Probiotics are defined as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’Reference Reid, Jass, Sebulsky and McCormick1. Prebiotics are a more recent concept and are defined as chemical substances that act as substrates specifically for the host's intrinsic probiotic bacteria, and thus encourage their growthReference Hamilton-Miller2. A combination of probiotic and prebiotic is termed synbiotic, which has been used to improve various aspects of human healthReference Flickinger, Wolf and Garleb3, Reference Gibson and Roberfroid4.
High-fat diets have been associated with hypercholesterolaemia as indicated by higher than normal serum total cholesterolReference Kenney5. Studies have shown that 1 mmol higher than normal cholesterol concentration could increase the risk of CHD by 35 % and the risk of coronary death by 45 %, and a small reduction in the serum cholesterol of 1 % could reduce the risk of CHD by 2–3 %Reference Chyou and Eaker6. Numerous dietary approaches have been used to improve hypercholesterolaemia including the usage of synbiotics. Several possible mechanisms for cholesterol removal by probiotics and prebiotics have been proposed including assimilation of cholesterol by growing cells, binding of cholesterol to cellular surface, incorporation of cholesterol into the cellular membraneReference Liong and Shah7, deconjugation of bile via bile salt hydrolase, co-precipitation of cholesterol with deconjugated bileReference Liong and Shah8, binding action of bile by fibreReference Marlett9 and production of SCFA by oligosaccharidesReference Hara, Li and Sasaki10. Although numerous studies have shown promising results in reducing TAG, total cholesterol and LDL-cholesterol concentrations after consumption of probiotic and/or prebioticsReference Jin, Ho, Abdullah and Jalaludin11, their effects on serum lipids have been controversial with numerous studies indicating inconclusive resultsReference Lin, Ayres, Winkler and Sandine12. Also, most of these studies involved continuous feeding of high-cholesterol diets containing high concentrations of probiotics and/or prebiotics. In recent years, consumers are becoming nutritionally conscious, and adopt diets containing MUFA and PUFA, and minimize intake of total fat. Very little information is available on the cholesterol-lowering effect of synbiotic in hypercholesterolaemic subjects after cessation of high-fat diets.
A basic understanding of the metabolism of the plasma lipoproteins, and the major lipids that they transport is of primary importance. Because the metabolism of the plasma lipoproteins is highly interrelated, it is important to consider each of the lipoproteins and their properties instead of focusing only on the main lipid classes to better understand the complete lipid profileReference Kwiterovich13.
When rabbits, guinea pigs and dogs were fed high-cholesterol diets, morphological defects of the erythrocytes including the formation of spur cells was observedReference Cooper, Leslie, Knight and Detweiler14, where the enterocytes are covered with spike-like formations that may vary in width, length and distribution. Although the role of this abnormality remains unknown, the occurrence of spur cells contributes to the entrapment and destruction of erythrocytes in the spleen and has been reportedly caused by increased membrane rigidityReference Cooper, Leslie, Knight and Detweiler14. Any changes in the erythrocyte membranes would involve modifications on the lipid order due to the fact that the erythrocyte membrane is an amphiphilic phospholipid layer containing protein and cholesterol. Fluorescence probes are used to evaluate modifications in the membrane packing order via incorporation into different sites in the bilayer, namely the apolar regions, interface between the polar head and apolar tail, and in the polar surfaceReference Barshtein, Bergelson, Dagan, Gratton and Yedgar15.
We have previously screened and developed a synbiotic product consisting of Lactobacillus acidophilus ATCC 4962, mannitol, fructooligosaccharides (FOS) and inulin that specifically targeted removal of cholesterol in laboratory media containing cholesterolReference Liong and Shah16. However, the effect of such synbiotic on plasma lipid profiles and erythrocytes in in vivo models has not been studied. Thus, the aims of the present study were to evaluate (1) the effect of such a synbiotic on plasma lipoproteins and the possible mechanisms via lipid transporters, and (2) the effect of the synbiotic on the morphology of erythrocytes and the possible mechanisms via alteration on membrane fluidity, by using pigs given high- and low-fat diets. The effect of the synbiotic during a continuum of the high-fat diet and after the cessation of the high-fat diet will also be evaluated.
Material and methods
Source of probiotic culture and prebiotics
L. acidophilus ATCC 4962 is a human-derived strain that was obtained from the Australian Starter Culture Research Center (ASRC; Werribee, Australia). The stock culture was stored in 40 % (v/v) glycerol at − 80°C. The organism was subcultured three times in sterile de Mann, Rogosa, Sharpe (MRS) broth using 1 % (v/v) inoculum and 20 h of incubation at 37°C prior to use and was stored at 4°C between transfers. A freeze-dried culture (containing approximately 9·0 log10 colony-forming units/g) was used in the present study. For freeze-drying, the cell pellet obtained from harvesting the fermentation broth was suspended in 0·1 m-phosphate buffer (pH 6·8) containing 2·0 % (w/v) of food grade cryoprotectant Unipectin™ RS 150 (Savannah Bio Systems, Balwyn East, Australia), frozen at − 20°C and freeze-dried (Dynavac FD300; Airvac Engineering Pty Ltd, Rowville, Australia) at − 20°C and − 100 kPa. Three commercially available prebiotics were used including mannitol (Mannogem; SPI Polyols Inc., New Castle, DE, USA), FOS (Raftilose P95; Orafti Pty Ltd, Tienen, Belgium) and inulin (Raftilene ST; Orafti). The FOS had a purity of 95 % and the remaining 5 % contained glucose, fructose and sucrose. The degree of polymerization of FOS ranged from 2 to 7, with an average of 4. The inulin had a purity of 92 %, and an average degree of polymerization of 10. The mannitol had a degree of polymerization between 1 and 2 and was affirmed Generally Regarded As Safe (GRAS; Code of Federal Regulations, Title 21 CFR 180.25).
