Obesity is one of today's most blatantly visible public health problems, with an escalating global epidemic of overweight and obesity taking over many parts of the world(1). Even in many low-income countries, obesity is now rapidly increasing, and often coexists in the same population with chronic undernutrition(Reference Popkin2). Obesity significantly increases the risk of developing the metabolic syndrome, which carries with it the risk of developing CVD and type 2 diabetes mellitus. The metabolic syndrome consists of a combination of different abnormalities(Reference Isomaa, Almgren and Tuomi3), such as abdominal obesity, atherogenic dyslipidaemia, insulin resistance, glucose intolerance, impaired endothelial function, elevated blood pressure (BP) and inflammation(Reference Grundy4). Endothelial dysfunction and hypertension are associated with increased cardiovascular (CVD) morbidity and mortality and have been implicated in the development of atherosclerosis(Reference Cook, Hugli and Egli5). A small reduction in BP of 2–5 mmHg results in reductions in total mortality and mortality as a result of stroke and CVD(Reference Appel, Brands and Daniels6). It has been proposed that diet may play an important role in lowering BP and improving endothelial function(Reference Streppel, Arends and van't Veer7–Reference Coudray, Demigne and Rayssiguier9).
Epidemiological studies(Reference He, Klag and Whelton10, Reference Stamler, Caggiula and Grandits11) and clinical trials(Reference Birketvedt, Aaseth and Florholmen12, Reference Eliasson, Ryttig and Hylander13) have shown that the intake of dietary fibre either in the form of food or supplements(Reference Streppel, Arends and van 't Veer14) may be inversely correlated with BP levels and might be able to decrease the risk of hypertension development(Reference Ascherio, Rimm and Giovannucci15). Accordingly, an increase in dietary fibres has been recommended by the WHO as a safe and practical approach to CVD risk reduction in the hypertensive population(16).
In an 8-week parallel-design study, treated hypertensive patients were randomised to one of four groups, consuming a low-protein (12·5 % energy), low-fibre (15 g/d) diet alone or having supplements of soya protein (25 % energy), psyllium (12 g fibre/d) or both protein and fibre(Reference Burke, Hodgson and Beilin17). Results of this study suggest that a diet containing fibre-rich grain, fruit and vegetables significantly decreases the need for anti-hypertension medication and improves BP control in individuals with hypertension(Reference Burke, Hodgson and Beilin17).
Clinical trials on dietary fibre supplementation have shown a wide variation in BP responses. In trials with concentrated fibre supplements(Reference Eliasson, Ryttig and Hylander13, Reference Whelton, Hyre and Pedersen18, Reference Solum, Ryttig and Solum19), reductions in BP were larger than those found in trials with fibre-rich foods. Furthermore, a meta-analysis on published randomised trials indicates that increased dietary fibre consumption (from 7 to 13·3 g/d), from mixed supplements pills, guava fruit or psyllium(Reference Eliasson, Ryttig and Hylander13, Reference Burke, Hodgson and Beilin17, Reference Schlamowitz, Halberg and Warnoe20, Reference Singh, Rastogi and Singh21), may provide a safe and acceptable means to reduce BP in patients with hypertension(Reference Whelton, Hyre and Pedersen18) compared with those consuming < 7 g/d. The study by Eliasson et al. (Reference Eliasson, Ryttig and Hylander13) showed that dietary fibre tablets containing a mixture of beet, barley and citrus in a ratio of 60:30:10 (7 g/d) significantly reduced diastolic blood pressure (DBP) in mildly hypertensive patients (from 18 to 70 years old) compared with a control group taking placebo (1 g fibre (starch)/d) tablets. These changes were independent of changes in body weight(Reference Eliasson, Ryttig and Hylander13). In the study by Burke et al. (Reference Burke, Hodgson and Beilin17), hypertensive patients were randomised to one of four groups in an 8-week factorial study of parallel design in which they either (1) followed a low-protein, low-fibre diet, (2) had supplements of soya protein to increase protein intake to 25 % energy, (3) had psyllium supplementation to provide an additional 12 g soluble fibre/d or (4) had both protein and fibre. Results showed that the combination of dietary protein and soluble fibre supplements lowered BP additively (group 4) in hypertensive individuals(Reference Burke, Hodgson and Beilin17). In another study(Reference Singh, Rastogi and Singh21), seventy-two hypertensive patients consuming a soluble diet containing 0·5–1·0 kg of guava daily (13·3 g fibre/d) for 4 weeks had a decreased systolic blood pressure (SBP) and DBP compared with those in the control group (consuming their usual diet). Another study has shown a significant reduction in both SBP and DBP in individuals (presenting with high BP) consuming a diet supplemented with 8 g/d of water-soluble fibre from oat bran compared with those consuming a low-fibre diet (1·3 g/d)(Reference He, Streiffer and Muntner22).
