To achieve optimal growth, preterm infants need better nutrition in the neonatal period than any other time of their life because of decreased intra-uterine nutrient deposition( Reference Hay, Lucas and Heird 1 ). The American Academy of Pediatrics recommends that breast milk should be the preferred feeding for all infants. Formula milk is recommended when human milk supply is inadequate or the mother is unable to breast-feed( Reference Eidelman, Schanler and Johnston 2 ). Meanwhile, preterm infants often require additional supervisions and support systems compared with term infants, because their buccal coordination and swallowing mechanisms are not fully matured( Reference Wang, Dorer and Fleming 3 ). In addition, despite lower weight and shorter length than term infants upon discharge, preterm infants have been found to show a catch-up growth and abnormal adiposity at term corrected age, which indicates a potential risk factor for CVD( Reference Roggero, Giannì and Amato 4 , Reference Uthaya, Thomas and Hamilton 5 ). Therefore, it is essential to monitor growth and body composition changes continuously in relation to different nutrition interventions, because growth pattern and body composition appear to have a long-term effect on health outcomes( Reference Roggero, Giannì and Piemontese 6 ).
It is quite clear that a period of rapid growth would be likely to have negative effects on long-term health outcomes( Reference Sighal and Lucas 7 ), and unbalanced catch-up growth of fat mass (FM) could attribute to this association. At the same time, two types of rapid catch-up growth in preterm infants exist( Reference Ong 8 ): one is paralleled by an increase in ‘predominantly FM’ and the other by ‘predominantly fat-free mass (FFM)’. Moreover, evidence suggests that FFM and FM can also provide precise determinations of body composition( Reference Mazess, Barden and Bisek 9 ). Therefore, important implications can be obtained by measuring FM and FFM.
Despite the critical inter-relationship between early nutrition, growth, development, and subsequent health, a few data are available on changes of body composition in preterm infants during the 1st year of life. In addition, systematic reviews that determine the effect of formula milk feeding compared with maternal breast milk feeding on rate of growth and developmental outcomes in preterm or low birth weight infants have been inconclusive, because no data from randomised trials of formula milk v. maternal breast milk for feeding preterm or low birth weight infants could be obtained( Reference Henderson, Anthony and McGuire 10 ). Meanwhile, the study of Fewtrell et al.( Reference Fewtrell, Kennedy and Murgatroyd 11 ) suggested that breast-feeding or high-sn-2 infant formula has no significant effect on bone mass in the long term.
The purpose of our study was to assess the effects of breast-feeding v. formula-feeding on body composition by collecting all the evidence available from cohort studies comparing the effects of breast-feeding v. formula-feeding on preterm infant body composition.
Methods
Protocol and registration
Our systematic review was registered at http://www.crd.york.ac.uk/PROSPERO/, with the registration number CRD42015023335.
Eligibility criteria
-
(1) The participants were preterm infants (≤37 weeks of gestation at birth and/or 2500 g) without congenital malformations or complications affecting body composition.
-
(2) The types of exposure were breast-feeding (exclusive or predominant) and formula-feeding (exclusive or predominant). Broadly, formula milk can be considered as follows: (i) ‘term’ formulae, designed for term infants providing 280–293 kJ/100 ml (67–70 kcal/100 ml); (ii) ‘preterm’ formulae, designed to provide nutrient intakes to match intra-uterine accretion rates with energy enrichment (about 335 kJ/100 ml (80 kcal/100 ml)), variably protein and mineral enrichment( Reference Fewtrell and Lucas 12 ). The effects of breast-feeding and formula-feeding on body composition were measured at the same time points.
-
(3) The outcomes were FM (kg), FFM (kg) and the percentage of FM. We excluded studies in which body composition was measured by skinfold thickness because of its poor ability to predict body composition( Reference de Bruin, van Velthoven and Stijnen 13 ).
-
(4) The types of studies were cohort studies; no language restrictions were placed. Review articles and commentaries were excluded.
Information sources
This systematic review was designed and carried out according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses( Reference Moher, Liberati and Tetzlaff 14 ). This included electronic searches of databases such as PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), Cochrane Central Library Issue, Ovid(medline), Embase and other resources (such as Google Scholar). All databases were searched from their earliest records to May 2015 and were updated in August 2015.