Animals and diets
Twenty-four crossbred (Large White × Landrace) pigs (initial weight 33 (sd 8 kg)) were used. The study was approved by the Animal Ethics Committee of the Department of Primary Industry (Werribee, Australia). Pigs were housed in individual pens in a randomized block design according to their initial live weight and four treatments. The animals were kept on a basal diet containing 15 % fat for 2 weeks to induce hypercholesterolaemia. After this period, pigs were given either a high-fat (15 % fat) or a low-fat (5 %) diet for 8 weeks. The pigs on the synbiotic diet were supplemented with powdered L. acidophilus ATCC 4962 (1·00 g/pig per d), FOS (1·25 g/pig per d), mannitol (1·56 g/pig per d) and inulin (2·20 g/pig per d), while pigs on the control were not supplemented with synbiotic. The composition of the synbiotic was based on our previous in vitro optimization study where inoculum size of L. acidophilus ATCC 4962, and concentrations of mannitol, FOS and inulin were the significant factors for optimum removal of cholesterolReference Liong and Shah16. The compositions of the high- and low-fat diets are shown in Table 1. Pigs were kept in a room with controlled temperature (20–22°C) and humidity (50–55 %) and maintained in a cycle of light for 12 h (06·00–18.00 hours) and dark for 12 h (18·00–06.00 hours). The body weight of each pig was recorded weekly and the amount of the basal diet adjusted individually according to their energy intake per weight; 16·9 MJ/kg and 14·6 MJ/kg for high-fat diet and low-fat diet, respectively. Pigs were fed on a daily basis and allowed to consume ad libitum water during the experimental period.
Table 1 Composition of the low-fat (5 % fat) and high-fat (15 % fat) basal diet*
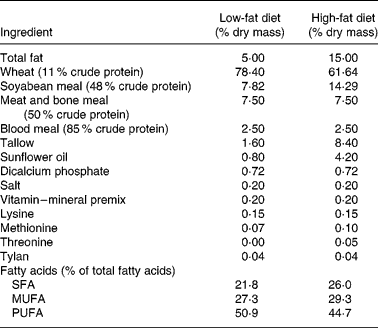
* Feed was adjusted weekly according to energy intake per weight of pigs; 14·6 MJ/kg for those on the basal diet containing 5 % fat and 16·9 MJ/kg for those on the basal diet containing 15 % fat.
Preparative procedures
All pigs were fasted overnight prior to blood collection weekly. Immediately after bleeding, pigs were fed their respective diets and non-fasting blood was collected after 3 h of feeding. Fasting and non-fasting blood samples were obtained by venepuncture. Heparin was used as anticoagulant. All data are presented using fasted blood samples unless stated otherwise.
The whole blood was centrifuged for 20 min at 2714 g (Sorvall RT7; Newtown, CT, USA) to separate the plasma from the erythrocyte pellet. The plasma was recentrifuged for 30 min at 18 879 g (Beckman Coulter, Fullerton, CA, USA) to remove chylomicrons.
A discontinuous density gradient ultracentrifugal procedure was used to fractionate plasma lipoproteins as previously describedReference Chapman, Goldstein, Lagrange and Lapaud17. Chylomicron-free plasma was adjusted with NaCl–KBr solution (1·346 g/ml) to desired densities of 1·006, 1·063 and 1·21 g/mlReference Duhamel, Forgez, Nalpas, Berthelot and Chapman18. Gradients were centrifuged at 40 000 rpm for 20 h at 15°C (Beckman model TI ultracentrifuge with type 70·1 rotor) and subfractionated by successive downwards aspiration using a Pasteur pipette. VLDL was isolated at density < 1·006 g/ml and the infranet obtained was refloated at a density of 1·063 g/ml to obtain LDL (density 1·006–1·063 g/ml). Similarly, HDL was obtained at density 1·063–1·21 g/ml.
For the preparation of Hb-free erythrocyte membrane (erythrocyte ghosts), erythrocyte pellets were washed twice with saline to remove any remaining plasma and buffy coat, followed by lysing in 30 volumes of 5 mm-Na2HPO4 buffer, pH 8·0Reference Dumaswala, Dumaswala, Levin and Greenwalt19 and isolating by centrifugation. Erythrocyte ghosts were washed with the same buffer until Hb-free.
