Haemodialysis is an established treatment for patients with advanced chronic kidney disease (CKD). Haemodialysis efficiently clears small water-soluble solutes, such as urea, but it is less effective at removing larger charged molecules and those bound to proteins. As such, these azotaemic solutes can accumulate over time. Several of them, including tryptophan and trimethylamine( Reference Jankowski, Westhof and Vaziri 1 ), undergo metabolism by the gastrointestinal microbiome( Reference Anders, Andersen and Stecher 2 ).
Dietary changes, particularly switching from a meat-based diet to a vegetarian one, can alter the gastrointestinal microbiome( Reference Anders, Andersen and Stecher 2 ), and this may reduce the levels of trimethylamine and p-cresol( Reference Patel, Luo and Plummer 3 ). Retention of these azotaemic toxins and other toxins that include advanced glycosylation end products (AGE) have been linked to increased CVD risk for CKD patients.
The plasma levels of these toxins may not necessarily reflect the total body burden. Circulating AGE, for example, are deposited in tissues such as the skin, and measurement of tissue deposition is a much more reliable marker of long-term exposure to AGE than serum values are. These tissue depositions in the skin can be measured by skin autofluorescence (SAF)( Reference Graaff, Arsov and Ramsauer 4 ).
Previous studies have reported increased tissue AGE accumulation in diabetes, CKD and CVD( Reference Meerwaldt, Hartog and Graaff 5 ). Haemodialysis patients are at an increased risk for AGE deposition because AGE are normally cleared by the kidney. We dialysed a multicultural group of CKD patients and compared SAF between vegetarians and non-vegetarians to determine whether dietary intake influences skin AGE deposition.
Materials and methods
We measured SAF in 332 adult haemodialysis patients who were dialysing in north central London. Patients dialysed using high-flux dialysers( Reference Vernon, Peasegood and Riddell 6 ) and anticoagulated with low-molecular-weight heparin( Reference Davenport 7 ), and they were directly asked about dietary habits and smoking history. Tissue AGE were measured using a UV technique (DiagnOptics). SAF measurement is non-operator dependent, and it has been validated in patients with skin reflection of >6 % in Fitzpatrick classes 1 to 4 and 6–78 % in Fitzpatrick classes 5 to 6. The measurements were performed at a standard temperature on the volar surface of the non-fistula forearm, skin blemishes and hairs were avoided, and an additional light source was used for pigmented skin colour( Reference Chaudhri, Fan and Davenport 8 ). The mean of three readings was recorded and reported in arbitrary units (AU).
We complied with UK National Health Service (NHS) guidelines for clinical service development, and SAF was also measured as part of an observational study of alternative methods for assessing dialysis adequacy (study registration ISRCTN70556765, National Research Ethics Committee Camden and Islington (13/LO/0912)), in accordance with the Declaration of Helsinki.
Statistical analysis
All categorical data are reported as numbers (percentage), and numeric variables were reported as means and standard deviations. A stepwise backward multivariate model was derived using variables with a P< 0·1 univariate correlation with SAF and then excluding non-significant variables unless they improved the model fit (SPSS version 18.0; University of Chicago).
Results
SAF was measured in 332 adult dialysis patients (Table 1). SAF was lower in the twenty-seven vegetarians than it was in the non-vegetarians (Fig. 1). Simple univariate correlation (Table 2) showed that increased SAF deposition was associated with older age, Caucasian ethnicity, longer dialysis vintage, a history of diabetes, CVD, peripheral vascular disease, serum cholesterol and C-reactive protein as well as the prescription of lanthanum which contained phosphate binders, whereas SAF deposition was reduced for those who ate a vegetarian diet. There was no association between SAF and current smoking status. SAF measurements were higher for Caucasians as compared to South Asians and African Afro-Caribbeans (3·52 (sd 0·94) v. 3·01 (sd 0·81) v. 2·4 (sd 0·6) AU, P< 0·001, respectively), even with similar proportions of diabetic patients in the three cohorts. Of the Caucasian subjects, 3 % were vegetarian, as compared to 24 % of the South Asians and 9 % of the African Afro-Caribbeans. Although the proportion of vegetarian South Asians was greater, there was no statistical difference between the Caucasians and the African Afro-Caribbeans (χ2 2·49, P= 0·12). For each racial group, the SAF was lower in the vegetarian cohort (Caucasians 2·97 (sd 0·74) v. 3·53 (sd 0·95) AU, South Asians 2·70 (sd 0·58) v. 3·12 (sd 0·85) AU, African Afro-Caribbeans 2·37 (sd 0·46) v. 2·45 (sd 0·61) AU, respectively).
