), dimeric, trimeric and polymeric tannin structures(Reference Shi, Yu and Pohorly4). Findings from in vitro studies have demonstrated that these compounds act as free-radical scavengers and may prevent the oxidation of LDL-cholesterol(Reference Viana, Barbas and Bonet5-Reference Miyagi, Miwa and Inoue7); therefore, they have an important role in decreasing the progression of CVD(Reference Aviram and Fuhrman8). Their free radical-scavenging ability has been indicated to be even fifty times greater than that of vitamins C and E and β-carotene(Reference Bagchi, Bagchi and Stohs9,Reference Bagchi, Garg and Krohn10) . A number of these studies have also shown positive effects of flavonoids upon novel vascular risk factors such as inflammation(Reference Bumrungpert, Kalpravidh and Chuang11). However, in vivo experience has provided less clear results(Reference Adisakwattana, Moonrat and Srichairat12-Reference Hansen, Marckmann and Dragsted17). Although a recent investigation has shown that GSE significantly reduced plasma cholesterol in rats fed a high-fat diet(Reference Adisakwattana, Moonrat and Srichairat12), few clinical trials have explored this issue in humans, some with promising results. For instance, in an Italian study, conducted on twenty-four heavy smokers, no significant change in plasma lipid profiles was found after 4 weeks supplementation with 75 mg GSE twice daily; however, GSE resulted in a decreased susceptibility of LDL-cholesterol to oxidation(Reference Vigna, Costantini and Aldini13). Conversely, combined administration of niacin-bound Cr and GSE decreased total cholesterol (TC) and LDL-cholesterol after 2 months among forty hypercholesterolaemic individuals(Reference Preuss, Wallerstedt and Talpur14). In addition, administration of GSE for 8 weeks in mild hyperlipidaemic patients resulted in improved lipid profiles(Reference Razavi, Gholamin and Eskandari15). This was also reported in diabetic patients(Reference Kar, Laight and Rooprai16). Nonetheless, Hansen etal. reported no significant effect of GSE on serum lipids in sixty-nine healthy individuals(Reference Hansen, Marckmann and Dragsted17). The origin, dosage and composition of polyphenolic extracts from the grape as well as different study designs along with the health conditions of the study participants might provide some reasons for these discrepancies.
Despite several publications on the effects of GSE on serum lipid profiles, we are aware of no study summarising earlier publications in this regard. Given the controversial findings in previous publications, this study was done to systematically review earlier publications on the impact of GSE administration on lipid profiles and to perform a meta-analysis of relevant randomised controlled trials in this regard.
Methods
This study was performed based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement(Reference Moher, Shamseer and Clarke18).
Search strategy
A systematic search was carried out in the online databases of PubMed, ISI Web of Science, Scopus, ProQuest, Science Direct and Embase for relevant publications until March 2019. The keywords used in our search strategy were (‘Grape seed extract’ [Mesh] OR ‘Polyphenols’[Mesh] OR ‘Proanthocyanidins’ [Mesh] OR ‘Vitis’ [Mesh] OR ‘Grape seed’ [tiab] OR ‘Polyphenols’ [tiab] OR ‘Proanthocyanidins’ [tiab] OR ‘Vitis’ [tiab]) AND (‘Lipids’ [Mesh] OR ‘Cholesterol’ [Mesh] OR ‘Cholesterol, VLDL’ [Mesh] OR ‘Cholesterol, HDL’ [Mesh] OR ‘Lipoproteins, HDL’ [Mesh] OR ‘High-Density Lipoproteins, Pre-beta’ [Mesh] OR ‘Cholesterol, LDL’ [Mesh] OR ‘Triglycerides’ [Mesh] OR ‘Lipoproteins’ [Mesh] OR ‘Hypercholesterolemia’ [Mesh] OR ‘Hyperlipidemias’ [Mesh] OR ‘Dyslipidemias’ [Mesh] OR ‘lipids’ [tiab] OR ‘cholesterol’ [tiab] OR ‘triglyceride*’ [tiab] OR ‘triacylglycerol’ [tiab] OR ‘HDL’ [tiab] OR ‘LDL’ [tiab] OR ‘Hypercholesterolemia’ [tiab] OR ‘Hyperlipidemias’ [tiab] OR ‘Dyslipidemias’ [tiab]) AND (‘Clinical Trial’ OR ‘trial’ OR ‘intervention’). We considered no restriction on time of publication and language. In addition, the reference lists of the relevant papers were also hand-searched to identify further relevant studies. In the search strategy, unpublished studies were excluded.
