Prostate cancer (PCa) accounts for 28 % of total cancer incidence(Reference Jemal, Siegel and Xu1) and is the second most common cause of cancer-related death of men in the USA(2). Prostate-specific antigen (PSA) testing is widely used for the detection of asymptomatic and early-stage PCa(Reference Jemal, Murray and Ward3). There is great controversy surrounding PSA screening, because a considerable number of men with elevated serum PSA, who were referred for biopsy, were not diagnosed with PCa(Reference Etzioni, Howlader and Shaw4). Indeed, serum PSA concentrations may be affected by many factors unrelated to prostate disease, including age(Reference Saraiya, Kottiri and Leadbetter5), race(Reference Catalona, Partin and Slawin6), type 2 diabetes mellitus(Reference Wallner, Morgenstern and McGree7), dietary factors(Reference Sonn, Aronson and Litwin8), certain clinical cardiac problems(Reference Açıkgöz, Can and Doğan9) and obesity(Reference Grubb, Black and Izmirlian10). Consumption of some medications such as non-steroidal anti-inflammatory drugs, acetaminophen(Reference Singer, Palapattu and van Wijngaarden11) and statins(Reference Krane, Kaul and Stricker12) can also affect serum PSA levels. In general, overall PCa mortality is high in Northern Europe and North America, and is low in Japan and other Asian nations(Reference Jemal, Bray and Center13). But, for Japanese migrants to the USA, the incidence of mortality increases as a function of the number of years lived in the USA(Reference Astorg14). The major contributory factor thought to account for this increased frequency in PCa death is the Western diet(Reference Berquin, Min and Wu15). The fatty acid composition of Western diets has changed dramatically during the 20th century. Typically, the Western diet includes less n-3 PUFA and more n-6 PUFA(Reference Weaver, Ivester and Seeds16). Currently, the n-6:n-3 ratio in Western diets is approximately 15(Reference Simopoulos17). This ratio in the Japanese diet is 4:1(Reference Sugano and Hirahara18). DHA, EPA and α-linolenic acid are the main n-3 PUFA. Linoleic acid, γ-linolenic acid (GLA) and arachidonic acid are the main n-6 PUFA. Several investigations have reported the influence of PUFA on the development of PCa. n-3 PUFA inhibit human prostate cancer cell lines(Reference Rose and Connolly19), while n-6 PUFA increase the growth of human prostate tumour cell lines(Reference Rose and Connolly19, Reference Connolly, Coleman and Rose20). Akinsete et al. (Reference Akinsete, Ion and Witte21) reported that the administration of a high amount of n-3 slows down prostate tumorigenesis in mice. Kelavkar et al. (Reference Kelavkar, Hutzley and Dhir22) investigated the effects of the n-6:n-3 ratio in the diet on prostate tumour growth and recurrence. They concluded that the n-3 fatty acid stearidonic acid (precursor of EPA) induces apoptosis and declines proliferation in cancer cells, causing diminished PSA doubling time. Kobayashi et al. (Reference Kobayashi, Barnard and Henning23) examined whether altering the dietary content of n-3 and n-6 PUFA affects the growth of androgen-sensitive PCa xenografts. They found decreased serum PSA in the n-3 diet group.
Coenzyme Q10 (CoQ10) is an antioxidant and improves endothelial function in some subjects at risk of CVD(Reference Watts, Playford and Croft24). Chai et al. (Reference Chai, Cooney and Franke25) investigated the association of circulating CoQ10 levels with PCa risk. They concluded that moderate levels of circulating CoQ10 may reduce PCa risk; however, the findings did not reach statistical significance. Most preliminary studies with n-3, n-6 and CoQ10 have been investigative and suggested conflicting results regarding clinical efficacy in patients with PCa, without addressing the effects of specific PUFA and CoQ10 on serum PSA levels separately(Reference Kelavkar, Hutzley and Dhir22, Reference Kobayashi, Barnard and Henning23, Reference Chai, Cooney and Franke25, Reference Hoenjet, Dagnelie and Delaere26). Therefore, we need further rigorous randomised studies.
Materials and methods
Study subjects
Recruitment and data collection were performed between July 2009 and November 2010. A total of 600 healthy men aged 40–70 years were recruited via local advertisements for a study of PCa. They were judged to be in good health based on a complete physical examination, medical and surgical history, electrocardiogram and laboratory tests, including serum biochemistry, haematology and urinalysis. None of the participants had lower urinary tract symptoms, a history of systemic inflammatory illness or urogenital disorders. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Medical Ethics Committee at the site. Verbal consent was witnessed and formally recorded.