Analytical procedures
Chylomicron-free plasma was analysed for total, HDL- cholesterol, LDL-cholesterol and TAG using commercial kits (Thermo Electron Corp., Melbourne, Australia). Plasma subfractions of VLDL, LDL and HDL were analysed for TAG, protein, phospholipids, cholesteryl esters (CE) and free cholesterol concentrations. TAG and total cholesterol were analysed using commercial kits (Thermo Electron Corp.). CE were determined using the Amplex Red Reagent Kit (Molecular Probes, Eugene, OR, USA) and free cholesterol was determined as the difference between total cholesterol and CEReference Dhaliwal and Steinbrecher20. The protein content was determined with bovine serum albumin as the standardReference Lowry, Rosebrough, Farr and Randall21. A factor of 0·8 was used to convert bovine serum albumin protein into lipoprotein proteinReference Sardet, Hansma and Ostwald22. The total phospholipid content was measured as phosphate as previously describedReference Daly and Ertingshausen23 and a factor of 25 was used for conversion into serum phospholipidsReference Cooper, Leslie, Knight and Detweiler14.
The erythrocyte count was carried out using a Newbauer haemocytometer. The morphology of erythrocytes was assessed using Wright's stain smearsReference Cooper, Leslie, Fischkoff, Shinitzky and Shattil24. The erythrocyte total cholesterol and phospholipids were determined after lipid extraction with acetone–ethanol (1:1). The total cholesterol and phospholipids were measured as described earlier.
The erythrocyte membrane lipid order was determined by measuring the fluorescence anisotropy (FAn) of lipid probes inserted into the erythrocyte ghosts. Three fluorescence probes were used, namely 1,6-diphenyl-1,3,5-hexatriene (DPH), 1-(4-trimethylammonium)-6-phenyl-1,3,5-hexatriene (TMA-DPH) and 8-anilino-1-napthalenesulphonic acid (ANS). DPH and TMA-DPH were dissolved in tetrahydrofuran to a final concentration of 2 μm while ANS was dissolved in ethanol to a final concentration of 6 μmReference Barshtein, Bergelson, Dagan, Gratton and Yedgar15. The probe stock solutions were diluted (1:1000) in 0·155 m-NaClReference Cooper, Leslie, Knight and Detweiler14 with vigorous mixing before being incubated with erythrocyte ghosts. Working probe solutions were incubated with erythrocyte ghost solution (optical density 0·3) at a ratio of 3:1 at temperature of 37°C. The incubation time for DPH and TMA-DPH was 60 min, while that for ANS was 90 min. FAn was measured using a luminescence spectrophotometer (LS-50; Perkin Elmer, Wellesley, MA, USA). Excitation wavelength for DPH and TMA-DPH was 365 nm, while for ANS was 390 nm. Emission was determined at 445 nm for DPH and TMA-DPH, and 490 nm for ANSReference Barshtein, Bergelson, Dagan, Gratton and Yedgar15. An unlabelled erythrocyte ghost was used as a blank. FAn was calculated according to the equation:
where I vv and I vh are the fluroscence intensities obtained from a vertical polarizer, and a vertical and horizontal analyser, while G is the instrumental grating factor. G = I hv/I vh where I hv is the intensity measured from a horizontal polarizer and a vertical analyserReference Barshtein, Bergelson, Dagan, Gratton and Yedgar15.
Statistical analyses
A repeated measures analysis was used to compare the average means of the four treatment groups in a 2 × 2 factorial design (SPSS version 10.0; SPSS Inc., Chicago, IL, USA). The factors used were with or without synbiotic treatment, dietary fat content of 5 % and 15 %, and experimental period. ANOVA was used to perform multiple comparisons between means. All data are presented as means and their standard errors (n 6).
Results
Pigs were healthy in general during the 8 weeks of the experimental period. The growth rate of pigs, and their feed consumption and feed efficiency are shown in Table 2. The supplementation of synbiotic did not exhibit a significant effect on growth rate (P = 0·225), weekly feed intake (P = 0·411) and feed efficiency (P = 0·474). Due to different fat content in the basal diet, feed was adjusted as per energy intake of the pigs; 14·6 MJ/kg for those on the basal diet containing 5 % fat and 16·9 MJ/kg for those on the 15 % fat diet. This has contributed to insignificant effect of dietary fat on the growth rate (P = 0·124), feed intake (P = 0·456) and feed efficiency (P = 0·093).
Table 2 Effect of the supplementation of synbiotic and dietary fat on the growth rate, feed intake and feed efficiency of hypercholesterolaemic pigs (six per group) for 8 weeks* (Mean values)

F, effect of dietary fat; T, effect of treatment.
* The control diet contained no synbiotic while the synbiotic diet contained Lactobacillus acidophilus ATCC 4962 (1·00 g/pig per d), fructooligosaccharide (1·25 g/pig per d), mannitol (1·56 g/pig per d) and inulin (2·20 g/pig per d).
† Standard error of the difference for T × F.
‡ Feed was adjusted weekly according to energy intake per weight of pigs; 14·6 MJ/kg for those on the basal diet containing 5 % fat and 16·9 MJ/kg for those on the basal diet containing 15 % fat.
