There is increasing evidence that weaning is a critical stage of postnatal growth and gut development in various mammals including rats( Reference Cheung, Yuan and Dyce 1 , Reference Wang, Zhou and Chen 2 ), pigs( Reference Kang, Toms and Yin 3 , Reference Wang, Guo and Zhou 4 ) and humans( Reference Buddington 5 , Reference Jubran, Grant and Duffner 6 ). After weaning in mammals, the immature gastrointestinal tract (GIT) must adapt to the intake of solid diets, bacterial colonisation and the resulting mucosal surface stimulation from both dietary and bacterial antigens. Consequently, post-weaning stress in mammals contributes to growth checks, gut disorders, infections and diarrhoea, simultaneously leading to post-weaning maldigestion and malabsorption( Reference Lallès, Boudry and Favier 7 , Reference Lallès, Bosi and Smidt 8 ).
Indeed, weaning stress involves complex psychological, social, environmental and dietary stresses that interfere with GIT development and adaptation. Interestingly, during and shortly after weaning, the dramatically decreased intake of epidermal growth factor (EGF), which is present at approximately 124 μg/l in sow milk( Reference Lallès, Boudry and Favier 7 ), may be an important cause for reduced digestive and absorptive functions and decreased mucosal defences( Reference Xu, Wang and Zhang 9 ). Accumulating evidence indicates that an exogenous EGF supplements may be effectively taken up by early-weaned animals including rats and piglets( Reference Cheung, Yuan and Dyce 1 – Reference Kang, Toms and Yin 3 ). Administering EGF to weaned piglets orally can increase the activities of jejunal lactase, sucrose and alkaline phosphatase and improve the growth performance( Reference Lee, Chang and Yu 10 ). EGF supplementation in piglet or rat diets has also been shown to facilitate the recovery of intestine health from gut disorders, infection and diarrhoea and to promote protection against colonisation of intestinal pathogens( Reference Lee, Chuang and Chiou 11 ). Kang et al.( Reference Kang, Toms and Yin 3 ) and Wang et al.( Reference Wang, Zhou and Chen 2 , Reference Wang, Guo and Zhou 4 ) also reported that supplementing liquid formula with EGF increased growth performance, intestinal development and Ig levels in early-weaned pigs or rats( Reference Cheung, Yuan and Dyce 1 , Reference Wang, Zhou and Chen 2 ).
In addition, changes in diet, use of antibiotics and intestinal colonisation have likely modified intestinal microbial communities and contributed to the increased prevalence of gut disorders, infection and diarrhoea after weaning( Reference Abraham and Medzhitov 12 ). Alterations in intestinal microbiota have been identified in early-weaned piglets or rats with weaning stress and are associated with high mortality. Thus, homoeostasis exists among members of the microbiota such that potential pathogenic and non-pathogenic organisms can play a key role in the regulation of the intestinal immune system( Reference Nicholson, Holmes and Kinross 13 , Reference Tremaroli and Bäckhed 14 ). To our knowledge, the intestinal microbiota in mammals is a complex biological system comprising a vast repertoire of microbes with considerable metabolic activity relevant to both bacterial growth and host health( Reference Reichardt, Barclay and Weaver 15 ). The intestinal tract of mammals is home to 1013 to 1014 commensal bacteria composed of hundreds of species that benefit the host by supplying nutrients, metabolising otherwise indigestible food and preventing colonisation by pathogens( Reference Kunisawa and Kiyono 16 ). Therefore, minimising contact between luminal micro-organisms and the intestinal epithelial cell surface is an ideal strategy for maintaining homoeostasis with the microbiota of the mammalian intestinal( Reference Zareie, Johnson-Henry and Jury 17 – Reference Resta-Lenert and Barret 19 ). These findings indicate that micro-organisms including Lactobacillus GG, Lactobacillus acidophilus, Bifidobacterium bifidum and Saccharomyces cerevisiae may help decrease weaning stress in mammals( Reference Wang, Zhou and Chen 2 , Reference De Roos and Katan 20 , Reference Mennigen and Bruewer 21 ).