In animal models, soluble fibre supplementation has been suggested to improve the endothelial function and BP(Reference Galisteo, Sanchez and Vera23). A diet supplemented with 3·5 % plantago ovata husks (psyllium) for 25 weeks has been shown to reduce SBP in obese diabetic Zucker rats(Reference Galisteo, Sanchez and Vera23) as well as in spontaneously hypertensive rats supplemented a 3 and 10 % diet for 30 d(Reference Obata, Ikeda and Yamasaki24). In addition, Goto Kakizaki type 2 diabetic rats presented reduced SBP following long-term consumption of cereal dietary fibre (8 g barley/100 g of diet) for 16 weeks(Reference Li, Wang and Kaneko25). Both results suggest that a high-fibre diet decreases BP in hypertensive, obese and diabetic rats.
The differences in BP response might be explained by fibre dose, type of fibre consumed or better compliance with dietary supplements than with high-fibre diets(Reference He and Whelton26). Intake of fibre in the USA and many other Western countries is about 15 g daily(Reference Alaimo, McDowell and Briefel27), which is only half the amount recommended by the American Heart Association (25–30 g/d from foods)(Reference Marlett, McBurney and Slavin28). It is estimated that Australian adults consume 18–25 g dietary fibre/d(29). This falls short of the Australian Government recommendations of 25 g dietary fibre/d for adult women and 30 g dietary fibre/d for men(Reference NHaMR Council30). Given this large population segment with inadequate fibre intake, the demonstration of the beneficial effects of fibre on BP may have an impact on public health policy.
Overweight and obesity is associated with an increased risk of poor BP control(Reference Hense31) and hyperlipidaemia. The augmentation index (AI) is an indicator of arterial stiffness and has been shown to be higher in those with hypercholesterolaemia(Reference Wilkinson, Prasad and Hall32). It has been suggested that the increase in arterial stiffening may be associated with an increase in SBP and DBP(Reference Blacher and Safar33–Reference Tomiyama, Hashimoto and Hirayama35). Arterial stiffness is one of the major risk factors of CVD(Reference Qureshi, Brown and Salciccioli36), presenting an independent risk factor for this condition(Reference Qureshi, Brown and Salciccioli36, Reference Casey, Pierce and Howe37). It is an age-related phenomenon(Reference Greenwald38, Reference McEniery, Wilkinson and Avolio39) and its progression is faster in patients with clusters of the metabolic syndrome(Reference Ostmark, Harrisson and Wooley40–Reference Safar, Thomas and Blacher42), resulting in luminal enlargement with wall thickening (remodelling) and a reduction in elastic properties (stiffening) at the level of large elastic arteries, namely arteriosclerosis(Reference Izzo and Shykoff43). Besides the metabolic syndrome(Reference Scuteri, Najjar and Muller44), patients with hypertension(Reference Arnett, Tyroler and Burke45) or diabetes(Reference Amar, Ruidavets and Chamontin46) exhibit increased carotid wall thickness and stiffness and this ‘accelerated’ arterial ageing is well confirmed to be a risk for CVD(Reference Benetos, Waeber and Izzo47). It is currently unknown whether fibre supplementation can improve arterial stiffness.