The effect of synbiotic supplementation and dietary fat on lipid profiles is shown in Table 3. The plasma total cholesterol decreased in pigs supplemented with synbiotic on both dietary fats, while those without supplementation increased over 8 weeks (P = 0·001). Pigs on the high-fat diet also showed higher plasma total cholesterol concentration than those on the low-fat diet (P = 0·016). However, those given synbiotic and the low-fat diet showed a larger decrease (18·16 %) than those on the high-fat diet (1·51 %) over 8 weeks. Pigs on the control diet had an increased fasted plasma TAG of 17·64 % (low-fat diet) and 49·69 % (high-fat diet) over 8 weeks. Conversely, those on the synbiotic diet had a decrease of 15·48 % (low-fat diet) and 41·40 % (high-fat diet) (P = 0·002). Changes in plasma TAG were more prevalent between treatment groups and dietary fat in non-fasted samples. Pigs given the high-fat diet had significantly higher non-fasted plasma TAG than those on the low-fat diet (P = 0·001), while those given the synbiotic had a lower concentration than those without supplementation (P < 0·001). The supplementation of synbiotic significantly lowered plasma LDL-cholesterol compared to the control (P = 0·045) over 8 weeks; pigs without the supplementation of synbiotic showed an increase of 46·63–59·19 % while pigs given the synbiotic showed an increase of only 13·11–28·11 %. Dietary fat had no effect on the concentration of HDL-cholesterol.
Table 3 Effect of the supplementation of synbiotic and dietary fat on plasma lipid profiles of hypercholesterolaemic pigs (six per group) for 8 weeks* (Mean values)
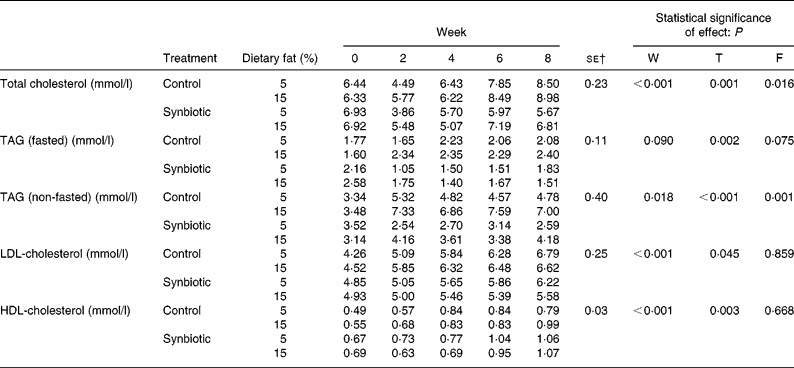
F, effect of dietary fat; T, effect of treatment; W, effect of experimental period.
* The control diet contained no synbiotic while the synbiotic diet contained Lactobacillus acidophilus ATCC 4962 (1·00 g/pig per d), fructooligosaccharide (1·25 g/pig per d), mannitol (1·56 g/pig per d) and inulin (2·20 g/pig per d).
† Standard error of the difference for T × F.
The subfractions of major lipoproteins are shown in Fig. 1. TAG content of VLDL decreased over 8 weeks (Fig. 1(A, D)) for pigs on both dietary treatments and synbiotic supplementation. The low-fat diet had significantly lower concentration of TAG in VLDL than the high-fat diet (P = 0·008) over 8 weeks, while the supplementation of synbiotic similarly lowered the concentration of TAG in VLDL over the experimental period compared to pigs without supplementation (P = 0·008). This was accompanied by an increasing concentration of CE in VLDL over the experimental period for all treatment groups.
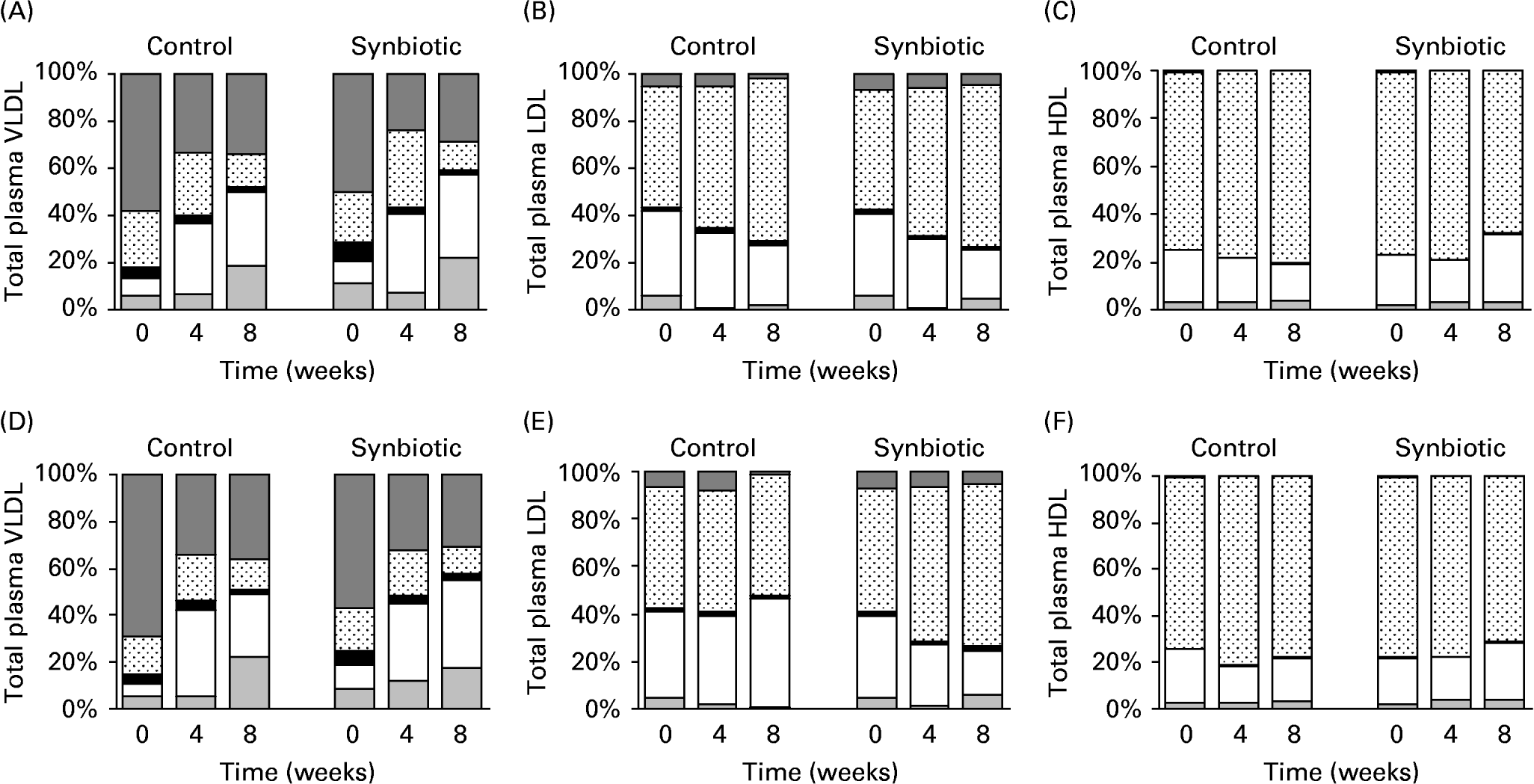