Unfortunately, there is only limited evidence to consider the combination of the delivery of some milk-borne growth factors (e.g. EGF, insulin, insulin-like growth factor 1 and insulin-like growth factor 2) and a micro-organism approach, which may offer possibilities for formulating dietary supplements for child or animal models during their weaning transition stages. Indeed, the ability of S. cerevisiae to express and secrete the biologically active EGF has been previously demonstrated in early-weaned rats( Reference Wang, Zhou and Chen 2 ). In order to explore a suitable form of protein expression, the present study will test the effects of diets supplemented with S. cerevisiae, expressing either intracellular EGF (IE-EGF) extracellular EGF (EE-EGF) or tagged EGF (T-EGF) on the duodenal development and Ig levels of weaned piglets.
Methods
Production of recombinant intracellular epidermal growth factor, extracellular epidermal growth factor and tagged epidermal growth factor-expressing Saccharomyces cerevisiae
IE-EGF-, EE-EGF- and T-EGF-expressing S. cerevisiae strains were designated as INVSc1-IE(+), INVSc1-EE(+) and INVSc1-TE(−), respectively. The INVSc1-IE(+), INVSc1-EE(+), INVSc1-TE(−) and an empty vector-expressing S. cerevisiae (INVSc1(EV)) (as S. cerevisiae control) were generated and cultured as previously described( Reference Wang, Zhou and Chen 2 ).
Animal experiments
The animal procedures that were used in this study were in accordance with the guidelines of the China Animal Protection Association, and all the procedures were approved by the Southwest University Nationalities Animal Care Committee. In total, 200 piglets (Landrace×Duroc) were weaned at 21–26 d of age and randomly assigned to the five following treatment groups: (1) basal diet with medium (control), (2) basal diet with fermented INVSc1(EV), (3) basal diet with fermented INVSc1-TE(−), (4) basal diet with fermented INVSc1-EE(+) and (5) basal diet with fermented INVSc1-IE(+). Thus, forty piglets were assigned to each group, with 4 pens (experimental unit)/ group and 10 piglets/pen. The piglets of each pen had an average initial body weight (BW) between 6·37 and 6·39 kg.
The concentration of EGF in the fermentation product was approximately 30 mg/l, as previously described( Reference Wang, Zhou and Chen 2 ). Throughout the 21-d trial, the diets of the INVSc1(EV), INVSc1-EE(+), INVSc1-TE(−) and INVSc1-IE(+) groups were supplemented with 50 ml (per kg diet) fresh culture from the INVSc1(EV), INVSc1-EE(+), INVSc1-TE(−) and INVSc1-IE(+) strains, respectively. Meanwhile, the control group was only given sterile water (50 ml/kg diet). Thus, all treatments included 60·00 μg/kg BW EGF/d.
The diets (Table 1) were formulated in powder form without any in-feed antibiotics according to the National Research Council guidelines( 22 ) for 5–10 kg piglets and contained similar nutrient levels but differed in terms of whether they contained S. cerevisiae expressing IE-EGF, EE-EGF or T-EGF.
Table 1 Composition and nutrient levels of the basal diet (as fed-basis)
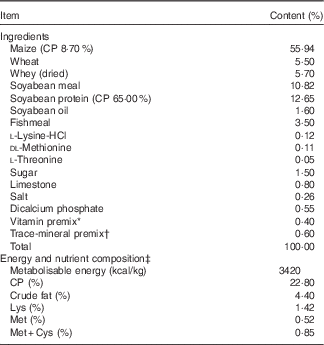
CP, crude protein.
* Vitamins were provided in the following amounts per kilogram of the diet: vitamin A, 3 mg; vitamin D3, 37·50 μg; vitamin E, 33·33 mg; vitamin K3, 2·50 mg; vitamin B12, 60 μg; vitamin B1, 4·50 mg; vitamin B2, 12 mg; niacin, 60 mg; pantothenic acid, 36 mg; folic acid, 1 mg; vitamin B6, 10 mg; biotin, 0·50 mg; and vitamin C, 200 mg.
† Trace minerals were provided in the following amounts per kilogram of the diet: Fe, 100 mg; Cu, 6 mg; Mn, 4 mg; Zn, 100 mg; I, 0·30 mg; Co, 0·14 mg; and Se, 0·30 mg.
‡ Calculated values unless indicated otherwise.
The piglets had ad libitum access to water and feed, and the remaining feed was weighed at 08.00 hours each day. The BW and average daily feed intake (ADFI) were recorded weekly to estimate the average daily gain (ADG) and feed:gain ratio (F:G). In addition, signs of diarrhoea, sickness and abnormal behaviour were also recorded during the 21-d feeding trial.
