Postmenopausal osteoporosis, which is also considered the ‘silent disease’, consists one of the most important public health issues. Almost 9 million fractures of osteoporotic patients are listed every year around the world(Reference Cooper, Cole and Holroyd1). This disease affects older women in particular, who sustain osteoporotic fractures 1·6 more times than men, and is one of the diseases that obliges people to be absent from work and social commitments for a long period of time. It is estimated that one out of two women and one out of four men at the age of 50 years and older will face at least one osteoporotic fracture(Reference Pisani, Renna and Conversano2). Among the factors which seem to lead to lower bone mineral density (BMD) and increased risk of fracture are genetic background, lack of exercise, high body fat mass, smoking, high alcohol intake and prolonged use of glucocorticoids(Reference Dey and Bukhari3–Reference Øyen, Gram Gjesdal and Nygård5). On the other hand, physical exercise (in particular weight-bearing exercise and walking), starting early before menopause onset, seems to be beneficial for the maintenance or increase of BMD(Reference Howe, Shea and Dawson6,Reference Shatrugna, Kulkarni and Kumar7) .
The use of rapid and efficient therapies is of high importance for the prevention of fractures as well as for the reduction of the cost for health providers(Reference Pike, Birnbaum and Schiller8). Therapies which have been used successfully throughout the years (calcitonin, hormone replacement therapy, selective oestrogen receptor modulators, bisphosphonates, denosumab, parathyroid hormone and strontium ranelate)(Reference Cooper, Reginster and Cortet9) have been reported to cause adverse effects especially when administered for a long period(Reference Solomon, Rekedal and Cadarette10,Reference Cosman11) . Poor compliance and other concomitant ageing disorders of the patients make the assessment of long-term efficacy and adverse effects very difficult. This could possibly explain why there are currently limited studies to examine these factors for long periods of treatment and follow-up(Reference Cooper, Reginster and Cortet9,Reference Langdahl12) .
For these reasons, nutritional measures which are alternative to pharmaceutical treatment have been proposed for postmenopausal women, such as Ca and/or vitamin D supplementation, fruits and vegetables or high-protein diets(Reference Kemmler, Lauber and Weineck13–Reference Mustafa, Alfky and Hijazi17). Nowadays, non-pharmaceutical products are believed to be useful for supporting or substituting already existing treatments(Reference Higgs, Derbyshire and Styles15,Reference Leung, Ko and Siu18,Reference Lappe, Kunz and Bendik19) . This trend has motivated researchers to focus on plant extracts, which have compounds that have been reported to show oestrogenic and/or antioxidant properties and other positive effects related to prevention of diseases, such as osteoporosis, cancer and CVD(Reference Patisaul and Jefferson20–Reference Psaltopoulou, Naska and Orfanos22). Amongst them are the olive constituents oleuropein and hydroxytyrosol (phyto-oestrogens), which have already been proved to have anti-ischaemic, antioxidative and hypolipidaemic actions(Reference Andreadou, Iliodromitis and Mikros23–Reference Marković, Torić and Barbarić27). A few ex vivo (Reference Nousis, Doulias and Aligiannis28) and in vivo studies have shown that these table olive wastewater extract (OE) compounds could have a potential positive effect on the prevention and maintenance of bone mass density in the postmenopausal osteoporosis model of the ovariectomised adult rat(Reference Puel, Quintin and Agalias29,Reference Chin and Ima-Nirwana30) . Therefore, the present study aimed to elucidate the effect of per os OE administration on bone density and strength in mature ovariectomised rats.
Experimental methods
Laboratory animals
The present study was evaluated by the research establishment’s Protocol Evaluation Committee and was approved by the General Directorate of Veterinary Services (permit no. 3002/15-05-2014), the Research Committee of the KAT Hospital (EΣ 244-7661, 26.06.2014), as well as by the General Assembly of the School of Medicine, University of Athens (04-06-2014) according to Greek legislation (Presidential Decree 56/2013, in compliance with the Directive 2010/63/EU). Before the beginning of the experiment, during the experimental design period and at the end before submitting for publication, the ‘PREPARE’ and ‘ARRIVE’ guidelines were followed(Reference Smith, Clutton and Lilley31,Reference Kilkenny, Browne and Cuthill32) .