Fig. 1 Subfractions of VLDL-, LDL- and HDL-cholesterol of pigs fed the control and synbiotic diets with 5 % fat (A, B, C) and 15 % fat (D, E, F) for 8 weeks. The control diet contained no synbiotic while the synbiotic diet contained Lactobacillus acidophilus ATCC 4962 (1·00 g/pig per d), fructooligosaccharide (1·25 g/pig per d), mannitol (1·56 g/pig per d) and inulin (2·20 g/pig per d). Results are expressed as percentage of total plasma lipoproteins: TAG (![]() ), protein (⊡), phospholipids (■), cholesteryl esters (□) and free cholesterol (
), protein (⊡), phospholipids (■), cholesteryl esters (□) and free cholesterol (![]() ).
).
By weight, a large portion of LDL is CE and free cholesterol with little TAGReference McEneny, McMaster, Trimble and Young25. This was shown in pigs on all treatments studied (Fig. 1(B, E)). Although the concentration of TAG was small in LDL and was decreasing over 8 weeks for all treatments, pigs supplemented with synbiotic had a significantly smaller decrease (P = 0·025) over 8 weeks (30·29–31·65 %) than those without supplementation (63·07–77·58 %), regardless of the dietary fat. However, dietary fat appeared to have no effect on the concentration of TAG in LDL. The supplementation of synbiotic also contributed to a larger decrease (P < 0·001) in the concentration of CE in LDL for both dietary fats (39·36–44·92 %) over 8 weeks than those without supplementation (25·01–30·42 %). Pigs on the high-fat diet also had a significantly larger decrease of CE in LDL than those given the low-fat diet (P = 0·05). There was a linear interaction (P = 0·002) between experimental period and supplementation of synbiotic such that the concentration of CE in LDL decreased with time in pigs supplemented with synbiotic while the control remained relatively constant.
The HDL particles contain high amounts of protein (approximately 50 %) with small amounts of TAG (5 %), cholesterol (20 %) and phospholipids (25 %)Reference Sanchez-Muniz, Merinero, Rodríguez-Gil, Ordovas, Rodenas and Cuesta26. These have been manifested in pigs on all treatment groups (Fig. 1(C, F)). The concentration of CE in HDL increased (P = 0·036) when pigs were supplemented with synbiotic regardless of dietary fat over the experimental period (25·86–35·09 %), while those without supplementation showed a decrease of 20·34–29·87 % over 8 weeks.
The morphology of erythrocytes was assessed using Wright's stain and is illustrated in Fig. 2. The erythrocyte morphology appeared to be normal for all treatments initially (Fig. 2(A–D)), however, at the end of the experimental period, the pigs on the control diet showed a distinct characteristic of spur cells (Fig. 2(E, G)), while the erythrocytes of those on the synbiotic diet were less affected. Further, the occurrence of spur cells was more prevalent in pigs on the high-fat diet (Fig. 2(F)) than those on the low-fat diet (Fig. 2(H)).