Inclusion criteria
We included the studies in the present meta-analysis if they met the following criteria: (1) studies that investigated the effect of GSE on any of the lipid profile parameters, including TC, HDL-cholesterol, LDL-cholesterol and TAG; (2) those that were of randomised controlled clinical trial; (3) those that presented sufficient information on plasma/serum lipid levels at study baseline and at the end of trial and (4) those trials that were done on healthy participants or individuals only with chronic diseases including dyslipidaemia, diabetes mellitus, the metabolic syndrome and breast cancer. In the case of duplicate publications from the same study group, a study with the larger sample size or more complete information was retained.
Data extraction
According to the predefined inclusion criteria, two investigators independently completed the search, data extraction and quality assessment, and any discrepancies between the two reviewers were resolved through discussion. Data of interest from each individual study were extracted as follows: first author, year of publication, country of origin, study design, the duration of the intervention, sample size, sex, mean age and BMI at study baseline, the type and dose of GSE supplementation, participants’ baseline health status and the means, and standard deviations of serum concentrations of lipids at study baseline and post-intervention. The data for blood lipids were converted into the same units (mmol/l), and mean differences in concentrations of plasma lipids (TC, LDL-cholesterol, HDL-cholesterol and TAG) between the control and GSE groups were calculated. If a study had reported the effect sizes for two different doses of GSE (low- and high-dose), each arm was considered as a separate study.
Excluded studies
In this meta-analysis, letters, comments, short communications, reviews, ecological studies and animal studies were excluded from the analysis. In our initial search, we found 531 articles. On the basis of title and abstract, we excluded 512 studies and nineteen remaining articles were reviewed in full text. Another eight papers were further excluded because of the following reasons: (1) studies that investigated the effect of whole grape or grape juice supplementation on lipid profiles (n 3); (2) those that evaluated the administration of grape powder (n 2) and (3) publications that assessed the impact of red wine supplementation on lipid profiles (n 3). Finally, eleven randomised clinical trials that met our inclusion criteria were included in this analysis (Fig. 1).

Fig. 1. Flow diagram of study selection.
Assessment of study quality
Study quality was assessed by using Jadad scale(Reference Jadad, Moore and Carroll19), in which the total score ranges from 0 to 5 points based on the following criteria: (1) randomisation; (2) appropriate method for randomisation; (3) double-blinding; (4) appropriate method for double-blinding and (5) description of dropouts and withdrawals. In the present study, trials scored one point for each area addressed in the study design, with a possible score of 0 to 5 (highest level of quality). Any discrepancies were resolved by discussion. We defined high-quality publications as those that had the Jadad score of 3 or more (Table 1).
Table 1. Baseline characteristics of all included clinical trials investigating impacts of grape seed extract (GSE) supplementation on plasma lipids

NR, not reported; TC, total cholesterol.
Statistical analysis
The overall effect sizes were calculated using mean differences and standard deviations of plasma lipids (TC, LDL-cholesterol, HDL-cholesterol and TAG). In studies that had reported standard errors, we computed sd using CI or P values based on the standard formula(Reference Borenstein, Hedges and Higgins20,Reference Hozo, Djulbegovic and Hozo21) . If the sd of the mean difference was not stated in the publication, we calculated it using the following formula: 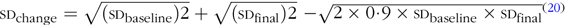 ${\rm{\textsc{SD}_{change}} = {\sqrt {({\textsc{SD}_{baseline}})2}} + {\sqrt {({\textsc{SD}_{final}})2}}} {\rm-{\sqrt {2 \times 0\hskip-2·\hskip-2{9} \times {\textsc{SD}_{baseline}} \times {\textsc{SD}_{final}}}}$(Reference Borenstein, Hedges and Higgins20). Then, the meta-analysis was conducted to calculate the overall weighted mean difference pooling study-specific mean difference through random effects model. We also applied meta-regression to examine the effective dosage of GSE supplementation. To test dose–response relations, we plotted the relation between GSE dosage (mg/d) and the absolute mean change in each outcome using fractional polynomial models with the best-fitting model considered the one with the lowest deviance(Reference Fan and Gijbels22). We applied Cochran’s Q test and I 2 to assess between-study heterogeneity(Reference Green and Higgins23). To detect probable sources of heterogeneity, we did subgroup analysis. The predefined categories for subgroup analysis were as follows: geographical region (USA/non-USA countries), study design (parallel/crossover), duration of intervention (<10 weeks/≥10 weeks), sample size (<50/≥50 participants), sex (male/female/both sex), mean age at study baseline (<50/≥50 years), mean BMI at study baseline (<27/≥27 kg/m2), GSE dosage (<300/≥300 mg/d), supplementation type (capsule/tablet/functional foods), participants’ baseline status (healthy/chronic condition) and dyslipidaemia (yes/no). In addition to main analyses, we conducted sensitivity analysis to find if the overall estimate depended on the effect size from a single study. Assessing the publication bias was done by visual inspection of funnel plots along with Eggerʼs test. All statistical analyses were done using Stata software, version 11.2 (StataCorp). P < 0·05 was considered as statistically significant.