Some mechanisms have been suggested to explain the potential effect of dietary fibre intake on BP. Fibre seems to decrease the lipid profile by increasing intestinal viscosity(Reference Jenkins, Kendall and Axelsen48), therefore reducing bile acid absorption and promoting cholesterol catabolism(Reference Salas-Salvado, Bullo and Perez-Heras49). A high fibre intake is associated with a decrease in total and LDL-cholesterol(Reference Jenkins, Wolever and Rao50, Reference Brown, Rosner and Willett51), thereby potentially lowering the incidence of coronary disease(Reference Bingham, Day and Luben52). It has been shown that serum cholesterol affects BP regulation(Reference Ferrara, Guida and Iannuzzi53) by impairing endothelium-dependent dilation(Reference Creager, Cooke and Mendelsohn54). Interestingly, many hypertensive patients also appear to have increased serum cholesterol levels(Reference Laurenzi, Mancini and Menotti55). However, whether this is only a statistical association or it also implies a pathophysiological link is still under discussion(Reference Ferrara, Guida and Iannuzzi53). Hypercholesterolaemia is associated with the loss of NO-induced vasodilation(Reference Hayakawa and Raij56) and the subsequent increase in BP(Reference Vallance, Collier and Moncada57). There is evidence that cholesterol induces endothelial dysfunction even at normal or high-to-normal ranges by reducing the bioavailability of endothelium-derived NO(Reference Creager, Cooke and Mendelsohn54, Reference Hayakawa and Raij56). Higher cholesterol levels may also be associated with more atherosclerotic vessels, not only in the carotid circulation by the increase in intima-media thickness, but also in other arteries(Reference Ferrara, Guida and Iannuzzi53). In addition, water-soluble fibre seems to reduce insulin resistance in both diabetic and healthy subjects(Reference Anderson, Zeigler and Deakins58, Reference Fukagawa, Anderson and Hageman59), which is suggested to be involved in the development of hypertension(Reference Ferrannini, Buzzigoli and Bonadonna60). Hence, there is a possibility that fibre intake can also improve BP and endothelial function in overweight individuals.
Currently, there is a lack of studies on the effects of increasing dietary fibre intake on BP and arterial stiffness. A direct comparison between a group taking fibre supplementation alone and a group taking increased fibre by adopting a healthy eating pattern needs to be undertaken to reveal the effects from these different sources on chronic disease risk factors in overweight and obese individuals.
Psyllium husk fibre is a viscous, mostly water-soluble fibre and has been shown to be an effective supplement in adjunct to dietary intervention to control blood cholesterol, TAG and insulin levels in our previous studies(Reference Pal, Khossousi and Binns61, Reference Khossousi, Binns and Dhaliwal62). Therefore, the aim of the present study was to investigate whether 12 weeks of additional dietary fibre intake in the form of a healthy diet or dietary fibre supplement (psyllium) would sustain improvements in vascular function and BP.
Methods
Subjects
Overweight and obese individuals with a BMI between 25 and 40 kg/m2 and aged between 18 and 65 years, presenting lipid profile and glucose/insulin levels in any range were recruited through local media (newspaper and television) from the community of Perth, Australia. Of ninety-four respondents, seventy-two were eligible to participate (thirty-seven men and thirty-five women) and commenced the study. Potential participants were screened by telephone and they attended at Curtin University to assess their suitability for the study, at which time the details of the study were explained. Exclusion criteria included smoking, lipid-lowering medication, use of steroids and other agents that may influence lipid metabolism, use of warfarin, diabetes mellitus, hypo- and hyperthyroidism, cardiovascular events within the last 6 months, psychological unsuitability, major systemic diseases, gastrointestinal problems, proteinuria, liver failure, renal failure, weight fluctuations over the past 6 months and vegetarianism. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Curtin University Human Research Ethics Committee (approval no. HREC 157/2004). All participants gave their written informed consent.
Study design
The present study was a randomised, single-blind, parallel design over a 12-week period. The randomisation was done based on sex and alphabetical sequences of the surnames. The participants were allocated into two main groups of males and females and their lists were sorted based on their surnames alphabetically on a spreadsheet. The first name on each list was allocated into group 1, the second name into group 2 and the third name into group 3, and the same sequence of 1, 2 and 3 was repeated for the rest of the names, respectively, to allocate the participants into three intervention groups randomly and to avoid any bias. The study participants were randomised to one of three groups: the control group (n 18), who consumed placebo with their usual diet; the fibre supplement group (FIB, n 18), who consumed a fibre supplement with their usual diet; a healthy eating with placebo (HLT, n 18), who consumed placebo with a healthy eating regimen. The fibre supplement consisted of psyllium serving, which is a soluble fibre gel-forming mucilage from the Plantago ovata plant whose bioactive fraction is a fibre composed of a highly branched arabinoxylan(Reference Blackwood, Salter and Dettmar63). The participants were instructed to take 12 g of Metamucil (Metamucil; P&G, Sydney, NSW, Australia) containing 7 g of psyllium, three times a day, in a total extra fibre ingestion of 21 g daily. A serving size of 12 g of Metamucil supplement (two rounded teaspoons = approximately 12 g) contains approximately 7 g of dietary psyllium fibre, with the other remaining 5 g being made up of citric acid, aspartame, sunset yellow FCF CI 15985, maltodextrin, artificial and natural orange flavour and Na. Psyllium has advantages over other types of soluble fibre because it is less readily fermented and therefore causes less flatulence and abdominal bloating(Reference Blackwood, Salter and Dettmar63). It was used in the present study as it would be easily tolerated and is readily marketed worldwide. The placebo consisted of 12 g of breadcrumbs with flavouring, which provided 1·5 g of soluble fibre, presenting low energy and fibre content and similarity in texture and appearance to the psyllium supplement. The participants were asked to consume either the placebo or fibre supplement mixed with 250 ml water, 5–10 min before breakfast, lunch and dinner.