Sample collection and processing
On days 0, 7, 14 and 21, respectively, two piglets (approach to average BW in each pen) from each pen (4 pens/group) were chosen to be killed with sodium pentobarbital (50·00 mg/kg BW) using intravenous injections to sample the small intestine. Immediately after slaughter, the contents of the entire duodenum (an approximately 10-cm section beginning at the pylorus) were rapidly removed with ice-cold PBS. After slitting lengthwise and further gentle rinsing with ice-cold PBS, mucosa from the duodenum was scraped with a glass slide and then rapidly placed in liquid N2 for further analysis. In addition, the 2·50-cm duodenal segments were rinsed with ice-cold PBS and fixed in 10 % neutral formalin to measure the overall duodenum morphology.
Assessment of the morphology of the duodenum
The samples of duodenum were prepared according to Liu et al.( Reference Liu, Piao and Thacker 23 ). The fixed samples were embedded in paraffin, and 4–5-μm sections were mounted on poly-Lys-coated slides, de-paraffinised and re-hydrated. The slides from each sample were stained with haematoxylin and eosin (HE) to examine the duodenum morphology. Furthermore, light microscopy analyses at ×40 magnification were performed on the paraffin-embedded duodenum tissue sections using HE staining to examine mucosa morphology of the duodenum after the respective treatments. Approximately ten selected villi or crypts per sample were measured for their architecture using image analysis software (Motic Images Advanced 3.2; Motic Co.). In addition, the villus height:crypt depth (VH:CD ratio) ratio was calculated.
Determination of digestive enzyme activities and Ig levels in duodenal mucosa
The levels of IgA, IgM and IgG in the duodenal mucosa of weaned piglets were determined using an ELISA Kit (RD Co.).
Enzyme activities of alkaline phosphatase (ALP), creatine kinase (CK), lactate dehydrogenase (LDH) and sucrase in the duodenal mucosa of weaned piglets were assayed by Enzyme Activity Detection Kits (NanJing JianCheng Bioengineering Institute).
RNA preparation and real-time reverse transcription-PCR
Total RNA was extracted using Trizol (Invitrogen Co.) according to the manufacturer’s protocol. The RNA concentration was measured using a NanoDrop ND-1000 spectrophotometer (Agilent Co.). The purity of total RNA was determined by A260:280 and A260:230 ratios. RNA quality was further analysed via agarose gel electrophoresis.
In this study, the A260:280 and A260:230 ratios ranged from 1·90 to 2·05 and from 2·00 to 2·10, respectively, which indicated that the samples were of good quality. For each sample, 1·00 μg of total RNA was reverse transcribed for complementary DNA (cDNA) synthesis using a PrimeScript™ RT Reagent Kit with a gDNA Eraser (Takara Bio Inc.) according to the manufacturer’s protocol.
Quantitative real-time reverse transcription-PCR
Real-time PCR reactions were performed in ninety-six-well plates with a Bio-Rad CFX96TM Real-time PCR Detection System. Reactions were carried out in a final volume of 15·0 μl, containing 7·5 μl of 2×SYBR Premix EX Taq II, 500 nM of each primer, 1 μl of cDNA and 5·5 μl of DNase (deoxyribonuclease)/RNase (ribonuclease)-free water. The cycling programme was 95°C (5 min), followed by 40 cycles of 95°C (10 s), 60°C (20 s) and 72°C (20 s). A melting curve analysis was carried out by heating samples from 65 to 95°C with continuous fluorescent acquisition. All reactions were performed in triplicate for each cDNA sample. Standard curves were established using a 10-fold dilution series of purified PCR fragments as templates.
Selection of reference genes, target gene and primer design
To further study the contributions of gene transcription of digestive enzymes (CK, ALP, LDH and sucrase) and epidermal growth factor receptor (EGF-R), real-time reverse transcription-PCR (RT-PCR) analyses were carried out. We determined the changes in relative abundance of mRNA of these genes in the duodenal mucosa (Fig. 4). In addition, five appropriate reference genes encompassing hydroxymethylbilane synthase (HMBS), hypoxanthine phosphoribosyltransferase 1 (HPRT1), 18S ribosomal RNA (18S), β-actin and β-2-microglobulin (B2M) in piglet intestines were also assessed in this study. The primer pairs in this study were designed by Primer 5.0 software based on the swine sequence information available in GenBank. Primer details are listed in Table 2. Before quantitative real-time RT-PCR, conventional PCR and agarose gel electrophoresis were performed to assay the gene-specific primers and verify the amplified products. All PCR products were sequenced and then aligned against the pig genome with the BLAST (Basic Local Alignment Search Tool) program to verify their identity.