${\rm{\textsc{SD}_{change}} = {\sqrt {({\textsc{SD}_{baseline}})2}} + {\sqrt {({\textsc{SD}_{final}})2}}} {\rm-{\sqrt {2 \times 0\hskip-2·\hskip-2{9} \times {\textsc{SD}_{baseline}} \times {\textsc{SD}_{final}}}}$(Reference Borenstein, Hedges and Higgins20). Then, the meta-analysis was conducted to calculate the overall weighted mean difference pooling study-specific mean difference through random effects model. We also applied meta-regression to examine the effective dosage of GSE supplementation. To test dose–response relations, we plotted the relation between GSE dosage (mg/d) and the absolute mean change in each outcome using fractional polynomial models with the best-fitting model considered the one with the lowest deviance(Reference Fan and Gijbels22). We applied Cochran’s Q test and I 2 to assess between-study heterogeneity(Reference Green and Higgins23). To detect probable sources of heterogeneity, we did subgroup analysis. The predefined categories for subgroup analysis were as follows: geographical region (USA/non-USA countries), study design (parallel/crossover), duration of intervention (<10 weeks/≥10 weeks), sample size (<50/≥50 participants), sex (male/female/both sex), mean age at study baseline (<50/≥50 years), mean BMI at study baseline (<27/≥27 kg/m2), GSE dosage (<300/≥300 mg/d), supplementation type (capsule/tablet/functional foods), participants’ baseline status (healthy/chronic condition) and dyslipidaemia (yes/no). In addition to main analyses, we conducted sensitivity analysis to find if the overall estimate depended on the effect size from a single study. Assessing the publication bias was done by visual inspection of funnel plots along with Eggerʼs test. All statistical analyses were done using Stata software, version 11.2 (StataCorp). P < 0·05 was considered as statistically significant.
Results
Among 531 retrieved publications, eleven papers met the inclusion criteria and were selected for the present meta-analysis(Reference Vigna, Costantini and Aldini13-Reference Kar, Laight and Rooprai16,Reference Clifton24-Reference Brooker, Martin and Pearson30) . Two studies had two arms with two different doses(Reference Sano, Uchida and Saito27,Reference Sivaprakasapillai, Edirisinghe and Randolph29) ; therefore, we had thirteen effect sizes for the analysis.