Table 1 Baseline characteristics of patients in dialysis cohort with prescription of calcium and lanthanum carbonate phosphate binders (Mean values and standard deviations; median and interquartile range; percentages)
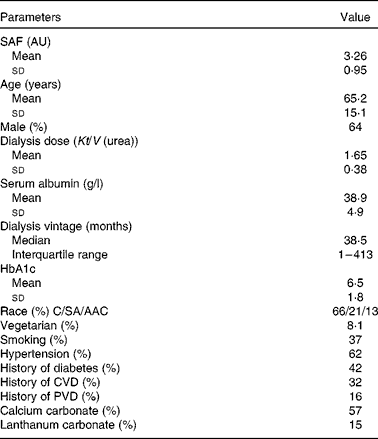
SAF, skin autofluorescence; AU, arbitrary units; HbA1c, glycosylated Hb; C, Caucasian; SA, South Asian; AAC, African Afro-Caribbean; PVD, peripheral vascular disease.
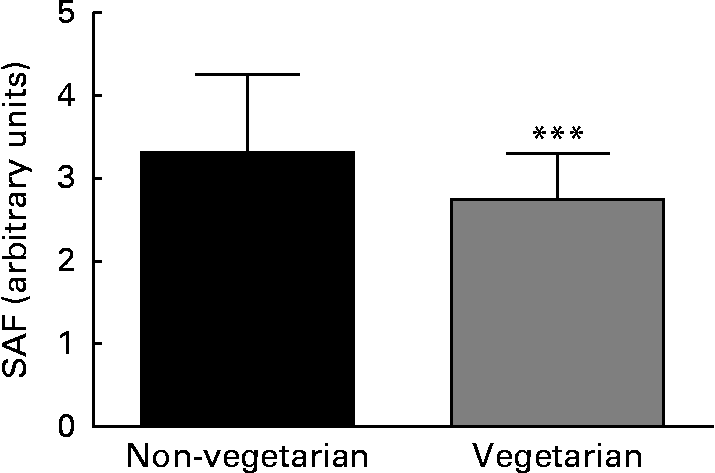
Fig. 1 Difference in skin autofluorescence (SAF) between vegetarian and non-vegetarian haemodialysis patients. *** Mean value was significantly different from that of the non-vegetarian group (P= 0·002).
Table 2 Univariate analysis of factors associated with skin autofluorescence (SAF)
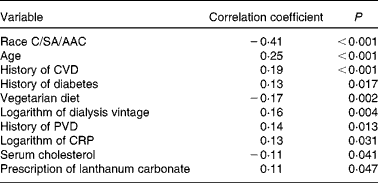
C, Caucasian; SA, South Asian; AAC, African Afro-Caribbean; PVD, peripheral vascular disease; CRP, C-reactive protein.
A multiple regression model (Table 3) showed that increased SAF was positively associated with Caucasian ethnicity, older age, female sex (although women did not have significantly lower values (3·38 (sd 1·0) v. 3·19 (sd 0·9) AU)), longer dialysis vintage, a history of diabetes and the prescription of Ca and lanthanum phosphate binders, whereas a vegetarian diet was associated with lower SAF.
Table 3 Multiple regression analysis of factors associated with skin autofluorescence (SAF) of dialysis cohort* (β Coefficients and 95 % confidence intervals)
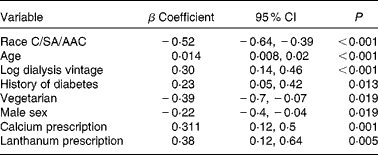
C, Caucasian; SA, South Asian; AAC, African Afro-Caribbean.
* Model fit R 2 0·334, adjusted R 2 0·309.