Inclusion/exclusion criteria
The inclusion criteria were age between 40 and 70 years and no drug or alcohol dependence. Exclusion criteria included age ( < 40 or >70 years); inability or reluctance to comply with study treatment; disease or medication that might interfere with the metabolism of PUFA or CoQ10, including diabetes mellitus and vitamin E consumption; significant liver (total bilirubin >2 mg/100 ml) and renal (serum creatinine >2 mg/100 ml) function impairments; history of a cardiovascular event; hypertension (resting blood pressure >140 mmHg systolic and/or >90 mmHg diastolic); history of pancreatitis; and the use of fish oil capsules or other n-3, n-6 PUFA or CoQ10 supplements within 6 months of the study. Men with baseline abnormal digital rectal examination or transrectal ultrasonography and serum total PSA level >2·5 ng/ml were excluded from the study and referred for further investigations.
Evaluations
All participants underwent complete physical examination and digital rectal examination. The following data were collected from each participant: medical and surgical history, occupational status and educational level, smoking history, height and weight and family history of PCa. The study consisted of five clinic visits: one screening visit, one visit during the baseline period and three visits during double-blind treatment. At screening (week − 8), patients meeting the initial eligibility criteria received detailed explanation regarding study protocol and purposes. Participation was voluntary throughout the study period and subjects were given the opportunity to withdraw at any time they desired. Serum PSA was measured three times at 1-week intervals at baseline (at weeks − 4, − 3 and − 2).
Each visit was preceded by a 12 h fasting, and blood samples were taken for complete blood count, measurement of serum total PSA, TAG, lipid profiles (total cholesterol, LDL and HDL), serum levels of testosterone, dihydrotestosterone (DHT), luteinising hormone (LH) and sex hormone-binding globulin (SHBG) and biochemical safety measures (liver function tests). All men were requested to abstain from sexual activity for at least 24 h prior to blood sampling. Serum PSA levels were measured by a dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA) PSA dual-label free/total PSA kit (Wallac Oy). The detection sensitivity for PSA was 0·05 ng/ml (5 % CV at 2·3 ng/ml). Prostate volume was measured by using transrectal ultrasonography. At each follow-up visit (weeks 4, 8 and 12), serum levels of PSA were assessed two times.
Serum fatty acid composition was measured by using GC, as described previously(Reference Harris, Hibbeln and Mackey27), and expressed as concentrations (μg/l or μmol/l). CV were 3·5 % for EPA, 3·1 % for DHA and 3·2 % for GLA. Blood samples were analysed for total CoQ10 by HPLC using the methodology adapted from that of Tang et al. (Reference Tang, Miles and DeGrauw28). CV for total CoQ10 quantification was 3·3 %.
Because plasma volume and body surface area influence PSA concentrations significantly(Reference Loeb, Carter and Schaeffer29), we calculated body surface area and plasma volume using the following formulas for putting them in multivariate logistic regression analysis:
Of recruited subjects, 504 met study eligibility and consented to proceed with the study protocol.
Randomisation procedure
Included participants were randomly assigned 1:1 to n-3 fatty acids (1·12 g of EPA and 0·72 g of DHA per capsule; EPAX 5500TG, EPAX AS) (group 1, n 126), n-6 fatty acid (600 mg GLA per capsule; Vitex Pharmaceuticals Pty Ltd) (group 2, n 126), CoQ10 (100 mg per capsule; Nutri Q10, Nutri Century) (group 3, n 126) or a similar regimen of placebo (group 4, n 126) for 12 weeks. The placebo was maize oil, selected as it minimally affects the fatty acid content of the typical diet. The maize oil placebo contained 56 % linoleic acid, 28 % oleic acid, 12 % palmitic acid and 4 % of other fatty acids. Study medication was administered as two capsules to be taken twice daily. Patients were asked to maintain a stable, dietitian-advised, low-cholesterol diet for the whole duration of the trial. Adherence to dietary instructions was reinforced at each follow-up clinic visit. No other medication (herbal, synthetic or dietary supplementation) was allowed during the study period. Bottles of identical-appearing medication for each treatment group were set by an independent pharmacist and were assigned a participant number, which was provided by an independent statistician. Investigators, all study personnel, as well as the participants themselves were blinded to the randomisation table, the procedure and the code assignments.