Findings from systematic review
Characteristics of studies included in this systematic review are briefly described in Table 1. These clinical trials were published between 2000 and 2016 and had recruited 536 participants in total, with individual study sizes ranging from 19 to 96. Three publications were from the USA(Reference Preuss, Wallerstedt and Talpur14,Reference Park, Edirisinghe and Choy25,Reference Sivaprakasapillai, Edirisinghe and Randolph29) , two studies from the UK(Reference Kar, Laight and Rooprai16,Reference Brooker, Martin and Pearson30) , two from Iran(Reference Razavi, Gholamin and Eskandari15,Reference Argani, Ghorbanihaghjo and Vatankhahan28) and one study from Australia, Spain, Italy and Japan(Reference Vigna, Costantini and Aldini13,Reference Clifton24,Reference Yubero, Sanz-Buenhombre and Guadarrama26,Reference Sano, Uchida and Saito27) . All studies, except for one(Reference Sano, Uchida and Saito27), were double-blind controlled trials. Of eleven clinical trials, four had a crossover design(Reference Vigna, Costantini and Aldini13,Reference Razavi, Gholamin and Eskandari15,Reference Kar, Laight and Rooprai16,Reference Clifton24) and seven had a parallel design(Reference Preuss, Wallerstedt and Talpur14,Reference Park, Edirisinghe and Choy25-Reference Brooker, Martin and Pearson30) . The duration of intervention varied from 4 to 24 weeks. Ten trials reported mean age of participants, which varied between 44 and 62 years(Reference Vigna, Costantini and Aldini13,Reference Razavi, Gholamin and Eskandari15,Reference Kar, Laight and Rooprai16,Reference Clifton24-Reference Brooker, Martin and Pearson30) . In addition, seven studies, out of eleven trials, were conducted among individuals with a mean BMI ≥ 27 kg/m2(Reference Vigna, Costantini and Aldini13,Reference Preuss, Wallerstedt and Talpur14,Reference Kar, Laight and Rooprai16,Reference Clifton24,Reference Park, Edirisinghe and Choy25,Reference Sivaprakasapillai, Edirisinghe and Randolph29,Reference Brooker, Martin and Pearson30) ; others were carried out among subjects with a mean BMI < 27 kg/m2. Six studies were done on hyperlipidaemic patients(Reference Preuss, Wallerstedt and Talpur14-Reference Kar, Laight and Rooprai16,Reference Clifton24,Reference Argani, Ghorbanihaghjo and Vatankhahan28,Reference Sivaprakasapillai, Edirisinghe and Randolph29) and others on normolipidaemic subjects. Furthermore, out of eleven trials, one study was conducted among subjects with breast cancer(Reference Brooker, Martin and Pearson30), seven studies were done on individuals with high risk of CVD(Reference Preuss, Wallerstedt and Talpur14-Reference Kar, Laight and Rooprai16,Reference Clifton24,Reference Park, Edirisinghe and Choy25,Reference Argani, Ghorbanihaghjo and Vatankhahan28,Reference Sivaprakasapillai, Edirisinghe and Randolph29) and other three were done among healthy participants(Reference Vigna, Costantini and Aldini13,Reference Yubero, Sanz-Buenhombre and Guadarrama26,Reference Sano, Uchida and Saito27) . The method of intervention was using tablets or capsules in nine studies(Reference Vigna, Costantini and Aldini13-Reference Kar, Laight and Rooprai16,Reference Yubero, Sanz-Buenhombre and Guadarrama26-Reference Brooker, Martin and Pearson30) and functional foods in two researches(Reference Clifton24,Reference Park, Edirisinghe and Choy25) . Dosages of GSE used in these studies were different from 150 to 2000 mg/d. Regarding the study quality, presented in Table 1, one publication had a Jadad score of 2(Reference Sano, Uchida and Saito27) and others had a score of ≥3(Reference Vigna, Costantini and Aldini13-Reference Kar, Laight and Rooprai16,Reference Clifton24-Reference Yubero, Sanz-Buenhombre and Guadarrama26,Reference Argani, Ghorbanihaghjo and Vatankhahan28-Reference Brooker, Martin and Pearson30) .
Findings from the meta-analysis
All of the eleven trials included in the systematic review were also considered in the present meta-analysis, out of which levels of TC, LDL, HDL and TAG were evaluated in eleven, nine, ten and eight studies, respectively.
Combining thirteen effect sizes from eleven studies, we found that GSE supplementation did not significantly influence serum levels of TC (−0·18 mmol/l; 95 % CI −0·38, 0·03; Fig. 2(a)). Stratification by the health condition of the participants did not change this finding (Fig. 2(b)). A significant between-study heterogeneity was observed (Cochran’s Q, P < 0·001, I 2 = 98·3 %). To investigate the potential sources of inter-study heterogeneity, we conducted subgroup analyses based on the country of origin, study design, health status of subjects, mean age and BMI of participants at study baseline, sex, and sample size, the type and dose of GSE supplementation as well as the duration of intervention (Table 2). In these analyses, we found that GSE supplementation resulted in decreased levels of TC among participants with a BMI of <27 kg/m2 compared with control group (−0·40 mmol/l; 95 % CI −0·72, −0·07). In addition, GSE supplementation had favourable effects on TC levels in a subgroup of studies which used capsules as their intervention type (−0·28 mmol/l; 95 % CI −0·53, −0·03) as well as those that had used the dosage of <300 mg/d (−0·30 mmol/l; 95 % CI −0·55, −0·06), were done on a sample of more than fifty individuals (−0·40 mmol/l; 95 % CI −0·72, −0·07), with a duration of <10 weeks (−0·28 mmol/l; 95 % CI −0·54, −0·03) (Table 2).