Table 2 Effect of the different forms of epidermal growth factor on growth performance of weaned piglets (Mean values with their standard errors)
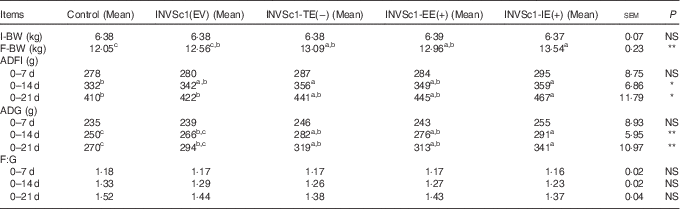
I-BW, initial body weight; F-BW, final body weight; ADFI, average daily feed intake; ADG, average daily gain; F:G, feed:gain ratio.
a,b,c Means values within the row unlike superscript letters were significantly different (P<0·05), NS at P>0·05; * significant at the 5·00 % level; ** significant at the 1·00 % level.
Data analysis
Three classic software programs, geNorm, NormFinder and BestKeeper, each with a unique advantage, were used to evaluate the reference genes encompassing HPRT1, HMBS, 18S, B2M and β-actin. Next, the ratio of the expressions of the target genes (e.g. ALP, CK, LDH, Sucrase and EGF-R) relative to the reference genes was calculated using the 2−ΔΔCT method( Reference Schmittgen and Livak 24 ), in which ΔΔCT=((CTtarget gene−CTreference gene)treatment groups−(CTtarget gene−CTreference gene)control group).
The data for growth performance, morphology, digestive enzyme activities and Ig levels in duodenal mucosa, as well as relative gene expressions, were assessed using each pen as the experimental unit in the present study. The results were analysed using multi-way ANOVA with different treatments and growth stages as two factors using the PROC ANOVA procedure of SAS software package (version 9.0). Duncan’s multiple-range test was further performed for multiple comparisons when the multi-way ANOVA results were significant. A P-value<0·05 was considered to be statistically significant, and P<0·10 was considered a trend.
Results
Effects of different forms of epidermal growth factor-expressing Saccharomyces cerevisiae on growth performance of weaned piglets at different growth stages
As shown in Table 3, from 0 to 14 d or 21 d, the growth performance including ADG and ADFI of weaned piglets significantly increased in the INVSc1-EE(+), INVSc1-TE(−) and INVSc1-IE(+) groups compared with the control and INVSc1(EV) groups (P<0·05).The effects of different forms of EGF-expressing S. cerevisiae including the INVSc1-EE(+), INVSc1-TE(−) and INVSc1-IE(+) on the growth performance of weaned piglets were further analysed. From 0 to 14 d or 21 d, the ADG, ADFI and F:G showed no differences in the INVSc1-IE(+) group compared with the INVSc1-TE(−) and INVSc1-EE(+) groups (P>0·10).
Table 3 Primers and relative information of the reference and target genes

ALP, alkaline phosphatase; CK, creatine kinase; LDH, lactate dehydrogenase; EGF-R, epidermal growth factor receptor; B2M, β-2-microglobulin; HMBS, hydroxymethylbilane synthase; HPRT1, hypoxanthine phosphoribosyltransferase 1; 18S, 18S ribosomal RNA.
Effects of different forms of epidermal growth factor-expressing Saccharomyces cerevisiae on the mucosa morphology of the duodenum in weaned piglets
The morphologies of the duodenum segment are shown in Fig. 1 and Table 4. On 7, 14 and 21 d, the VH, CD and the VH:CD ratio of the duodenum segment significantly increased in the INVSc1-EE(+), INVSc1-TE(−) and INVSc1-IE(+) groups compared with the control and INVSc1(EV) groups (P<0·05). In addition, on 7, 14 and 21 d, the VH, CD and the VH:CD ratio of the duodenum segment did not differ among these groups including INVSc1-IE(+), INVSc1-TE(−) and INVSc1-EE(+) (P>0·10).