Fig. 2 Morphology of erythrocytes after staining using Wright's stain from pigs (n 6) on the high-fat diet without (A) and with (B) synbiotic at the initial feeding period, pigs on the low-fat diet without (C) and with (D) synbiotic at the initial feeding period, pigs on the high-fat diet without (E) and with (F) synbiotic over 8 weeks, and pigs on the low-fat diet without (G) and with (H) synbiotic over 8 weeks. The synbiotic supplementation contained Lactobacillus acidophilus ATCC 4962 (1·00 g/pig per d), fructooligosaccharide (1·25 g/pig per d), mannitol (1·56 g/pig per d) and inulin (2·20 g/pig per d).
Phospholipid content of erythrocyte membranes increased (P < 0·001) over the experimental period for all treatments (Table 4). The administration of synbiotic significantly (P = 0·005) contributed to higher concentrations of phospholipids compared to that without supplementation. Pigs given the high-fat diet also showed higher concentration of erythrocyte phospholipids than those on the low-fat diet (P = 0·001). The diet with higher fat content contributed to higher concentration of cholesterol in the erythrocytes compared to that with lower fat content (P < 0·001). There was a significant interaction (P = 0·017) between the dietary fat and supplementation of synbiotic; there was a largest increase in the concentration of cholesterol over 8 weeks (157·67 %) when pigs were not supplemented with synbiotic and were given the high-fat diet, while the concentration of cholesterol in erythrocytes had the smallest increase (44·58 %) when pigs were supplemented with synbiotic and were given the low-fat diet. The ratio of cholesterol/phospholipids (C/P; mol/mol) was significantly lower (P = 0·001) when pigs consumed synbiotic compared to those that did not. The C/P was also lower (P = 0·007) when pigs were given the low-fat diet compared to those on the high-fat diet. There was a significant interaction between the experimental period, supplementation of synbiotic and dietary fat (P = 0·004) where a smaller increase in C/P over 8 weeks was observed in pigs supplemented with synbiotic but was given the high-fat diet (5·00 %) compared to those given the low-fat diet (13·58 %).
Table 4 Effect of the supplementation of synbiotic and dietary fat on concentration of cholesterol and phospholipids in erythrocyte membranes of hypercholesterolaemic pigs (six per group) for 8 weeks* (Mean values)
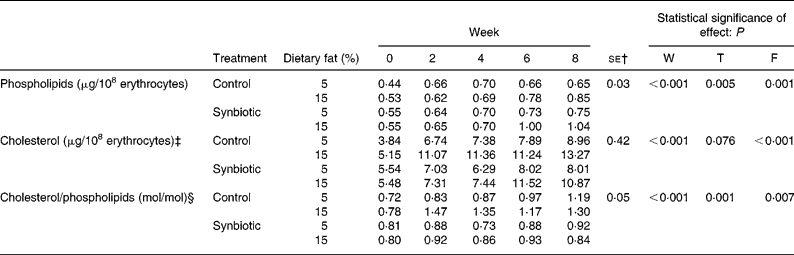
F, effect of dietary fat; T, effect of treatment; W, effect of experimental period.
* The control diet contained no synbiotic while the synbiotic diet contained Lactobacillus acidophilus ATCC 4962 (1·00 g/pig per d), fructooligosaccharide (1·25 g/pig per d), mannitol (1·56 g/pig per d) and inulin (2·20 g/pig per d).
† Standard error of the difference for T × F.
‡ T × F interaction, P = 0·017.
§ W × T × F interaction, P = 0·004.
Considering that the administration of dietary fat affected erythrocyte membrane lipid content and the supplementation of synbiotic could alter such effects, we further evaluated the locations of cholesterol enrichment using fluorescence probes (Table 5). FAn of DPH increased over just 2 weeks for all treatments studied. However, pigs supplemented with synbiotic and given the low-fat diet showed an increase of DPH over 4 weeks (P < 0·01). The supplementation of synbiotic also reduced the FAn (P < 0·001) in pigs for both dietary fat treatments (10·87–19·57 %) while those without supplementation showed an increase (2·27–39·39 %) over the experimental period. The FAn was also higher (P = 0·012) when pigs were on the high-fat diet than those on the low-fat diet.
Table 5 Effect of the supplementation of synbiotic and dietary fat on fluorescence anisotropy of erythrocyte ghosts in hypercholesterolaemic pigs (six per group) for 8 weeks* (Mean values)
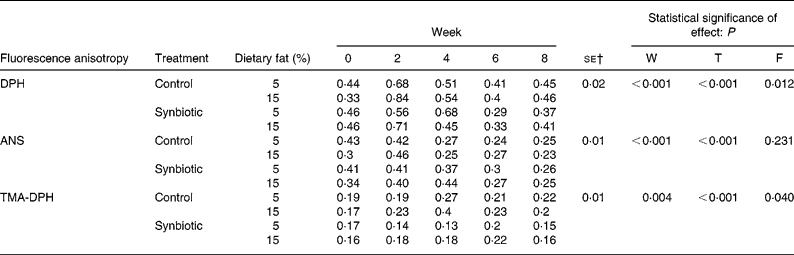
ANS, 8-anilino-1-napthalenesulphonic acid; DPH, 1,6-diphenyl-1,3,5-hexatriene; F, effect of dietary fat; T, effect of treatment; TMA-DPH, 1-(4-trimethylammonium)-6-phenyl-1,3,5-hexatriene; W, effect of experimental period.