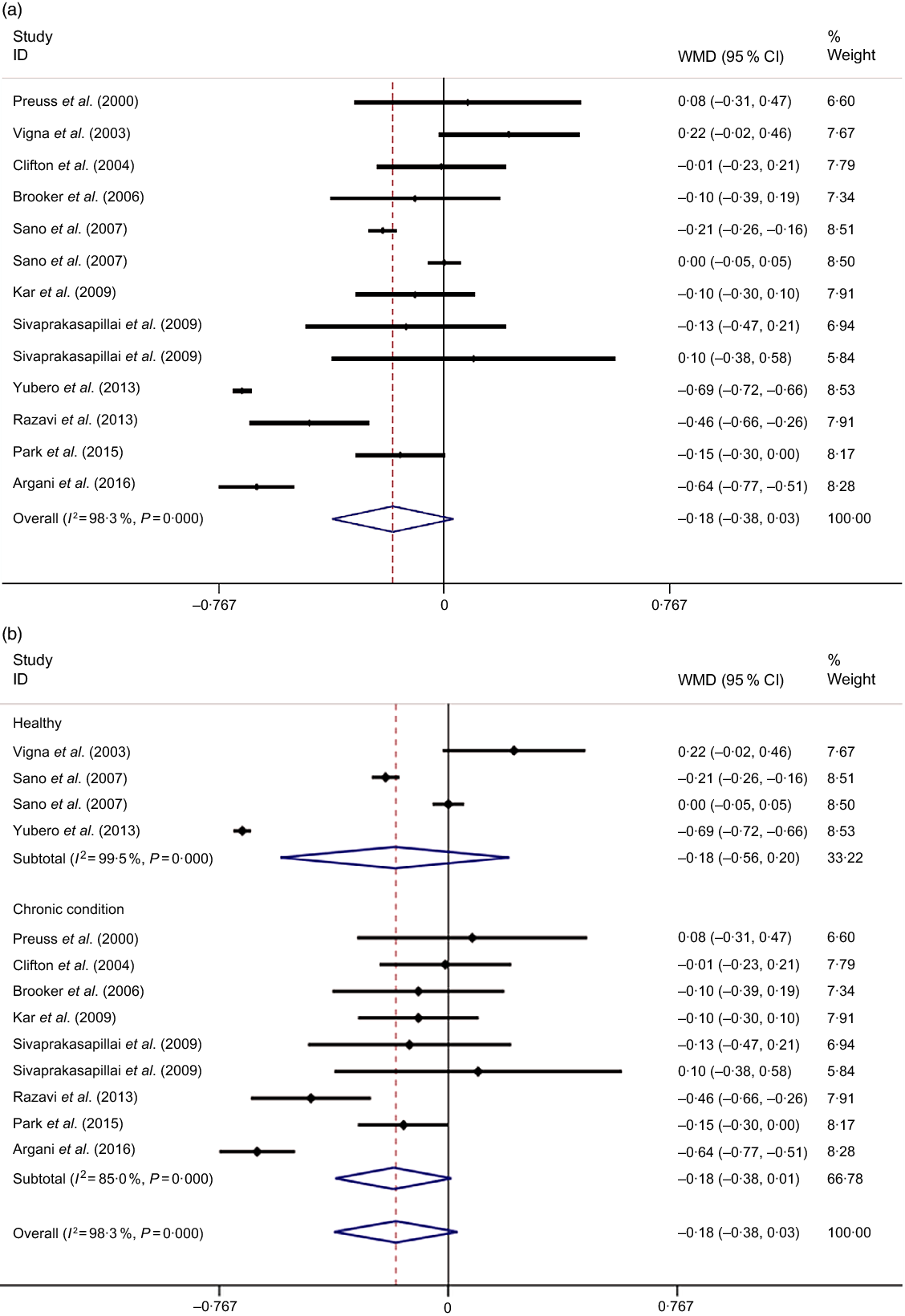
Fig. 2. Forest plot for the effect of grape seed extract supplementation on serum levels of total cholesterol using a random effects model in all participants (a) and stratified by the health condition of participants (b). Weights are from random effects analysis.