Search
Subject terms, keywords and truncation symbol were used in the search strategy. The search method was adjusted in accordance with each database, using a combination of key words such as ((‘Premature Birth’) OR (‘Infant, Premature’) OR (‘Labor, Premature’)) AND ((‘Infant formulas’) OR (‘artificial formula’) OR (‘breast feeding’) OR (‘human milk’)) AND ((‘body composition’) OR (‘Body Fat Distribution’)), as well as Medical Subject headings (MeSH) terms; for example, (‘Breast feeding’) AND (‘Infant formula’) AND (‘Infant premature’) AND (‘Body composition’). The search strategy details are given in the online Supplementary material. References of eligible articles and previous reviews were manually searched for studies probably suitable for inclusion.
Study selection
We identified relevant studies by examining the titles and abstracts of all studies or by obtaining a full text of the article if no abstract was available. The potentially eligible articles were reassessed by retrieving and evaluating the full text. Screening was conducted independently by two reviewers (P. H. and J. Z.). Inter-reviewer reliability for the study selection process was determined by the Kappa test. The consistency of our study was 61·3 %. In case of disagreement for inclusion or exclusion, the issue was discussed until consensus was achieved by the reviewers (P. H. and J. Z.).
Data collection process
Data were collected by two independent review authors (P. H. and J. W.). Efforts were made to contact authors for additional data if the articles were suitable for a meta-analysis. Authors were asked to provide mean values and standard deviations for primary outcomes including FM, FFM and the percentage of FM. We attempted to send the second request if our first request did not yield a response. The study was excluded from the meta-analyses if the author was unable to provide additional data.
Data items
The following data were extracted from the included studies: study design, year of publication, location, demographic characteristics of the participants, definition of exposure, measuring technology of body composition, outcome and potential sources of bias.
Risk of bias in individual studies
All the authors assessed the risk of bias independently using the Newcastle–Ottawa Scale (NOS): (1) representativeness of the exposed cohort, (2) comparability of groups, (3) blinding of investigators who measured outcomes, (4) the time and completeness of follow-up, (5) contamination bias and (6) other potential sources of bias. Articles were scored as follows: ≥7=high quality (NOS).
Summary measures
We calculated the mean difference and 95 % CI of outcome at each postnatal age point between the formula-fed and breast-fed groups.
Synthesis of results
We performed a meta-analysis on studies that reported the outcomes (FM, FFM and the percentage of FM) between formula-fed and breast-fed groups at the following time points: 32-week corrected gestational age, 36-week gestational age, term, 3-month corrected age, 6-month corrected age and 12-month corrected age. We calculated the mean difference and 95 % CI of outcome at each postnatal age point between the formula-fed and breast-fed groups. The χ 2 test for Cochrane’s Q statistic and I 2 were used to test heterogeneity( Reference Higgins and Thompson 15 ). A fixed-effects meta-analysis was undertaken with RevMan 5 software (The Cochrane Collaboration) by using the inverse variance method (P≥0·05; χ 2 test). In contrast, a random-effects meta-analysis was carried out. This method was performed separately for each postnatal age point.
Risk of bias across studies
Publication bias was assessed using funnel plots.
Additional analyses
We intended to conduct subgroup analyses for sex and the same measuring technique of outcome.
Results
Study selection and characteristics
The characteristics of included and excluded studies are outlined in Fig. 1. A total of 630 articles were retrieved by search strategy, and eight articles were additionally identified by assessing the references of the retrieved articles and other sources. In total, 206 articles were removed as they were duplicate records of the same report through reference management software and manual screening. After examination of titles and abstracts, thirty articles were considered relevant; six studies were included and twenty-four studies were excluded after full-text review for the following reasons: the articles included breast-fed infants or formula-fed infants separately( Reference Aimone, Rovet and Ward 16 , Reference Koo and Hockman 17 ); the primary outcomes were not reported( Reference Kagan, Stanincova and Felix 18 – Reference Goswami, Rochow and Fusch 23 ); the measurement method of body composition was skinfold thickness only( Reference Dewey, Hawck and Brown 24 ); articles were reviews( Reference Roggero, Giannì and Piemontese 6 , Reference Greer 25 – Reference Juretic and Guszak 27 ); the subjects were not preterm infants( Reference de Zegher, Sebastiani and Diaz 28 , Reference Butte, Wong and Hopkinson 29 ); the language used was not English( Reference Zamrazilová, Hainer and Cerná 30 – Reference Beliaeva, Namazova-Baranova and Tarzian 32 ); the studies were randomised-controlled trials( Reference Zachariassen 33 , Reference Ryumina, Baibarina and Grosheva 34 ); and the studies were conference publications( Reference Pludowski, Czech-Kowalska and Gruszfeld 35 – Reference Belyaeva, Tarzyan and Skvortsova 37 ).