During the 12-week, double-blind treatment phase, patients attended clinic visits at weeks 4, 8 and 12. At every clinic visit, patients were asked to report any possible adverse events or unusual symptoms. Compliance was checked in two ways. First, a capsule-dispensing record was completed and the information was coupled with unused capsule counts. In addition, plasma levels of EPA, DHA, GLA and CoQ10 were measured at baseline ( − 2 weeks) and at weeks 4, 8 and 12 during the treatment period.
End points
The primary end point was percentage change from baseline in mean serum PSA levels at 12 weeks. Secondary end points included changes in fasting serum DHA, EPA, GLA, CoQ10, testosterone, DHT, LH and SHBG levels.
Statistical analysis
Data are expressed as means and standard deviations. The sample size was determined by using an effect size of 0·5 in a one-way ANOVA to give a power of 0·90 with a two-sided α of 0·01. Assuming a potential dropout rate of 20 %, a final sample size of 120 subjects was randomly assigned to each of four study groups (n 480). Because of the exponential distribution of PSA concentrations, serum PSA values were reported as the geometric mean. Student's t- or Mann–Whitney's tests were applied for continuous variables. χ2 or Fisher exact tests were used for categorical data. After testing data for normality (Kolmogorov–Smirnov statistic with Lillefors correction), Student's paired t or Wilcoxon signed-rank test was used to compare values between baseline and treatment at 12 weeks. We used Kruskal–Wallis one-way ANOVA or ANOVA on ranks to compare baseline or treatment effects among study groups. Statistical significance between two groups was examined by unpaired Student's t test. Statistical significance between more than two groups was assessed by one-way ANOVA. A list-wise deletion approach was used to handle missing data, therefore, excluding participants who missed one or two follow-up visits(Reference Howell30). OR and 95 % CI were computed in the multivariable logistic regression model. We put demographic (age, body surface area, plasma volume, prostate volume, occupational status, educational level and smoking status) and clinical (serum testosterone, DHT, LH, TAG, cholesterol, LDL and HDL) covariates into the multivariable logistic regression model. To assess the correlations between serum PSA levels and the continuous and categorical predictor variables, Spearman's correlation coefficient and χ2 tests were used, respectively. Two-tailed P< 0·05 was considered statistically significant. The statistical analysis was carried out by SPSS version 18.0 for Windows (SPSS Inc.).
Results
Characteristics of the participants
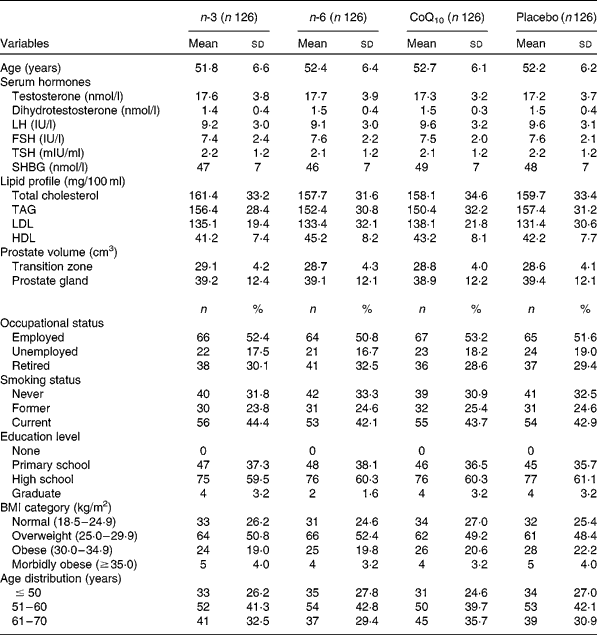
Table 1 demonstrates demographic and clinical characteristics of study participants. Baseline characteristics of randomised subjects revealed no statistically significant differences between the four groups. A total of 600 subjects were screened for eligibility, with 504 subjects (84·0 %) being randomised (126 in each group). Of 504 randomised subjects, seventy-five dropped out of the study. Study withdrawal and absenteeism resulted in 429 subjects completing the whole study period. Overall, the mean age of participants was 52·1 (sd 6·2) years. According to the FFQ completed by the study subjects before and after the study, intake of n-3, n-6 and CoQ10 from foods did not change significantly in the intervention or placebo groups during the study.