The subjects attended a briefing session on how to complete food records and comply with the study protocol. Dietary intake over the course of the trial was monitored through the completion of 3 d weighed food diaries at baseline, 6 and 12 weeks. Ad libitum healthy eating plans, as designed by a registered dietitian, were prescribed for the HLT group for 12 weeks, which included healthy meals and snacks based on the Dietary Guidelines for Australian Adults(Reference Lee, Skurk and Hennig64). These study participants were asked to record their food and drink intake for 3 d every 3 weeks to monitor the adherence to the guidelines(Reference Lee, Skurk and Hennig64) and were given feedback and strategies if necessary. These participants were closely monitored to ensure their compliance with the dietary guidelines.
The participants in the control and FIB groups were asked to maintain their usual dietary intake for the duration of the study. To monitor compliance, all the participants were provided with a measuring cup to measure either the fibre supplement or the placebo, they were required to complete a diary to record their supplement consumption and they were asked to return the empty and non-empty containers of the supplements at their 6- and 12-week visits.
Assessments
Subjects were asked to visit the Curtin University for measurements, in a fasted state and wearing light clothing, on three occasions, one for baseline measures, at 6 weeks and at 12 weeks. Body weight (UM-018 Digital Scales; Tanita, Tokyo, Japan) was recorded in light clothing. Height was measured to the nearest 0·1 cm, without shoes, using a stadiometer (26SM 200 cm; SECA, Hamburg, Germany). Waist circumference was measured in the standing position at the narrowest area between the lateral lower rib and the iliac crest. Hip measurement was taken at the largest circumference of the lower abdomen. The waist and hip measurements were taken three times at baseline, week 6 and week 12. The average at each time point was then reported. BP was measured with an automated, calibrated sphygmomanometer (Dinamap; Compact T, Critikon, Germany), with subjects in a supine position after resting for at least 10 min. The measurements were taken on the same arm three times at 1 min intervals. These readings were then averaged.
Pulse-wave analysis for augmentation index
Pulse-wave analysis was used to assess arterial stiffness and vascular function using the SphygmoCor™ (AtCor Medical, Sydney, NSW, Australia). SphygmoCor is a non-invasive device that enables aortic root pressure to be measured during a normal clinical consultation. All pulse-wave analysis measurements were taken in a quiet, temperature-controlled room (22°C), after a period of at least 5 min of rest. Patients fasted overnight and were asked to refrain from drinking drinks containing caffeine. Only high-quality readings, defined as an in-device quality index of 90 % (derived from an algorithm including average pulse height, pulse height variation, diastolic variation and the maximum rate of rise of the peripheral waveform) and acceptable curves by visual inspection by the investigator, were included in the analysis.
Measurements were taken in the fasting state at baseline, week 6 and week 12. For each assessment, at least three measurements were taken and results were averaged. The AI is negative in healthy young people, but with ageing or increasing cardiovascular risk, arteries stiffen and the AI becomes increasingly positive. The AI was calculated using the following formula: AI (%) = augmentation pressure/(SBP − DBP) × 100. The reference range provided by the Sphygmocor software (AtCor Medical) was determined from an analysis of 405 healthy individuals in a study by Wilkinson et al. (Reference Wilkinson, Franklin and Hall65). Mean population AI at age 50 years was 18·43 (90 % CI − 0·17, 39·97) %. All measurements were performed by the same operator. These measurements were taken three times with a quality index of at least 90 % or over and the readings were averaged.
Statistical analysis
Statistical analysis was undertaken using SPSS 11 for Windows (SPSS, Inc., Chicago, IL, USA). Data are expressed as means with their standard errors and assessed for normality to ensure that the assumptions of the analysis are met. Data were analysed using multivariate ANOVA with baseline values considered as covariates. If significant between-group effects were present, post hoc comparisons between the treatment groups were made using the least significant difference method. Statistical significance was considered at P < 0·05.
A sample size of at least fifteen subjects per group will provide sufficient power (0·90 %) to detect an estimated within- and between-group effect size of 0·56 at a 5 % significance level. Recruiting a total of seventy-two subjects (n 18) will allow for 20 % dropouts.