Discussion
We measured SAF in a multicultural and ethnically diverse haemodialysis population. As expected from previous reports, diabetics and those subjects with CVD had an increased skin deposition of AGE, as measured by SAF( Reference Meerwaldt, Hartog and Graaff 5 ). Previous reports have investigated SAF in various ethnicities, ranging from Northern Europeans to Middle Easterners to Chinese and Japanese subjects, and the DiagnOptics skin autofluorescence device has been validated in patients with different skin pigmentations( Reference Koetsier, Nur and Chunmao 9 ). In addition to finding an association of SAF and age with both CVD and diabetes, as reported earlier( Reference Meerwaldt, Hartog and Graaff 5 ), we also found an association with dialysis vintage, which is in keeping with the azotaemic toxin and skin AGE accumulation that occurs over time on dialysis( Reference Davenport and Farrington 10 ). Compared to previous reports, we were not able to demonstrate an association between smoking and SAF; this may have been a result of the number of patients who had stopped smoking once they started dialysis.
We also found that racial differences might have an effect on SAF, and we observed that SAF measurements were higher for Caucasians as compared to South Asians and African Afro-Caribbeans, even with similar proportions of diabetic patients in the three cohorts. Although in theory these differences in SAF measurements could have been explained by differences in skin pigmentation affecting the penetration of UV light, our device is fitted with an additional light source which is used to overcome the effects of darker skin pigmentation on UV light penetration. However, there was one factor that was consistently associated with reduced SAF: a vegetarian diet. Although SAF was significantly lower for vegetarians, there was no statistical difference in SAF between the individual racial groups, but this could have been the result of the small numbers in the present cohort.
We also noted that the prescription of calcium carbonate and lanthanum carbonate phosphate binders was associated with higher SAF measurements. There is growing evidence that poor phosphate control may increase vascular calcification and the risk of CVD. Although patients were asked about dietary intake, formal dietary assessments of phosphate-rich foodstuffs were not undertaken( Reference Morey, Walker and Davenport 11 ). In the United Kingdom, higher-phosphate diets are associated with greater protein intake, which suggests a link between increased animal protein and tissue AGE deposition.
Although AGE can accumulate in the body through endogenous production coupled with reduced renal clearance, exogenous AGE in the diet can also increase AGE accumulation, especially in patients with CKD. Exogenous AGE are formed by both nutrient composition, such as the protein and fat content in foodstuffs, as well as the manner in which food is cooked; for example, AGE are increased when food is baked or roasted( Reference Uribarri, Woodruff and Goodman 12 , Reference Koschinsky, He and Mitsuhashi 13 ). Studies examining the effects of dietary AGE reported only a 10 % change in SAF following the introduction of a high-fat diet( Reference Stirban, Nandrean and Negrean 14 ). In the present study we asked our patients to refrain from eating before attending dialysis. The modest effect of dietary AGE may have resulted because exogenous AGE is less likely to bind and become incorporated into the tissue matrix in the skin, and as such exogenous AGE do not have the same predictive association with CVD risk. However, because autofluorescence patterns are similar for both endogenous and exogenous AGE, we were unable to distinguish whether the reduction in SAF was the result of a reduction in endogenous or exogenous AGE. Serum AGE concentrations have been reported to fall with standard haemodialysis treatments. We dialysed patients with high-flux dialysers that were designed for the increased removal of medium-sized molecules( Reference Davenport 15 ) in combination with higher-volume haemodiafiltration( Reference Tattersall, Ward and EUDIAL group 16 ), so we expected to have a greater clearance of smaller molecular weight AGE not bound to tissue matrix. We had not previously observed a reduction in SAF following haemodiafiltration( Reference Vernon, Peasegood and Riddell 6 ), which would suggest that we measured AGE incorporated into the skin tissue matrix. Whether the differences observed in SAF in the vegetarian patients were related to dietary differences in exogenous AGE ingestion or were secondary to differences in protein intake and metabolism, which led to a change in the production rate of endogenous AGE, remains to be determined. The present study suggests that there may be benefits to a vegetarian-based diet for dialysis patients, who are at an increased risk of tissue AGE deposition because of a failure of renal clearance. The study supports the results of a short-term study which reported that dietary manipulation reduced serum N-carboxymethyl-lysine, an AGE( Reference Uribarri, Peppa and Cai 17 ). The present study is the first to report lower tissue AGE deposition being associated with a vegetarian diet, most likely due to a lower exogenous dietary AGE intake. Our observational finding requires further prospective study to determine whether dietary manipulation can reduce tissue AGE deposition and reduce CVD risk in dialysis patients.
Acknowledgements
The study was funded by the Royal Free Hospital. A. D. conceived the study; A. N. measured skin autofluroescence and analysed the data.
The authors have no conflicts of interest.