Table 1 Frequency distributions of selected variables for cases and controls (Mean values and standard deviations; number of participants and percentages)

CoQ10, coenzyme Q10; LH, luteinising hormone; FSH, follicle-stimulating hormone; TSH, thyroid-stimulating hormone; SHBG, sex hormone-binding globulin.
Primary end point
In Table 2, the effects of the intervention on serum levels of PSA are demonstrated. Placebo treatment non-significantly reduced serum PSA level by 2·3 (95 % CI 1·9, 2·7) %. For simplicity, we refer to the supplements by the major fatty acid names, namely EPA for n-3 fatty acids and GLA for n-6 fatty acid. EPA treatment significantly reduced serum PSA level by 30·0 (95 % CI 25, 36) % (P= 0·004) from baseline. In contrast, GLA therapy significantly increased serum PSA concentration by 15·0 (95 % CI 11, 20) % (P= 0·02). CoQ10 therapy also significantly reduced serum PSA level by 33·0 (95 % CI 27, 40) % (P= 0·002). The magnitude of decrease in serum PSA levels was significantly different when results from EPA and CoQ10 therapy were compared, despite both therapies resulting in a comparable decrease in serum PSA levels (P= 0·01 by ANOVA). Geometric means (95 % CI) of serum levels of PSA in the intervention and placebo groups are shown in Table 2.
Table 2 Serum prostate-specific antigen (ng/ml) levels in study groups before and after intervention (Means, geometric means, arithmetic mean values and 95 % confidence intervals)

NA, not available; CoQ10, coenzyme Q10.
* Covariables included in the multivariate logistic regression model were age, plasma volume, BMI, prostate volume, smoking status, occupational status, educational level and serum testosterone, dihydrotestosterone, luteinising hormone and sex hormone-binding globulin.
† Mean geometric values were used for statistical analyses.
‡ P values refer to the difference in change compared with placebo. All P values are from two-sided χ2 tests.
Secondary end points
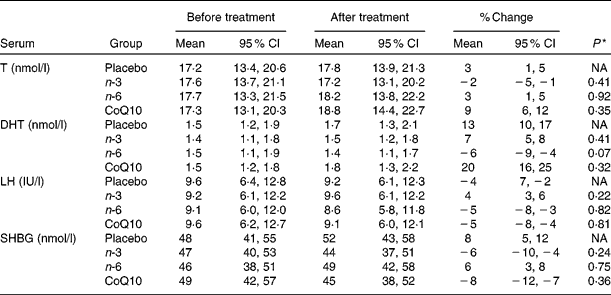
In the intervention group, serum levels of DHA increased by 93 (95 % CI 84, 104) % (P= 0·0001), EPA by 144 (95 % CI 125, 172) % (P= 0·0001), GLA by 140 (95 % CI 126, 177) % (P= 0·0001) and CoQ10 by 91 (95 % CI 84, 99) % (P= 0·0001), whereas DHA and EPA showed a significant decrease by 28 (95 % CI 22, 35) % (P= 0·02) and 17 (95 % CI 14, 21) % (P= 0·03), respectively, in subjects who took GLA. In contrast, in the placebo group, none of the serum levels of DHA, EPA, GLA and CoQ10 changed significantly during the study period. Serum levels of LH, SHBG, testosterone and DHT before and after the intervention are demonstrated in Table 3. In the intervention groups and placebo group, changes in plasma levels of these hormones did not reach statistical significance (for details, see Table 3).
Table 3 Serum concentrations of testosterone (T), dihydrotestosterone (DHT), luteinising hormone (LH) and sex hormone-binding globulin (SHBG) in study groups before and after intervention (Mean values and 95 % confidence intervals)

NA, not available; CoQ10, coenzyme Q10.
* P values refer to the difference in change compared with placebo. All P values are from two-sided χ2 tests.
Secondary analysis
As expected, serum PSA levels were significantly correlated with age (r 0·48; 95 % CI 0·25, 0·67; P= 0·001). To confirm whether multivariate analyses required adjustment for a dose–response, the recorded values of PSA, BMI, plasma volume, age, smoking history, prostate volume and duration of therapy coded as a continuous variable in months were analysed. The multivariable analysis of PSA demonstrated that serum values of PSA were strongly correlated with duration of EPA (r − 0·62; 95 % CI − 0·42, − 0·77; P= 0·003), GLA (r 0·42; 95 % CI 0·31, 0·58; P= 0·02) and CoQ10 use (r − 0·77; 95 % CI − 0·56, − 0·87; P= 0·001). There were also significant correlations between serum values of DHA, EPA, GLA and CoQ10 and serum PSA levels. No significant correlation existed between serum PSA concentrations and testosterone (r 0·24; 95 % CI − 0·031, 0·48; P= 0·24), DHT (r 0·31; 95 % CI − 0·032, 0·54; P= 0·62), LH (r 0·18; 95 % CI − 0·032, 0·37; P= 0·44) and SHBG levels (r 0·17; 95 % CI − 0·032, 0·48; P= 0·49).