The present clinical trial was registered with the Australian New Zealand Clinical Trials Registry. The registration no. is ACTRN12609000540213 and trial web address is https://www.anzctr.org.au/ACTRN12609000540213.aspx
Results
Subjects
A total of seventy-two individuals were randomly assigned to one of the three test groups: control, FIB or HLT. Of these, fifteen participants withdrew from the study due to unrelated illness, work commitments, poor compliance and personal reasons. The remaining fifty-seven participants (twenty-five males and thirty-two females) completed the 12-week study (control: n 15; FIB: n 16; HLT: n 12). Baseline values of clinical and vascular characteristics in the three treatment arms were not significantly different (Table 1). Although there were some reports of minor bloating initially, all subjects tolerated the supplements well with no adverse effects reported.
Table 1 Clinical and vascular characteristics of subjects at baseline
(Mean values with their standard errors)*
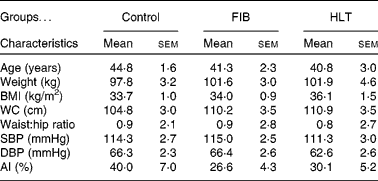
FIB, fibre supplement group; HLT, healthy eating with placebo; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; AI, augmentation index.
* Mean values were not significantly different between subject characteristics at screening.
Diet analysis
The self-reported food and drink intake at baseline, 6 and 12 weeks is shown in Table 2. There were no significant differences in total energy, carbohydrate, protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat and fibre between the three groups at baseline.
Table 2 Reported dietary intake data assessed by weighed food records†
(Mean values with their standard errors, n 57)

EI, energy intake; FIB, fibre supplement group; HLT, healthy eating with placebo.
a,b,c Mean values with unlike superscript letters were significantly different between the groups at 6 and 12 weeks (P < 0·05).
* Mean values were significantly different from baseline (P < 0·05).
† Nutritional data recorded in 3 d food diaries at baseline, week 6 and week 12.
Fibre intake was higher (P < 0·001) in the FIB group by 20·5 g and in the HLT group by 11 g at week 12 when compared with the control group. Fibre intake in the FIB group was also higher (P < 0·001) than the HLT group by 9·6 g at week 12.
Total fat intake was lower (P < 0·001) in the HLT group by 6 % at week 6 and by 24 % at week 12 when compared with the control group. When compared with the FIB group, total fat intake was lower (P < 0·001 and P = 0·020, respectively) in the HLT group by 10 % at week 6 and lower (P < 0·001) by 18 % at week 12. Saturated fat intake was increased (P < 0·05) in the control group at 12 weeks when compared with baseline. The HLT group also showed an increase (P < 0·05) in saturated fat at 12 weeks when compared with baseline. Saturated fat intake was reduced (P = 0·029) by 26 % in the HLT group when compared with the control group at week 12. Polyunsaturated fat intake was also lower (P = 0·018) in the HLT group by 23 % when compared with the control group at week 12. Monounsaturated fat intake in the HLT group was lower (P = 0·008) by 24 % at week 6 and lower (P = 0·047) by 20 % at week 12 when compared with the control group.
Effects of fibre on blood pressure
Resting BP was measured in the fasting state, at baseline, week 6 and week 12, and there were no significant differences in SBP or DBP. Between-group analysis revealed that SBP was lower in the FIB group by 7 % (P = 0·04) compared with the control group at week 6. SBP was lower in the HLT group by 9 % (P = 0·02) compared with the control group at week 12 (Fig. 1(a)). There were no differences in SBP between the other intervention groups at week 12 compared with the control group.

Fig. 1 Blood pressure levels at baseline, week 6 and week 12 between the three intervention groups (control (–●–) n 15, fibre supplement group (FIB; –■–) n 16, healthy eating with placebo (HLT; –▲–) n 12). (a) Systolic blood pressure (SBP, mmHg) and (b) diastolic blood pressure (DBP, mmHg). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different between the FIB v. control groups (P < 0·05). † Mean values were significantly different between the HLT v. control groups (P < 0·05).
Analysis between the groups at week 6 showed that DBP was lower by 7 % (P = 0·04) and 3 % (P = 0·04) in the FIB and HLT groups, respectively, compared with the control group. When DBP results were analysed at week 12, there was no difference between the groups (Fig. 1(b)).