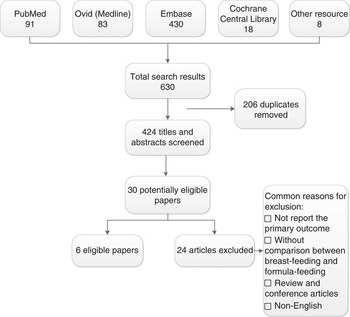
Fig. 1 Flow diagram of the search results.
Altogether, six studies were included in this systematic review( Reference Cooke, Griffin and McCormick 38 – Reference Amesz, Schaafsma and Cranendonk 43 ) (Table 1). We made efforts to contact the authors of three studies because data in their articles for meta-analysis were incomplete( Reference Pludowski, Czech-Kowalska and Gruszfeld 35 – Reference Belyaeva, Tarzyan and Skvortsova 37 ); one author replied but was unable to provide additional data( Reference Pludowski, Czech-Kowalska and Gruszfeld 35 ), and we therefore excluded the three studies from the meta-analysis. All studies were longitudinal. The main technique used to measure body composition was dual-energy X-ray absorptiometry (DXA)( Reference Cooke, Griffin and McCormick 38 , Reference Wauben, Atkinson and Shah 40 – Reference Amesz, Schaafsma and Cranendonk 43 ), and the other techniques used included body electrical impedance analysis( Reference Costa-Orvay, Figueras-Aloy and Romera 39 ). Measurements of outcomes were conducted at a range of time points from birth to 12-month corrected age (Table 1). Only one study reported whether investigators were blinded when measuring the outcomes( Reference Costa-Orvay, Figueras-Aloy and Romera 39 ). The feeding methods were prospectively defined in all studies, although the definitions of feeding group varied (Table 1). FM, FFM and the percentage of FM of the feeding group for each study are shown in Table 2. Variables not normally distributed were not extracted( Reference Amesz, Schaafsma and Cranendonk 43 ).
Table 1 Characteristics of the six included studies in the systematic review
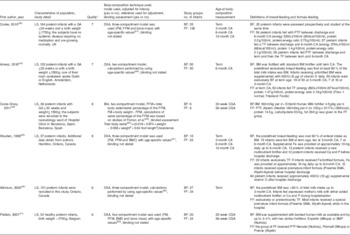
LS, longitudinal study; GA, gestational age; DXA, dual-energy X-ray absorptiometry; FM, fat mass; FFM, fat-free mass; CA, corrected age; BF, breast-fed; FF, formula-fed; BM, breast milk; PTF, preterm formula; TF, standard term formula; BIA, body electrical impedance analysis; CGA, corrected gestational age; BMC, bone mineral content; BMD, bone mineral density.
* Quality of the cohort studies were assessed using Newcastle–Ottawa Scale, ≥7=high quality.
Table 2 Body composition data of included studies in the systematic review (Mean values and standard deviations)

BF, breast-fed; FF, formula-fed; CA, corrected age; CGA, corrected gestational age.
Risk of bias within studies
The risk of bias of each study is presented in Table 1.
Synthesis of results
Both FFM and percentage of FM in the formula-fed group were significantly higher compared with the breast-fed group at 36-week corrected gestational age. The mean difference of the percentage of FM was 0·20 (95 % CI −1·11, 1·51) kg at 32-week corrected gestational age. No significant mean differences in FM were found between the formula-fed group and the breast-fed group at 32- and 36-week corrected gestational age (Fig. 2–4); two articles( Reference Costa-Orvay, Figueras-Aloy and Romera 39 , Reference Pieltain, De Curtis and Gérard 41 ) reported the outcomes at 32- and 36-week corrected gestational age. Percentage of FM was not provided in one study( Reference Costa-Orvay, Figueras-Aloy and Romera 39 ) even after we contacted the author; therefore, we excluded this study from the analysis for the percentage of FM. Formula-fed infants had significantly higher FM than breast-fed infants at term. No significant differences were detected in FFM or the percentage of FM (Fig. 2–4). The variables were not normally distributed, and thus the outcomes are not presented as mean values and standard deviations( Reference Amesz, Schaafsma and Cranendonk 43 ). No significant differences were found in FM, FFM or the percentage of FM between the formula-fed and the breast-fed groups at 3-month, 6-month and 12-month corrected age (Fig. 2–4). The mean differences in FM were −0·05 (95 % CI −0·28, 0·17) kg at 3-month, −0·03 (95 % CI −0·32, 0·25) kg at 6-month, −0·09 (95 % CI −0·37, 0·19) kg at 12-month corrected age. The mean differences in FFM were 0·24 (95 % CI −0·19, 0·66) kg at 3-month and 0·08 (95 % CI −0·26, 0·42) kg at 12-month corrected age.