* The control diet contained no synbiotic while the synbiotic diet contained Lactobacillus acidophilus ATCC 4962 (1·00 g/pig per d), fructooligosaccharide (1·25 g/pig per d), mannitol (1·56 g/pig per d) and inulin (2·20 g/pig per d).
† Standard error of the difference for T × F.
The experimental period significantly affected the FAn of ANS (P < 0·001); the low-fat diet contributed to a continuous decrease over 8 weeks regardless of synbiotic supplementation while the high-fat diet contributed to a decrease over 2–4 weeks. Also, pigs supplemented with synbiotic required a longer period before reducing the FAn of ANS compared to those without supplementation, accompanied by a smaller decrease of 26·47–36·59 % over 8 weeks while those without supplementation decreased by 23·33–41·86 % (P < 0·001).
There were significant changes (P = 0·004) in the FAn of TMA-DPH over the experimental period; pigs without the supplementation of synbiotic showed reduced FAn of TMA-DPH over 4 weeks for both the high- and low-fat diets, while the FAn for pigs supplemented with synbiotic only reduced over 6 weeks. The administration of synbiotic also reduced the FAn in pigs on both dietary fat treatments compared to those that did not consume the synbiotic (P < 0·001), accompanied by a lower increase over 8 weeks (0·00–11·76 %) compared to an increase of 15·79–17·65 % when pigs were not given the synbiotic. The high-fat diet also significantly contributed to a higher FAn (P = 0·040) of TMA-DPH than the low-fat diet.
Discussion
It has been previously found that modifications in dietary fats and fatty acids may affect plasma lipoprotein profilesReference Tholstrup, Sandstrom, Bysted and Holmer27. However, the present study indicated that only plasma total cholesterol and TAG (non-fasted) concentrations were significantly affected (P = 0·016 and P = 0·001, respectively) by the different diets while the other lipoprotein parameters were not affected. Although the high-fat diet had higher fat content, higher SFA and lower PUFA concentrations than the low-fat diet, these differences also had insignificant effect on the growth rate (P = 0·124), feed intake (P = 0·456) and feed efficiency (P = 0·093) in all pigs.
The current study showed that the supplementation of synbiotic reduced plasma TAG (fasted and non-fasted), total cholesterol and LDL-cholesterol in pigs. Hypercholesterolaemia has been associated with higher than normal total cholesterol (6·21 mmol/l) concentration. Although pigs given the high-fat diet remained hypercholesterolaemic over the experimental period, the administration of synbiotic contributed to a decreased plasma cholesterol concentration. When the low-fat diet was used, pigs without the supplementation of synbiotic remained hypocholesterolaemic over 8 weeks, while those given the synbiotic showed a reduction to normal concentration over 8 weeks. Higher than normal concentration of LDL-cholesterol (4·41 mmol/l) is also an attribute to hypercholesterolaemia. Although the supplementation of synbiotic improved the LDL-cholesterol content, it failed to achieve a normal concentration in all pigs.
There is very little information on the source and nature of abnormalities in lipoproteinsReference Jin, Ho, Abdullah and Jalaludin11, Reference Lin, Ayres, Winkler and Sandine12. Hence, we wanted to evaluate further the compositions of these lipoprotein classes in order to better understand the alteration of lipid components as affected by the synbiotic product that was developed in our previous studyReference Liong and Shah16.
VLDL is primarily synthesized in the liver and contains about 50–60 % of TAG, which is the major lipid class that is transported from the liver into the bloodstreamReference Kwiterovich28. Results from the present study showed that pigs on all dietary treatments had normal concentrations of TAG in the subfraction of VLDL at the initial point of the feeding trial, but were reduced over 8 weeks. VLDL particles are the precursors for LDL particles where the TAG in VLDL are exchanged for CE in the core of LDLReference Kwiterovich13. Results from the present study showed that pigs supplemented with the synbiotic decreased CE and increased TAG in LDL, complemented by a lower concentration of TAG in VLDL, indicating higher conversion of VLDL into LDL.
In addition, synbiotic also beneficially decreased the concentration of CE in LDL. Higher concentration of CE in LDL induced by diet was found to be associated with an increased risk for atherosclerosis in African green monkeysReference Carr, Parks and Rudel29. More importantly, such TAG-enriched LDL particles are more susceptible to further hydrolysisReference Kwiterovich28 and are removed from blood via binding to LDL receptors, where the CE particles are hydrolysed into free cholesterolReference Horton, Goldstein and Brown30. Loss of CE from the core of LDL forms smaller and denser LDL particles. Although smaller LDL appeared more atherogenic than larger LDL particlesReference Haffner31, smaller LDL formed as a result of dietary intervention was removed by plasma more rapidly than larger particlesReference Fernandez, Abdel-Fattah and McNamara32. Results from the present study clearly showed that the administration of the synbiotic decreased the concentration of CE coupled with increased concentration of TAG in the LDL subfraction, which may have contributed to the improved plasma LDL-cholesterol in pigs supplemented with synbiotic.