Effects of fibre on vascular function
Central AI was measured in the fasting state, at baseline, week 6 and week 12, and there were no significant differences within the groups at 6 or 12 weeks from baseline. Analysis of data between the groups showed that the AI was lower by 22 % in the FIB group compared with the control group (P = 0·02) at week 6, but it was not different between the groups at week 12 (Fig. 2).
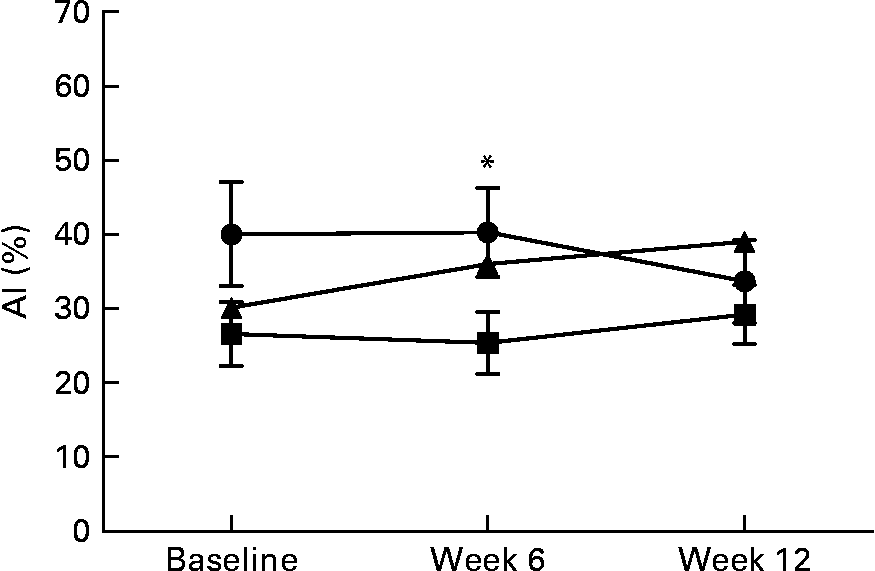
Fig. 2 Central augmentation index (AI; %) at baseline, week 6 and week 12 between the three intervention groups (control (–●–) n 15, fibre supplement group (FIB; –■–) n 16, healthy eating with placebo (–▲–) n 12). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different between the FIB v. control groups (P < 0·05).
Discussion
The aim of the present study was to investigate whether 12 weeks of consumption of a fibre supplement or a healthy eating diet would induce improvements to BP and arterial stiffness in overweight and obese individuals compared with a standard diet. SBP was significantly lower in the FIB group at week 6 compared with the control group. However, these changes were temporary and the only significant difference in SBP at week 12 was between the HLT and control groups. DBP was lower in the FIB group compared with the control group at week 6, but this did not last and there were no significant differences in DBP between the groups at the end of the study. Collectively, the present study showed that fibre supplementation in the form of psyllium had no effect on BP and endothelial function; however, increased fibre intake in the form of a healthy diet was beneficial to lowering BP.
Some mechanisms have been suggested to explain the potential effect of dietary fibre intake on BP, since dietary fibre presents numerous effects on the digestion and absorption of foods. The mechanism by which fibre lowers CVD risk remains to be determined; however, studies have suggested that fibre decreases the lipid profile by increasing intestinal viscosity(Reference Jenkins, Kendall and Axelsen48), therefore reducing bile acid absorption and promoting cholesterol catabolism(Reference Salas-Salvado, Bullo and Perez-Heras49). In particular, a high fibre intake is associated with a decrease in the serum concentrations of total and LDL-cholesterol(Reference Jenkins, Wolever and Rao50, Reference Brown, Rosner and Willett51) and, at the same time, with a lower incidence of coronary disease(Reference Bingham, Day and Luben52). In addition, water-soluble fibre seems to reduce insulin resistance and insulin plasma concentrations in both diabetic and healthy subjects(Reference Anderson, Zeigler and Deakins58, Reference Fukagawa, Anderson and Hageman59). Insulin resistance is implicated in the pathogenic mechanism for the development of hypertension(Reference Ferrannini, Buzzigoli and Bonadonna60).
Although little is known about the potential mechanisms by which dietary fibre may lower BP, there are a few studies investigating the beneficial effects of dietary fibre intake on BP. Studies with animals have suggested that a high-fibre diet decreases BP in hypertensive, obese and diabetic rats(Reference Galisteo, Sanchez and Vera23, Reference Obata, Ikeda and Yamasaki24). In fact, prospective observational studies that have analysed the relationship between dietary fibre intake and the risk of developing hypertension have indicated that an increased consumption of dietary fibre intake is inversely related to BP and may contribute to prevent hypertension(Reference Burke, Hodgson and Beilin17, Reference Whelton, Hyre and Pedersen18, Reference Hense31, Reference Galisteo, Duarte and Zarzuelo66), in both hypertensive or normotensive subjects.