Fig. 1 Duodenal morphology of weaned pigs that received different diets. Representative light micrograph of a cross-section of the duodenum from the control group (piglets that were fed the basal diet); the INVSc1(EV) group (piglets that were fed the basal diet plus the fermented INVSc1(EV) strain); the INVSc1-TE(−) group (piglets that were fed the basal diet plus the fermented INVSc1-TE(−) strain); the INVSc1-EE(+) group (piglets that were fed the basal diet plus the fermented INVSc1-EE(+) strain); and the INVSc1-IE(+) group (piglets that were fed the basal diet plus the fermented INVSc1-IE(+) strain). Images were taken at 40× magnification using the light microscope; the scale bar is equivalent to 100 μm.
Table 4 Effect of different forms of epidermal growth factor on the duodenum morphology of weaned piglets (Mean values with their standard errors)

VH:CD ratio, villus height:crypt depth.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05), NS at P>0·05; * significant at the 5·00 % level; ** significant at the 1·00 % level; *** Significant at the 0·10 % level.
Effects of different forms of epidermal growth factor-expressing Saccharomyces cerevisiae on Ig levels of the duodenal mucosa in weaned piglets
Throughout the 21-d feeding trial (on 7, 14 and 21 d), the Ig levels (IgA, IgG and IgM) of the duodenal mucosa were significantly stimulated in the INVSc1-TE(−), INVSc1-EE(+) and INVSc1-IE(+) groups compared with the INVSc1(EV) and control groups (P<0·01) (Fig. 2). In addition, on 7, 14 and 21 d, a trend (0·05<P<0·10) was observed in which the INVSc1-IE(+) group showed a significant increase in Ig levels (IgA, IgG and IgM) compared with the NVSc1-TE(−) and INVSc1-EE(+) groups.

Fig. 2 Effects of different forms of epidermal growth factor on Ig levels in the duodenal mucosa in weaned piglets. a,b,c,d Mean values with unlike letters were significantly different (P<0·05). ![]() , Control;
, Control; ![]() , empty vector-expressing Saccharomyces cerevisiae (INVSc1(EV));
, empty vector-expressing Saccharomyces cerevisiae (INVSc1(EV)); ![]() , tagged epidermal growth factor protein-expressing S. cerevisiae (INVSc1-TE(−));
, tagged epidermal growth factor protein-expressing S. cerevisiae (INVSc1-TE(−)); ![]() , extracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-EE(+));
, extracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-EE(+)); ![]() , intracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-IE(+)).
, intracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-IE(+)).
Effects of different forms of epidermal growth factor-expressing Saccharomyces cerevisiae on the digestive enzyme activities of duodenal mucosa in weaned piglets
As shown in Fig. 3, on 7, 14 and 21 d, the activities of digestive enzyme markers (CK, ALP, LDH and sucrase) of the duodenal mucosa were significantly higher in the INVSc1-TE(−), INVSc1-EE(+) and INVSc1-IE(+) groups compared with the INVSc1(EV) and control groups (P<0·05). Moreover, on 7, 14 and 21 d, the activities of digestive enzymes including CK, ALP, LDH and sucrase showed no significant differences in the INVSc1-IE(+) group compared with the INVSc1-TE(−) and INVSc1-EE(+) groups (P>0·10).

Fig. 3 Effects of different forms of epidermal growth factor on digestive enzyme activities of the duodenal mucosa in weaned piglets. CK, creatine kinase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase. a,b,c,d Mean values with unlike letters were significantly different (P<0·05). ![]() , Control;
, Control; ![]() , empty vector-expressing Saccharomyces cerevisiae (INVSc1(EV));
, empty vector-expressing Saccharomyces cerevisiae (INVSc1(EV)); ![]() , tagged epidermal growth factor protein-expressing S. cerevisiae (INVSc1-TE(−));
, tagged epidermal growth factor protein-expressing S. cerevisiae (INVSc1-TE(−)); ![]() , extracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-EE(+));
, extracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-EE(+)); ![]() , intracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-IE(+)).
, intracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-IE(+)).
Effects of different forms of epidermal growth factor-expressing Saccharomyces cerevisiae on the mRNA expressions of digestive enzymes and epidermal growth factor receptor in the duodenal mucosa of weaned piglets
The identification results of candidate reference genes and target genes are shown in Tables 5 and 6, and online Supplementary Figs. S1 and S2. In this study, B2M, HMBS and HPRT1 were the optimal reference genes for analysing the mRNA expressions of target genes after being assessed using three software programmes – geNorm, NormFinder and BestKeeper. Fig. 4 shows the mRNA expressions of digestive enzymes (CK, ALP, LDH and sucrase) and EGF-R in the duodenal mucosa of weaned piglets among all groups. On 7, 14 and 21 d, weaned piglets supplemented with INVSc1-EE(+), INVSc1-TE(−) and INVSc1-IE(+) incrementally induced the mRNA expressions of digestive enzymes (CK, ALP, LDH and sucrase) and EGF-R compared with the control and INVSc1(EV) groups (P<0·01). In addition, mRNA expression levels of digestive enzymes and EGF-R in the duodenal mucosa were significantly higher in the INVSc1-IE(+) group compared with INVSc1-EE(+) and INVSc1-TE(−) groups (P<0·05).