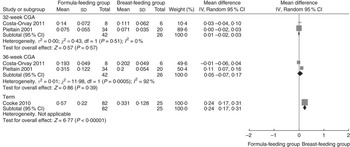
Fig. 2 Pooled mean differences in fat mass between the formula-fed group and the breast-fed group. CGA, corrected gestational age.

Fig. 3 Pooled mean differences in fat-free mass between the formula-fed group and the breast-fed group. CGA, corrected gestational age; CA, corrected age.

Fig. 4 Pooled mean differences in the percentage of fat mass between the formula-fed group and the breast-fed group. CGA, corrected gestational age; CA, corrected age.
Pooled differences
Fig. 2–4 indicate pooled differences in FM, FFM and the percentage of FM between the formula-fed and breast-fed infants by postnatal 1 year of corrected age.
Risk of bias across studies
The funnel plots of studies at 32- and 36-week gestational age indicated no considerable publication bias.
Discussion
Summary of evidence
On the basis of the current available evidence from six studies with data available from 642 infants, we found significant complex differences in body composition between breast-feeding and formula-feeding on preterm infants at 1 year of corrected age. The outcomes of the meta-analysis indicated that formula-fed infants had higher FM at 32-week corrected gestational age, 36-week corrected gestational age and term. By 3-month corrected age, this difference was no longer apparent, with a reverse trend and lower FM in formula-fed infants. Formula-fed infants had lower FFM than their breast-fed counterparts at 32-week corrected gestational age but higher FFM from 36-week corrected gestational age to 12-month corrected age. These findings are biologically plausible. Preterm infants were fed preterm formula before term. Preterm formula contains higher levels of protein and energy than term formula. At the initial stages of enteral feeding with high energy intakes( Reference Ashworth 44 ), rapid weight gain reflects an increase in FM( Reference MacLean and Graham 45 ), which is followed by an increase in muscle mass later. Meanwhile, because the ratio of protein:energy in the diet will influence body composition( Reference Wauben, Westerterp and Gerver 46 ), the lower protein:energy ratio of breast milk may lead to less FFM deposition with excess energy deposited as fat. For this reason, the lower protein intake in breast-fed infants may explain the observed higher FM compared with formula-fed infants after term.
Our observed differences in body composition between formula-fed infants and breast-fed infants are inconsistent with the results of the meta-analysis comparing the effects of breast milk on body composition with formula-feeding in healthy, term infants( Reference Gale, Logan and Santhakumaran 47 ). The reasons may be that preterm infants have fewer nutrient reserves at birth than full-term infants, and are often fed preterm formula or supplementation of mother’s milk with human milk fortifiers. Moreover, owing to immature metabolic pathways for nutrient utilisation and/or an immature growth hormone/insulin-like growth factor axis, the absorption and assimilation of nutrients are limited. Meanwhile, although late preterm infants (gestational age between 34 and 36 weeks) are usually able to breast-feed, they are more likely to experience difficulty in establishing successful breast-feeding than term infants because preterm infants’ oro-buccal coordination and swallowing mechanisms may not be fully matured( Reference Engle, Tomashek and Wallman 48 ). Furthermore, a systematic review was conducted by Arenz et al.( Reference Arenz, Rückerl and Koletzko 49 ), which analysed the association of breast-feeding with childhood obesity. Although the results showed that breast-feeding reduced the risk of obesity in childhood significantly, results from another systematic review carried out by Owen et al.( Reference Owen, Martin and Whincup 50 ) investigating the relation between breast-feeding and BMI throughout life suggested that mean BMI was lower among breast-fed infants, but the difference was small. Promotion of breast-feeding was not likely to reduce mean BMI. The outcome of our meta-analysis indicated that breast-fed infants had lower FM at 32-week corrected gestational age, 36-week corrected gestational age and term compared with formula-fed infants. By 3-month corrected age, this effect was no longer apparent, with a trend towards reversal and higher FM in breast-fed infants. Several drawbacks limited the validity of the meta-analyses, including the types of studies, publication bias, confounding factors and potential heterogeneity between studies.