In the present study, SBP and DBP were lower in the FIB group compared with the control group at week 6. However, this change appears to have been temporary as there were no significant differences in SBP and DBP between any groups at week 12. This discrepancy may have been due to lower BP in the control group at week 12. This may be due to day-to-day variability in BP(Reference Reeves67) or to some other factors such as psychological stress and mood which can affect BP levels(Reference Brown, James and Nordloh68). However, the reason why this would occur in the control group and not affect the other groups in the present study is unknown. The other possibility for no difference in BP between the groups after 12 weeks of study was that the average BP of the groups was within the healthy range and it is hard to highlight the BP differences in normotensive participants, as dietary fibre usually makes small changes in BP in these groups(Reference Galisteo, Duarte and Zarzuelo66, Reference Jenkins, Kendall and Vuksan69).
A good body of evidence supports the adoption of a diet high in fruits, vegetables and fibre and low in fat, which suggests that such a diet helps protect against CVD and diabetes(Reference Harriss, English and Powles70). Thus, the psyllium supplement consumed in the present study may not be as effective as the multiple sources of fibre from consuming non-viscous fibres and pectin from whole grain, fruit and vegetables. Furthermore, there is evidence that soluble fibre improves mineral absorption in the digestive system(Reference Coudray and Rayssiguier71, Reference Greger72), which may have a favourable effect on BP. In the Dietary Approaches to Stop Hypertension trial, the effect of dietary patterns on BP was assessed. The fruits-and-vegetables diet provided K, Mg and high amounts of fibre, and resulted in BP reductions of 2·8/1·1 mmHg compared with a typical US diet. Other studies have provided support for an increased effect of soluble fibre on BP(Reference Streppel, Arends and van 't Veer14).
In addition, several other mechanisms for a potential effect of dietary fibre intake on BP have been hypothesised. Dietary fibre has numerous effects on the digestion and absorption of foods and can change the glycaemic index of foods, thereby altering insulin response. Insulin plays a role in BP regulation and dietary fibre can modify the insulin and vascular endothelial function(Reference Bessesen73). Insulin resistance and its concomitant compensatory hyperinsulinaemia have been suggested as major underlying pathogenetic mechanisms for the development of hypertension(Reference Ferrannini, Haffner and Mitchell74). In this way, the effectiveness of both soluble and insoluble fibres in reducing insulin resistance and insulin levels in both diabetic and healthy persons(Reference King, Mainous and Egan75, Reference Qi, Rimm and Liu76) would contribute to treating or preventing hypertension. Our previous studies showed that simply adding psyllium fibre supplementation to a normal diet was sufficient to see improvements in total and LDL-cholesterol as well as body weight, BMI and percentage of body fat compared with the control group. However, there was no effect of psyllium fibre supplementation on insulin levels, but fasting insulin levels decreased at week 12 in the HLT group by 24 % (P = 0·03) compared with baseline(Reference Pal, Khossousi and Binns61). When compared with the control group at week 12, insulin levels decreased in the HLT group by 21 % (P = 0·001). Given that insulin plays a regulatory role on BP, the lack of change in insulin levels in the FIB group may partially explain why fibre supplementation did not affect BP in the FIB group.