Discussion
The present prospective randomised study concluded that men aged 40–70 years, who were administered EPA and CoQ10, had lower serum PSA levels. After adjusting for significant covariates, such as age, plasma volume, BMI and prostate volume, the significance of findings remained unchanged. An effect of PUFA and CoQ10 on serum PSA levels could potentially complicate PCa detection. Men who would be more affected by an unpredicted serum PSA decrease or rise are those with serum PSA concentrations near a cut-off value for which prostate biopsy is indicated. Due to ethical reasons, we only recruited men with serum levels of PSA < 2·5 ng/ml. There are a few well-documented risk factors for PCa, including family history, some specific genetic variants and being of African-American descent(Reference Fowke, Signorello and Chang31, Reference Safarinejad, Shafiei and Safarinejad32). However, a large number of men with PCa do not carry these risk factors, suggesting the presence of some unexplained components in the pathogenesis of PCa. Diet is one of the potential environmental risk factors for developing PCa(Reference Simopoulos33). Over the past two decades, epidemiological studies have addressed the impact of dietary fat on PCa risk(Reference Kolonel34). However, the actual mechanistic role of dietary fat in PCa has remained unknown. Immigrants from Poland and Japan to the USA demonstrate a significant rise in the risk of developing clinical PCa, comparable with the US population(Reference Astorg14). These findings underscore environmental factors, and possibly diet, as significant potential contributing factors. n-3 and n-6 PUFA are among commonly used supplements and may have some effects on prostate biology. Results of studies investigating the effect of n-3 PUFA use on PCa are conflicting. In seven prospective studies, the issue of fish intake and PCa risk have been investigated. Of these studies, three of them reported that high intake of fish might reduce the risk of PCa(Reference Augustsson, Michaud and Rimm35–Reference Terry, Lichtenstein and Feychting37), one reported a positive association(Reference Allen, Sauvaget and Roddam38), while three others had conflicting results(Reference Le Marchand, Kolonel and Wilkens39–Reference Park, Murphy and Wilkens41). We are not aware of other publications directly reporting on the association between EPA, GLA and CoQ10 use and serum PSA levels. However, several observational studies have suggested that n-3 and n-6 PUFA or CoQ10 may have a role in the pathogenesis of PCa. Berquin et al. (Reference Berquin, Min and Wu15) investigated the influence of fatty acids on PCa risk in animals. They found that n-3 PUFA decreased prostate tumour growth, delayed histopathological progression and increased survival, while n-6 PUFA had opposite effects. In another study, Kobayashi et al. (Reference Kobayashi, Barnard and Henning23) examined whether changing the dietary content of n-3 and n-6 PUFA alters the growth of androgen-sensitive PCa xenografts. They found that tumour growth rates, final tumour volumes and serum PSA levels were decreased in the n-3 group compared with the n-6 group. Friedrichs et al. (Reference Friedrichs, Ruparel and Marciniak42) reported that DHA and EPA are able to prevent progression of LNCaP cells, whereas arachidonic acid (n-6 fatty acid) actually accelerated cell growth. This is in line with the present findings. In the present study, GLA (n-6 fatty acid) administration resulted in increased serum PSA level by 15 %. In another study, Akinsete et al. (Reference Akinsete, Ion and Witte21) investigated whether changing from a diet that approximates n-6 fat content of the Western diet to a high-n-3 fat diet might decrease PCa risk in mice. They concluded that consumption of a high-n-3 diet slows down prostate tumorigenesis through decreasing oestradiol and testosterone levels. However, in the present study, serum concentration of testosterone did not alter significantly by EPA administration. In addition, serum PSA levels did not have significant correlations with serum testosterone levels. Also, in a study by Giltay et al. (Reference Giltay, Geleijnse and Heijboer43), n-3 fatty acid supplementation did not affect serum total testosterone levels.