Fig. 4 Effects of different forms of epidermal growth factor on mRNA levels of digestive enzyme and epidermal growth factor receptor (EGF-R) in the duodenal mucosa of weaned piglets. CK, creatine kinase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase. a,b,c,d Mean values with unlike letters were significantly different (P<0·05). ![]() , Control;
, Control; ![]() , empty vector-expressing Saccharomyces cerevisiae (INVSc1(EV));
, empty vector-expressing Saccharomyces cerevisiae (INVSc1(EV)); ![]() , tagged epidermal growth factor protein-expressing S. cerevisiae (INVSc1-TE(−));
, tagged epidermal growth factor protein-expressing S. cerevisiae (INVSc1-TE(−)); ![]() , extracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-EE(+));
, extracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-EE(+)); ![]() , intracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-IE(+)).
, intracellular expressing epidermal growth factor-expressing S. cerevisiae (INVSc1-IE(+)).
Table 5 Sequencing results of PCR products from the amplification of primers of reference and target genes designed for this experiment

ALP, alkaline phosphatase; CK, creatine kinase; LDH, lactate dehydrogenase; EGF-R, epidermal growth factor receptor; B2M, β-2-microglobulin; HMBS, hydroxymethylbilane synthase; HPRT1, hypoxanthine phosphoribosyltransferase 1; 18S, 18S ribosomal RNA.
Table 6 Sequencing results of genes using BLASTN (Basic Local Alignment Search Tool, nucleotides) from the National Center for Biotechnology Information (NCBI) against nucleotide collection

Discussion
A few new approaches to improve the health of the GIT in mammals during and shortly after weaning have been explored in recent years( Reference De Roos and Katan 20 , Reference Mennigen and Bruewer 21 , Reference Bron, van Baarlen and Kleerebezem 25 ). Interestingly, the combination of functional proteins (e.g. EGF, insulin and insulin-like growth factor) associated with repairing intestinal injury using micro-organisms (e.g. Lactobacillus, S. cerevisiae and B. bifidum) has increased the interest of researchers focused on addressing gastrointestinal dysfunction in humans, pigs and other mammals( Reference Cheung, Yuan and Dyce 1 , Reference Wang, Zhou and Chen 2 , Reference Lee, Chuang and Chiou 11 ). There is still limited research on the application of this method.
In the present study, the effects of IE-EGF-, EE-EGF- and T-EGF-expressing S. cerevisiae on the development and Ig levels of the GIT in weaned piglets were analysed. The results showed that supplementing diets with recombinant EGF-expressing S. cerevisiae stimulated the mRNA expressions of digestive enzymes (CK, ALP, LDH and sucrase) and EGF-R. The increased enzyme activities may have facilitated the development and immune function of the GIT in weaned piglets. The brush-border enzymes including ALP, CK, LDH and sucrase are used as cell markers of villus maturation, and their activities served as markers of small intestine damage in some studies( Reference Lee, Chuang and Chiou 11 , Reference James, Smith and Tivey 26 Reference Zijlstra, Odle and Hall – Reference Lackeyram, Yang and Archbold 28 ). ALP is known to be involved in fat absorption( Reference Zhang, Shao and Xie 29 ) and detoxification of luminal pathogenic bacterial lipopolysaccharides( Reference Zarepour, Bhullar and Montero 30 ). It is regarded as an enterocyte differentiation marker enzyme( Reference Hodin, Chamberlain and Meng 31 ). Previous studies have revealed decreased intestinal ALP expression and activity in weaned pigs( Reference Lackeyram, Yang and Archbold 28 ), suggesting the decrease may be responsible for growth decrease at this stage. Sucrase is also a brush-border membrane enzyme responsible for the terminal digestion of sucrose, and it has often been used as an indicator of gut maturity( Reference Lee, Chuang and Chiou 11 , Reference Bedford, Huynh and Fu 32 , Reference Xu, Wang and Zhang 33 ). In rats and piglets, treatment with EGF has been shown to stimulate sucrase activity( Reference Lee, Chuang and Chiou 11 , Reference James, Smith and Tivey 26 , Reference Xu, Wang and Zhang 33 , Reference Foltzer-Jourdainne, Garaud and Nsi-Emvo 34 ). This increased activity in EGF-treated piglets was suggested to be due to young enterocytes continuing to accumulate sucrase in their brush-border membranes when stimulated by exogenous EGF( Reference James, Smith and Tivey 26 ). The activities of CK and LDH are also generally considered as chemical indices of physical stress and indicators of muscle damage and muscle fatigue, even though they can be released into the blood under other circumstances( Reference Perez, Palacio and Santolaria 35 – Reference Wan, Yin and Xu 37 ). Therefore, the changes in CK and LDH activities are often used to reflect stress-coping characteristics and metabolic status of the animal. Some publications have demonstrated that EGF enhances intestinal brush-border enzyme activities encompassing CK and LDH( Reference Lee, Chuang and Chiou 11 , Reference Jaeger, Lamar and Cline 38 , Reference Wong and Wright 39 ).