Table 2. Subgroup analysis based on random effects models of grape seed extract (GSE) supplementation on plasma lipids
(Weighted mean difference (WMD), 95 % confidence intervals and I 2)

Pooling eleven effect sizes from nine studies revealed that GSE supplementation significantly reduced circulating LDL levels (−0·17 mmol/l; 95 % CI −0·34, −0·01; Fig. 3(a)). Stratification by the health condition of the participants changed this finding (Fig. 3(b)). A significant between-study heterogeneity was found (Cochran’s Q, P < 0·001, I 2 = 98·4 %). In the subgroup analyses, we found a significant LDL-lowering effect of GSE supplementation in studies performed on participants with a BMI of <27 kg/m2 (−0·33 mmol/l; 95 % CI −0·56, −0·10), those that used capsules as their intervention type (−0·34 mmol/l; 95 % CI −0·51, −0·16), used the dosage of <300 mg/d (−0·30 mmol/l; 95 % CI −0·54, −0·06), studies that were of parallel design (−0·20 mmol/l; 95 % CI −0·40, −0·00), publications that were done on a sample size of more than fifty individuals (−0·40 mmol/l; 95 % CI −0·58, −0·23) and duration of <10 weeks (−0·35 mmol/l; 95 % CI −0·56, −0·14) and those came from non-USA countries (−0·23 mmol/l; 95 % CI −0·43, −0·04).

Fig. 3. Forest plot for the effect of grape seed extract supplementation on serum levels of LDL-cholesterol using a random effects model in all participants (a) and stratified by the health condition of participants (b). Weights are from random effects analysis.
Combining twelve effect sizes from ten studies, we found that GSE supplementation did not influence serum HDL levels (0·01 mmol/l; 95 % CI −0·03, 0·04; Fig. 4(a)). Stratification by the health condition of the participants did not change this finding (Fig. 4(b)). There was a significant between-study heterogeneity (Cochran’s Q, P < 0·001, I 2 = 88·6 %). Potential sources of heterogeneity were assessed using subgroup analysis. As illustrated in Table 2, GSE supplementation resulted in an increment in serum HDL concentrations in studies with an intervention duration of <10 weeks (0·04 mmol/l; 95 % CI 0·001, 0·09).

Fig. 4. Forest plot for the effect of grape seed extract supplementation on serum levels of HDL-cholesterol using a random effects model in all participants (a) and stratified by the health condition of participants (b). Weights are from random effects analysis.
Pooling eleven effect sizes from eight studies, we found that GSE supplementation significantly decreased serum levels of TAG (−0·11 mmol/l; 95 % CI −0·18, −0·05; Fig. 5(a)). Stratification by the health condition of the participants changed this finding (Fig. 5(b)). A significant between-study heterogeneity was observed (Cochran’s Q, P < 0·001, I 2 = 73·4 %). In subgroup analyses, we observed that GSE supplementation significantly reduced TAG levels in studies that were carried out among non-healthy participants (−0·15 mmol/l; 95 % CI −0·24, −0·05), those that were performed on participants with a BMI of <27 kg/m2 (−0·16 mmol/l; 95 % CI −0·24, −0·09) as well as those that were done on people aged <50 years (−0·14 mmol/l; 95 % CI−0·26, −0·03). In addition, GSE supplementation had favourable effects on TAG levels in a subgroup of studies which used capsules as their intervention type (−0·14 mmol/l; 95 % CI −0·19, −0·08) as well as those that had used the dosage of <300 mg/d (−0·16 mmol/l; 95 % CI −0·26, −0·05), were done on a sample of more than fifty individuals (−0·19 mmol/l; 95 % CI −0·35, −0·04), publications that were of parallel design (−0·15 mmol/l; 95 % CI −0·22, −0·08) and those came from non-USA countries (−0·13 mmol/l; 95 % CI −0·21, −0·04).

Fig. 5. Forest plot for the effect of grape seed extract supplementation on serum levels of TAG using a random effects model in all participants (a) and stratified by the health condition of participants (b). Weights are from random effects analysis.
Findings from dose–response analysis about GSE supplementation on lipid profiles revealed that the overall pooled estimates on lipid profiles were independent of GSE dosage. We failed to detect a significant effect of specific dosage of GSE on lipid profiles, as examined by non-linear dose–response meta-analysis (Fig. 6).