The possible mechanism through which the n-3 PUFA exert their anti-tumour effect is by the anti-inflammatory effects of PUFA, through mediation of cyclo-oxygenase(Reference Reese, Fradet and Witte44). Cyclo-oxygenase is a key enzyme in fatty acid metabolism and inflammation(Reference Reese, Fradet and Witte44). Fradet et al. (Reference Fradet, Cheng and Casey45) reported that n-3 PUFA affect prostate inflammation and carcinogenesis through the cyclo-oxygenase-2 enzymatic pathway. This potential protective effect may be altered by genetic variants in cyclo-oxygenase-2(Reference Reese, Fradet and Witte44). Another mechanism is by the suppression of mammalian target of rapamycin (mTOR) signalling and androgen receptor expression(Reference Friedrichs, Ruparel and Marciniak42).
CoQ10 is considered an important cellular antioxidant and a potential anticancer agent(Reference Crane46). In addition to its role as an antioxidant, other roles include a non-specific stimulant for the immune system(Reference Hattersley47) and a role in membrane stabilisation, inhibition of intracellular phospholipases and stabilisation of Ca-dependent slow channels(Reference Pepping48). Very few studies have addressed the role of CoQ10 on PCa and serum PSA levels, with contradicting results. Two preliminary studies with CoQ10 have demonstrated potential clinical benefits in PCa patients(Reference Schroder, Dijk and Blom49, Reference Folkers, Osterborg and Nylander50). On the other hand, Chai et al. (Reference Chai, Cooney and Franke25) examined the association of serum CoQ10 levels with PCa risk in a case–control study. They did not find a statistically significant association between plasma CoQ10 levels and PCa risk. In another study, Hoenjet et al. (Reference Hoenjet, Dagnelie and Delaere26) assessed the effect of a nutritional supplement containing vitamin E, Se, vitamin C and CoQ10 on serum PSA levels in patients with hormonally untreated PCa and increasing serum PSA levels. The supplementation did not affect serum level of PSA.
The results from the present study suggest that men on EPA and CoQ10 supplementation may require a lower threshold (about 30 %) for PSA screening. However, these findings need to be interpreted with caution. The present study was not without limitation. The main limitation is that the men with PSA levels >2·5 ng/ml were excluded from the study. Currently, the standard cut-off of 4 ng/ml is still considered preferable for men aged between 50 and 70 years. As this is often used as a critical or focal point, exclusion of those patients with total PSA at 2·5–10 ng/ml is questioned. Furthermore, while 2 ng/ml may be normal in a 60-year-old man, that level is more likely to signify cancer in men aged 40–49 years. Thus, addressing the issue of whether fatty acids influence normal PSA levels (with a cancer risk of 1 %) does not appear to be as an important issue as whether those men with PSA of 4–10 ng/ml, who are at a greater risk for PCa, will have PSA levels altered by fatty acids. The present study is a preliminary one, and further studies with different design are needed to draw final conclusions. Another study limitation is that, although we demonstrated a PSA decrease, actually we did not demonstrate that this decrease in PSA delays PCa diagnosis or it results in a decrease in risk of PCa. In addition, although statistically PSA was altered with the duration of treatment, longer studies are necessary to reach an appropriate conclusion. Also, studies need to be carried out to determine whether removal of the treatment allows the PSA values to return back to pre-treatment levels. PSA velocity may be an important tool for assessment. Study strengths include a reasonable sample size, prospective randomised design and the ability to control for a variety of variables.
Conclusion
In conclusion, EPA and CoQ10 use are associated with decreased serum levels of PSA. It may be necessary to establish a different PSA cut-off value for men on these supplements. These findings may have possible clinical implications. Dietary supplements containing n-3 PUFA or CoQ10 may have a protective effect against developing PCa and/or a therapeutic effect in men with PCa.
Acknowledgements
We thank the many clinicians who endorsed the study and all patients and control individuals who participated in the study, without whom the present study would not be possible. We are grateful to Saba Safarinejad for statistical analyses. We have no disclosures to report. We have no conflict of interest, whether of a financial or other nature. We did not make any financial arrangement with a company. We have no commercial affiliations to report. The authors' contributions are as follows: M. R. S. contributed to the conception and design, acquisition of data, analysis and interpretation of data, drafting the article, revising it for intellectual content and final approval of the completed article. N. S. contributed to the acquisition of data, drafting the article and the final approval of the completed article. S. S. contributed to the acquisition of data, analysis and interpretation of data and revising it for intellectual content.