As shown in this study, the diets of weaned piglets supplemented with EGF can elevate the activities of ALP, CK, LDH and sucrase in the duodenal mucosa of weaned piglets. Numerous studies have reported the stimulatory effects of EGF on the activities of ALP, CK, LDH and sucrase in neonatal or weaned animals( Reference Lee, Chuang and Chiou 11 , Reference Bedford, Huynh and Fu 32 , Reference Jaeger, Lamar and Cline 38 ). This may be due to the suppression of villus cell apoptosis induced by EGF, which then leads to increased brush-border enzyme activities( Reference Knott, Juno and Jarboe 40 , Reference Banan, Zhang and Farhadi 41 ). A previous report has also demonstrated that EGF might regulate and control Cdx2 (a member of the caudalrelated homeobox gene family) in the proliferation and migration of small intestinal brush border( Reference Uesaka, Lu and Katoh 42 ). Here, the increases in the mRNA expressions of digestive enzymes including ALP, CK, LDH and sucrase coincided with the tendency to increase its activities in these piglets. In fact, our results are consistent with previous reports regarding the possible roles of EGF( Reference Lee, Chang and Yu 10 , Reference Bedford, Huynh and Fu 32 , Reference Xu, Wang and Zhang 33 ).
There are also studies on the effects of EGF on IgA, IgG and IgM secretion in the duodenal mucosa. In fact, the Ig including IgA, IgG and IgM are important immune effectors and were considered to reflect the health status of humans and animals in other studies( Reference Wang, Zhou and Chen 2 , Reference Schroeder and Cavacini 43 – Reference Lindner, Wahl and Föhse 46 ). IgA is critical for protecting mucosal surfaces against toxins, viruses and bacteria by means of neutralising or preventing them from binding to the mucosal surface( Reference Schroeder and Cavacini 43 ). Meanwhile, IgA also plays a role in the maintenance of mucosal homoeostasis, which may also affect the development of systemic immunity and determine the composition of the intestinal microbiota( Reference Lammers, Wieland and Kruijt 44 ). IgG, expressed on B cells, is the main antibody isotype in blood( Reference Hirano, Yasukawa and Harada 45 ). IgG antibodies directly contribute to an immune response including neutralisation of toxins and viruses( Reference Lindner, Wahl and Föhse 46 ). IgM is the first Ig expressed on the surface of B cells, and opsonises (coating) antigens for destruction by fixing complement. Meanwhile, IgM antibodies are also associated with the primary immune response and are frequently used to diagnose acute exposure to an immunogen or pathogen( Reference Schroeder and Cavacini 43 ). Our results further support other reports on the stimulatory effect of EGF on the levels of IgA, IgG and IgM in neonatal or weaned animals. For example, weaned piglet diets that were supplemented with recombinant EGF-expressed Pichia pastoris enhanced the levels of mucosal IgA and accelerated intestinal development to improve the health of the GIT during the post-weaning period( Reference Lee, Kuo and Chen 47 ). Wang et al.( Reference Wang, Zhou and Chen 2 ) also demonstrated that recombinant EGF-expressed S. cerevisiae could stimulate the levels of IgA, IgG and IgM in the blood to reduce weaning stress in early-weaned rats.