In subjects with the metabolic syndrome, the AI, an indicator of arterial stiffness, was shown to be significantly higher than in subjects with normal weight for the same age and mean arterial pressure(Reference Henry, Kostense and Spijkerman77, Reference Schram, Henry and van Dijk78). Thus, this measurement is one of the most appropriate for repeat determinations in clinical studies. Studies have shown an inverse relationship between healthy eating and arterial stiffness(Reference Seals, Moreau and Gates79). Compared with a low-fibre meal (12–16 % carbohydrate containing 0·5 g cereal fibre), a mix of high-carbohydrate, high-fibre meal (69–76 % carbohydrate containing 19 g cereal fibre) has been shown to improve flow-mediated dilation through increasing NO release in subjects with the metabolic syndrome(Reference Brock, Davis and Irving80). However, the role of dietary fibre in preventing and improving arterial stiffness in the short and long term has yet to be fully elucidated. Endothelial function has an important role in regulating BP and vascular resistance(Reference Wilkinson, Qasem and McEniery81). Consumption of psyllium husk has been shown to alter the endothelial function and SBP in Zucker rats(Reference Galisteo, Sanchez and Vera23). The hypotensive effect of psyllium was attributed, at least in part, to its ability to inhibit intestinal absorption of Na and improve the endothelial function by improving hyperlipidaemia and decreasing the inflammatory markers(Reference Obata, Ikeda and Yamasaki24). The present data analysis showed that the central AI did not change over 12 weeks of intervention from the baseline in any group. Data analysis between the groups showed that the AI was lower by 22 % in the FIB group compared with the control group at week 6; however, this did not last and there were no significant differences in the AI between the groups at the end of the study. This suggests that the mechanism of reduction of BP following dietary supplementation with psyllium is related to specific alterations in endothelial function. The present study is the first to investigate the effect of psyllium husk on arterial stiffness in human subjects. There is some evidence from animal studies which supports the beneficial effects of psyllium on endothelial function, such as diabetic rats fed daily with psyllium husk that showed improvement on endothelial function and, consequently, on SBP by altering the endothelium response to acetylcholine and improving inflammatory markers(Reference Galisteo, Sanchez and Vera23). Markers of the endothelial function such as NO were not measured in the present study, but it may be one of the mechanisms that altered BP temporarily in the intervention groups.
The amount of fibre supplementation of 21 g psyllium/d, as well as the duration of the study of 12 weeks, was considered suitable to detect differences in BP. Our choices were based on previous studies confirming this information. A meta-analysis based on the evidence from twenty-five randomised, controlled clinical trial, conducted in 1477 participants with or without hypertension observed that a fibre intake of 7·2–18·9 g/d reduced significantly both SBP ( − 2·3 mmHg) and DBP ( − 1·97 mmHg), respectively. Trials whose duration was 8 weeks or more in length showed a significant decrease in SBP and DBP ( − 3·12 and − 1·14 mmHg, respectively) mainly in hypertensive subjects but also in normotensive subjects(Reference Whelton, Hyre and Pedersen18). Therefore, the dose of fibre and the duration of the study length used in the present study are well above the range used in previous trials showing the beneficial effects of fibre on BP.
The present study has some limitations. Hypertensive subjects on various medications such as lipid lowering, hypertension, etc. had responded to our newspaper article but were then excluded given the confounding effects of these medications. Therefore, the average BP of the participants in the present study was within the healthy range. This may be the reason why no differences in BP was observed between the groups after 12 weeks of study, as it is hard to highlight the BP differences in normotensive participants. Dietary fibre usually makes only small changes in BP in normotensive groups(Reference Galisteo, Duarte and Zarzuelo66, Reference Jenkins, Kendall and Vuksan69).
The lack of significant differences in SBP and DBP between the FIB and HLT groups at the end of the study may also be due to lower BP in the control group at week 12, due to day-to-day variability in BP(Reference Reeves67) or due to some other factors such as psychological stress and mood which can affect BP levels(Reference Brown, James and Nordloh68). However, the reason why this would occur in the control group and not affect the other groups in the present study is unknown. Based on the variability in BP observed in the present study, the trial had very low power to detect clinically meaningful changes.
The lack of any improvement in arterial stiffness in the FIB and HLT groups may be due to the normal BP of the individuals and the short duration of the study. A meta-analysis of studies about the effects of healthy eating and dietary fibre on BP and endothelial function showed that these effects are more prominent in hypertensive individuals compared with normotensive individuals, and significant changes are usually measurable after a few months of intervention(Reference Whelton, Hyre and Pedersen18). Future studies with hypertensive subjects would clarify whether these results persist or not.
In summary, the present study did not show any improvements in BP or vascular function in overweight and obese individuals with psyllium fibre supplementation, although a healthy diet decreased SBP. There may be factors in a healthy diet affecting BP, which may not be related to an increase in fibre. Further studies are required in hypertensive overweight and obese patients to examine whether psyllium fibre supplementation can have beneficial effects on vascular health and BP.
Acknowledgements
The present trial was partially funded by the ATN Centre for Metabolic Fitness. A. K. coordinated the trial, conducted data collection, statistical analysis and provided input into the manuscript. C. B. provided input into the manuscript. S. D. supervised the statistical analysis. S. R.-B. contributed to the writing of the manuscript. S. P. conceived and designed the study, supervised the study and the statistical analysis and mentored the script. The authors declare that they have no conflicts of interest.