The major function of HDL is to transport cholesterol back to the liver, and this could involve a process referred to as reverse cholesterol transportReference Rader33. Briefly, free cholesterol is transported into HDL particles and subsequently esterified to form CE. HDL contains mainly lecithin and protein, with small amount of cholesterol and TAG. Thus, matured HDL with bigger core containing more CE are transported back to the liver and hydrolysed. This CE is transferred by CE transfer protein and internalized by the LDL receptor. The HDL is then released for further reverse cholesterol transportReference Kwiterovich28. Experimental data from the present study showed that the administration of synbiotic increased the concentration of CE in the HDL subfraction, which indicated that cholesterol was removed by HDL in the form of CE. This explains why there was reduced plasma total cholesterol in pigs supplemented with synbiotic.
Although results from the present study showed increased concentration of CE in the HDL particles, pigs supplemented with synbiotic also had lower plasma HDL-cholesterol than those without supplementation. Low plasma HDL-cholesterol is not an indicator of faulty removal of cholesterol through deficient activity of the reverse cholesterol transport pathwayReference Kwiterovich28. Instead, the CE in the core of HDL may also be exchanged by CE transfer protein for the TAG in VLDL, producing a TAG-enriched but CE-depleted HDL, which appeared to be catabolized more rapidly by the kidney. The HDL-cholesterol concentration is thus decreasedReference Kwiterovich13. Results from the present study showed that using the direct concentration of plasma HDL-cholesterol as an indicator for healthy lipoprotein profiles would be misleading, as a higher concentration of plasma HDL-cholesterol does not indicate high efficiency in cholesterol transport.
The occurrence of spur cells appeared to be improved by the supplementation of synbiotic as supported by the morphological representation and lower C/P ratio. Cholesterol in the membrane lipid bilayer prevents fatty acid chains from coming together and crystallizing; however, cholesterol also decreases the permeability of the bilayer to small water-soluble molecules, thus reducing fluidityReference Simons and Ehehalt34. It has been reported that the normal C/P ratio of erythrocytes for dogs is approximately 1·05Reference Cooper, Leslie, Fischkoff, Shinitzky and Shattil24. At the end of the experimental period, C/P ratio for the pigs with the supplementation of synbiotic was normal (0·84–0·92) while those without supplementation were higher than the normal ratio (1·19–1·30). Such abnormality in lipid composition would result in an increase in the surface area of the cells, thus increasing rigidity and decreasing fluidity. These led to a decreased ability of the cell to deform and thus the occurrence of spur cellsReference Duhamel, Forgez, Nalpas, Berthelot and Chapman18.
Although the present results and previous studiesReference Cooper, Leslie, Knight and Detweiler14 showed that dietary fat intervention contributed to the enrichment of cholesterol in erythrocyte membranes, we were unable to distinguish the location of such enrichment in the membranes. Thus, we have used three different fluorescence probes to allow examination in different locations of the membrane bilayer. These probes exhibit strong fluorescence increase upon binding to lipids and have sensitive anisotropy responses to lipid order. DPH is an apolar fluorophore mainly incorporated into different apolar regions of the membraneReference Kaiser and London35. The experimental data showed that pigs supplemented with the synbiotic had lower saturation of cholesterol within the apolar region of the erythrocytes. A higher FAn of DPH in pigs without synbiotic indicated enrichment of cholesterol in the region close to the acyl chains of the phospholipid tailsReference Roy, Mohanty and Dey36.
ANS has high affinity towards the interface between the apolar tail and the polar head of phospholipids, indicating changes in the surfaceReference Barshtein, Bergelson, Dagan, Gratton and Yedgar15, and has been associated with the insertion of positively charged molecules into the bilayer, thus increasing the packing order of the surface and the inner hydrocarbon regionReference Sujatha and Mishra37. The supplementation of synbiotic had little effect when pigs were given the low-fat diet. However, when pigs were on the high-fat diet, the supplementation of synbiotic had a reduced and delayed increase of cholesterol saturation in the upper phospholipids region, indicating improved membrane fluidity via increased permeability and decreased packing order.
TMA-DPH is a cationic probe with the fluorophore TMA incorporated in the head group and DPH incorporated in the acyl chainReference Ben-Yashar and Barenholz38, and been associated with reduced water penetrability into the bilayer, more ordered bilayer structure and higher cholesterol concentrations in the head groupReference Straume and Litman39. Results from the present study showed that the supplementation of synbiotic reduced the packing order and saturation of cholesterol in the polar heads of the membrane bilayer.
In conclusion, the supplementation of synbiotic reduced plasma TAG, total cholesterol and LDL-cholesterol in hypercholesterolaemic pigs. Evaluation of compositions of individual lipoprotein classes suggested that the synbiotic may reduce cholesterol in the form of CE via the interrelated pathways of lipid transporters (VLDL, LDL and HDL). The synbiotic also appeared to improve the occurrence of spur cells. The present study provided the experimental evidence that the supplementation of synbiotic reduced cholesterol saturation within the apolar tails of phospholipids, within the interfacial regions of the apolar tail and polar heads, and improved penetrability of the head group.