Moreover, our present study is also consistent with a previous hypothesis that IE-EGF exhibited higher bioactivity than EE-EGF and T-EGF in vivo and in vitro ( Reference Wang, Zhou and Chen 2 ). EGF-R are widely present in both basolateral surfaces and microvillar membranes of enterocytes and have been suggested to be ten times more prevalent in the intestine than EGF itself( Reference Miettinen, Perheentupa and Otonkoski 48 ), indicating that exogenously supplemented EGF may be taken up by animals or humans. In fact, EGF can bind effectively to its receptor, which contains an extracellular mitogen-binding site and a cytoplasmic domain possessing tyrosine kinase activity. Binding of the ligand to the receptor results in a second-messenger cascade that culminates in mitosis and (or) differentiation of the target cells. The EGF-R also mediates transforming growth factor α in mammalian tissues to exert its effects in the intestine via its receptors, which are present on the the luminal surface and basolateral membrane( Reference Xian and Shandala 49 ). Our results also show that the diets of weaned piglets supplemented with the same dose of IE-EGF significantly increased the mRNA expression of EGF-R in the duodenal mucosa compared with EE-EGF and T-EGF. Thus, for EGF to elicit trophic effects on the intestines of suckling neonates or weaned mammals, this protein must survive the digestive process and be present at a sufficiently high concentration to bind to EGF-R in order to stimulate cell growth and differentiation( Reference Cheung, Yuan and Dyce 1 , Reference Wang, Zhou and Chen 2 ).
A few studies have considered the differences in biological activities among different forms of the recombinant protein. A previous study on supplementation of exogenous EGF reported no change in pancreatic amylase activity via orogastric administration, whereas the opposite result was observed via intraperitoneal administration( Reference O’Loughlin, Chung and Hollenberg 50 ). The influences of dietary EGF supplementation occur via different routes, and EGF supplied orally is typically destroyed by digestive hydrolysis in the GIT, consequently losing its potency( Reference Chao, Liu and Chen 51 ). In newborns or mammals, gastric secretion is attenuated, and proteolytic digestion is incomplete( Reference Hartma, Hays and Baker 52 ). Approximately 70 % of the EGF reaches the middle of the small intestine as immunologically intact EGF( Reference Goldman, Atkinson and Hanson 53 ). Thus, a portion of EE-EGF is degraded by digestive enzymes in the oral cavity or the GIT( Reference Wang, Zhou and Chen 2 ). In our previous studies, we also found that the biological activities of IE-EGF were higher than those of EE-EGF and T-EGF in vivo and in vitro. In addition, Western blot analysis only confirmed the presence of IE-EGF or EE-EGF, but not of T-EGF. Thus, T-EGF cannot sufficiently bind the EGF-R to stimulate its biological activity, because the combination of the tagged peptide and EGF affects its primary structure( Reference Wang, Zhou and Chen 2 ). In this manner, IE-EGF expressed by recombinant S. cerevisiae is able to avoid these interference factors. Therefore, almost all of the recombinant EGF reaches the intestine in a fully biologically active form.
Conclusion
In conclusion, the present study demonstrated that including recombinant EGF-expressing S. cerevisiae to the diet of weaned piglets significantly improved Ig levels, enzyme activity and mRNA levels and EGF-R in duodenal mucosa, as well as intestinal development and growth performance. Moreover, the biological activities (e.g. the levels of IgA, IgG and IgM, mRNA expression of digestive enzymes and EGF-R) of IE-EGF were better than either EE-EGF or T-EGF. Thus, the results of the present study also further support the combination of functional protein delivery and a micro-organism approach to prevent the problems of intestinal diseases during weaning.
Acknowledgements
The authors thank the teachers and workers at Shenzhen Premix Inve Nutrition Co. Ltd.
This study was financially supported by the Animal Science Discipline Program of Southwest University for Nationalities (2014XWD–S0905).
S. W., C. G. and L. Z. conceived and designed the experiments. S. W., C. G., L. Z., Z. Z., W. Z., Y. H. and Z. Z. were involved in the generation, collection, assembly and analysis of the data. S. W., T. T. J. M. B. and T. G. M. F. G. were involved in the drafting and revision of the manuscript. S. W., C. G. and L. Z. approved the final version of the manuscript. All authors have contributed to, seen and approved the final, submitted version of the manuscript.
There were no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516000738