Fig. 6. Non-linear dose–response relationships between grape seed extract dosage (mg/d) and serum levels of lipids (mmol/l) in (a) total cholesterol, (b) LDL-cholesterol, (c) HDL-cholesterol and (d) TAG.  , 95 % Confidence interval;
, 95 % Confidence interval;  , predicted effect size;
, predicted effect size;  , weighted mean difference.
, weighted mean difference.
For all lipid profiles, no evidence of publication bias was seen through visual inspection of funnel plots (online Supplementary Fig. 1). Moreover, these findings were also confirmed by the Eggerʼs regression test (for TC: P = 0·15; LDL: P = 0·28; HDL: P = 0·07; TAG: P = 0·69). In addition, sensitivity analysis demonstrated that excluding individual studies did not alter the estimated pooled effect sizes in lipid profiles (online Supplementary Fig. 2).
Discussion
In the present meta-analysis of eleven trials, we observed that GSE supplementation resulted in a statistically significant reduction in serum levels of LDL-cholesterol and TAG, but it did not affect TC and HDL-cholesterol concentrations.
Although the efficacy of several medications, including statins, in lowering serum levels of LDL and reducing CHD events has been already established, finding a novel adjunct therapy with lower complications is still challenging. In the present meta-analysis, we found that GSE supplementation significantly decreased serum levels of LDL. In addition to traditional lipid profiles, some studies have investigated the effect of GSE supplementation on postprandial lipid profiles. In line with our findings, a crossover trial on postprandial lipids demonstrated that GSE supplementation resulted in enhanced postprandial plasma antioxidant capacity and thereby decreased levels of oxidised LDL(Reference Edirisinghe, Randolph and Cheema31). Similarly, Natella etal. reported that GSE supplementation remarkably reduced lipid peroxidation(Reference Natella, Belelli and Gentili32). However, in a long-term semi-experimental study on seventeen people, GSE supplementation did not lead to any significant change in serum LDL concentrations in healthy individuals; however, it resulted in a significant reduction in LDL in hypercholesterolaemic participants(Reference Vinson, Proch and Bose33). This inconsistency in findings about the effect of GSE supplementation on LDL levels might be attributed to differences in the composition of polyphenolic extract, along with trials’ designs and participants’ conditions.
This meta-analysis revealed that GSE supplementation reduced serum levels of TAG. This finding was against previous studies about the effect of GSE on postprandial levels of TAG in both healthy and hypercholesterolaemic participants(Reference Vinson, Proch and Bose33). Experimental studies have shown that polyphenolic compounds regulate pathways involved in lipoprotein metabolism which can in turn result in reduced serum TAG concentrations(Reference Yugarani, Tan and Teh34). These effects might be explained through alteration in microsomal transport protein activity and apoB secretion(Reference Wilcox, Borradaile and de Dreu35). For instance, Pal etal. detected the efficacy of grape polyphenols on lipoprotein production and clearance in cultured liver cells(Reference Pal, Ho and Santos36). In addition, the present study showed a significant reduction in TAG concentrations following GSE supplementation only among patients with chronic diseases. However, due to limited number of included studies and insufficient data about each disease, further studies are required to reach a firm conclusion in this area. Although the lipid-lowering effects of the polyphenols are well established in vitro, their in vivo effects are less documented. These compounds in GSE are extensively conjugated, and only a minor fraction remains unconjugated, thereby making it difficult to link biological effects to structure. This is particularly evident in the case of grape polyphenols which are mostly monomeric and their bioavailability is often underestimated due to poor detection and absorption(Reference Del Rio, Costa and Lean37,Reference Galvano, La Fauci and Vitaglione38) . However, consumption of other dietary sources of flavonoids, including dark chocolate and cocoa powder, has been shown to significantly influence serum TAG and LDL-cholesterol concentrations(Reference Grassi, Necozione and Lippi39,Reference Engler, Engler and Chen40) . For instance, in a meta-analysis of ten randomised trials involving 320 individuals, dark chocolate consumption led to a significant reduction in serum LDL-cholesterol concentrations in subjects with CVD risk factors(Reference Tokede, Gaziano and Djousse41). Moreover, the study of Jia etal. revealed that short-term consumption of a cocoa product lowered serum levels of LDL-cholesterol and TC(Reference Jia, Liu and Bai42). In addition, a growing body of evidence from both in vitro studies and animal studies also demonstrated the antidyslipidaemic and anti-inflammatory effects of cocoa and cocoa flavonoids(Reference Kondo, Hirano and Matsumoto43-Reference Ramos-Romero, Perez-Cano and Ramiro-Puig45). These favourable effects on the lipid profile might be explained by the reduction in the hepatic activity of 3-hydroxy-3-methlyglutaryl-coenzyme reductase, the limiting enzyme of cholesterogenesis(Reference Sung, Lee and Park46).