Some evidences support our outcomes. Investigators have reported that formula-fed infants having higher protein:energy ratio had a higher absolute FFM and lower percentage of FM( Reference Brunton, Saigal and Atkinson 51 ). Koo & Hockman( Reference Koo and Hockman 17 ) found that absolute FFM and FM were increased in infants fed standard formula. Furthermore, in the study of Cooke et al.( Reference Cooke, McCormick and Griffin 52 ), absolute FFM and FM were higher in the group of infants fed energy- and protein-enriched formulae, but the percentage of FM did not differ.
DXA is used to evaluate the composition of growth in a single scan. It is non-invasive and of low health risk to the infant, and has therefore been widely used in our included studies. We performed subgroup analyses of studies in which values were measured using this technique. This result supports our analysis of outcomes obtained using different in vivo measurement techniques of body composition.
Limitations
Our study has several limitations. First, the percentage of FM depends on FFM; therefore, it has been recommended to adjust FM and FFM for height to create independent measures( Reference VanItallie, Yang and Heymsfield 53 ) (fat-free mass index (FFMI) and fat mass index (FMI)). However, we could not collect data on FFMI or FMI because of lack of data in included articles. Second, as suggested by Cooke et al.( Reference Cooke, McCormick and Griffin 52 ), sex was an additional significant independent variable, resulting in an increase in FM and bone mineral content in female infants( Reference Pieltain, De Curtis and Studinski 54 ). Nevertheless, we were unable to examine the effect of sex because only a limited number of studies reported outcomes by sex.
Third, breast-feeding was assessed prospectively in all included studies, which limited the recall bias. However, the definition of feeding groups varied widely, and none of the studies used WHO criteria for exclusive breast-feeding, which indicates that a contamination bias may represent an important source of heterogeneity. Moreover, one article did not report the exact definition. Fourth, the infant formulae contained higher levels of long-chain PUFA (LCPUFA), Ca and P. Although the study of de Jong et al.( Reference de Jong, Kikkert and Fidler 55 ) suggested that LCPUFA supplementation does not alter neurological function, no data were available on its effect on body composition. Fifth, one study exploring the association between psychosocial risk factors and breast-feeding discontinuation suggested that maternal depression can lead to early discontinuation of breast-feeding( Reference Taveras, Capra and Braveman 56 ). Meanwhile, women who choose formula-feeding often lack confidence in their ability to breast-feed, because they think formula may be better for their infants or their milk is inadequately supplied. Therefore, the body compositions were probably affected by psychosocial factors. Finally, none of the included articles mentioned the blinding of data collectors( Reference Cooke, Griffin and McCormick 38 – Reference Amesz, Schaafsma and Cranendonk 43 ).
Conclusion
In summary, this systematic review suggests that formula-feeding is associated with altered body composition from delivery to term in preterm infants compared with breast-feeding. The effects of formula-feeding on preterm infant body composition from term to 1 year of corrected age are inconclusive. Our findings enable to confirm the possible contributions of breast-feeding and formula-feeding on risk of obesity in childhood and adult life.
The number of studies included in our analysis is limited, and the findings from our meta-analysis should be confirmed by future studies. Meanwhile, well-designed studies are required to define the effect of formula-feeding on body composition compared with breast-feeding.
Acknowledgements
The authors thank Guan-jian Liu from Evidence-Based Medicine Center of China for his assistance in data analysis. The authors also thank Qiu-ui Hao from West China Hospital of Sichuan University and Dong-tao Lin from College of Foreign Languages and Cultures of Sichuan University for editing the manuscript.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
B. L. assisted P. H. and J. Z. in formulating the research questions and supervising the quality of this manuscript. Designing the study and screening of the articles were conducted by two reviewers (P. H. and J. Z.). P. H. and J. W. collected data. J. Z. and Y. Y. analysed the data. P. H. and J. Z. wrote the article, and J. W. revised the article.
None of the authors has any conflicts of interest to declare.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516001720