The results of the present meta-analysis suggest that there is no significant effect of GSE supplementation on circulating TC and HDL-cholesterol levels. These findings were opposite to those reported from experimental studies. For instance, Vinson etal. reported that GSE induced a pronounced reduction in serum cholesterol levels in an atherosclerotic hamster model(Reference Vinson, Mandarano and Shuta47). Additionally, 28 d of GSE administration in rats reduced serum levels of lipids and prevented occurrences of fatty liver(Reference Giribabu, Eswar Kumar and Swapna Rekha48). In vivo experiments have also revealed that GSE supplementation might increase cholesterol exertion through reducing intestinal absorption(Reference Tebib, Besançon and Rouanet49,Reference Leifert and Abeywardena50) . The difference between human studies and those done on animals might be explained by the dosage of supplementation, duration of intervention, physiological difference and different study designs.
The lipid-lowering impacts of GSE were more evident in trials with <10 weeks of intervention. For instance, although for whole studies combined, we did not observe any significant effect of GSE supplementation on serum TC and HDL-cholesterol levels, the significant effect on these lipids was seen for studies with <10 weeks of intervention. Besides study duration, GSE dosages might also play a role in this regard. It seems that the dosages of <300 mg of GSE per d are more officious than higher doses to influence blood lipids. Another point is the significant effect of GSE on lipid profiles among subjects with a BMI of <27 kg/m2. Some of these discrepancies might be explained by differences in composition of GSE products. Moreover, different characteristics of populations being studied might also provide some reasons. Previous investigations have demonstrated that differences in gut microbiota can result in large inter-individual variability in plasma concentrations of all phenolic acids(Reference Espín, González-Sarrías and Tomás-Barberán51,Reference Feliciano, Mills and Istas52) .
Although the precise mechanisms of GSE on blood lipids remain unclear, the beneficial effects might be attributed to modulation of antioxidant enzymes’ expression, protection against oxidative damage in cells, antiatherosclerotic and anti-inflammatory effects(Reference Brewer53). These effects were specifically attributed to proanthocyanidins in GSE. These compounds may also reduce plasma lipid profiles by inhibiting specific cholesterol transporters such as the Niemann-Pick C1-like one cholesterol transporter(Reference Leifert and Abeywardena54). In addition, inhibition of pancreatic lipase, cholesterol esterase, cholesterol micellisation and bile acid binding is among other lipid-lowering mechanisms of GSE(Reference Adisakwattana, Moonrat and Srichairat12). Moreover, GSE supplementation has been indicated to suppress intestinal lipid absorption, chylomicron and VLDL secretion, and subsequently reduced lipid levels(Reference Tebib, Besançon and Rouanet49). The polyphenolic compounds of GSE are extremely varied which can explain the between-study heterogeneity. We found that GSE had no significant effect on lipids when administered in high dosages and for long time. It should be noted that GSE is a rich source of fatty acids, including SFA and PUFA. Therefore, it is possible that long-term intake of high dosage of GSE might neutralise its beneficial effects on lipids.
This is a comprehensive up-to-date meta-analysis that examined the effect of GSE supplementation on circulating lipid concentrations. However, several limitations should be considered. First, the sample size of included studies was not sufficiently large to detect significant effects. The effect of different forms of GSE supplements was not adequately examined, and further investigations are warranted to address questions specific to efficacy, bioavailability and complete metabolite profiles.
In conclusion, GSE supplementation seems to favourably affect serum levels of LDL as well as TAG levels, but it did not influence TC and HDL-cholesterol concentrations. However, given the limitations and small sample sizes of included studies, further investigations are needed to shed light on this issue. The take-home message of this study would be the recommendation to administer GSE as a secondary factor, along with medications, to control hyperlipidaemia.
Acknowledgements
The authors would like to thank the authorities in the Tehran University of Medical Sciences for financial support of the study.
This study was supported by the School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
J. A.-S., A. M., B. L. and A. E. contributed in conception, design, statistical analysis, data interpretation and manuscript drafting. All authors approved the final manuscript for submission.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520000902