Thirty 3-month-old intact female Wistar rats with similar body weights were obtained from the breeding facilities of the Hellenic Pasteur Institute, Athens, Greece for this study. The animals were housed in transparent polycarbonate open-top 45 cm × 30 cm × 20 cm cages, four to a cage under standard laboratory conditions (temperature between 19 and 22°C, relative humidity between 55 and 65 %, 15 air changes per hour and a 12-h light–12-h dark cycle). The rats were allocated into three groups at the age of 3 months and were left to reach the age of 9 months, when the first procedure took place: (a) Control (n 10), (b) Ovariectomised (OVX) (n 10) and (c) OVX + OE (n 10). Baseline measurements of body weight and BMD were taken at the age of 9 months. The Control animals remained intact and did not receive any treatment, but were necessary as age-matched animals, in order to compare with the other groups’ physiological age-related BMD changes, irrelevant to ovariectomy or to consumption of OE. The animals’ food (free from soya or soya by-products) intake was limited according to the Control’s group consumption in order to avoid post-ovariectomy obesity by ad libitum feeding and its possible confounding results. Food and water or extract intake were measured twice per week. During the course of the project, two of the Control group animals showed signs of respiratory infection and had to be euthanised; although necropsy was not conclusive for infection, their results were excluded from the analysis.
Extract
Production of extract
OE is the main by-product of the natural debittering procedure of table olives of Olea europaea. For the present study, 10 litres of OE was submitted to adsorption resin chromatography. The resin used was, per manufacturer’s specifications, XAD-4, which is the recommended type for the effective adsorption of phenolic compounds. Following the initial mandatory activation of the resin with ethanol and water, and the passing of the OE, the resin was washed with water in order to remove residual polysaccharides, lipids and salts, while the phenolic compounds remained adsorbed. In order to achieve their retrieval, 3 litres of ethanol was used as an elution solvent. The final dry extract produced, after desorption with ethanol and evaporation of the solvent, was 112 g (yield 1·12 %, w/v), similar to the one found in the literature(Reference Xynos, Abatis and Argyropoulou33).
HPLC analysis
The two main compounds identified in OE are the two phenylalcohols, tyrosol and hydroxytyrosol. Quantification of these compounds was accomplished based on a method already described in the literature(Reference Nardi, Bonacci and De Luca34). In the current study, a Thermo Finnigan® HPLC-DAD system (P4000 Pump, AS3000 Autosampler, PDA Detector UV8000, Chromquest TM 4.1 Software) and a Supelco® RP18 Discovery HS-C18 (250 mm, 4·6 mm, 5 um) column were employed. A two-solvent gradient elution system was used with starting conditions of 98 % water with 0·2 % acetic acid and 2 % acetonitrile. Linear gradient to 30 % acetonitrile was achieved in 40 min and maintained for 5 min. Initial conditions were reached in a further 5-min timeframe. Subsequently, total runtime was 50 min with a flow rate of 1 ml/min, while the injection volume was set at 20 ul. Temperature was kept at 25°C, and detection was performed at 280 nm.
The sample was weighed and dissolved to a final concentration of 2·4 mg/ml. Five-point calibration curves were constructed using commercially available formulations from Extra Synthase (Hydroxytyrosol: y = 87968x−11437, R 2 = 0·9992; Tyrosol: y = 55018x−29720, R 2 = 0·9998) and were used for the quantification of the analytes. The recorded chromatogram (Fig. 1) verified the presence of hydroxytyrosol and tyrosol in the extract with their concentration calculated at 9·75 % (w/w) and 0·34 % (w/w), respectively.

Fig. 1. HPLC-diode-array detector (DAD) chromatogram of the table olive wastewater extract (OE), recorded at 280 nm. Identified peaks correspond to hydroxytyrosol and tyrosol.
Extract administration
OVX + OE group rats were administered the OE for 6 months, diluted in their drinking water in a concentration of 150 mg/l, receiving a dose of 1·28 mg/kg of rat per d, which is the equivalent of 12 mg hydroxytyrosol per human consumption(Reference Nair and Jacob35) orally per d. This is higher than the minimum therapeutic dose of 5 mg/d suggested by EFSA(Reference Panel and Nda36). The extract was immediately provided after a 2-d rest following OVX and another 2-d acclimatisation period to the extract with an initially lower concentration(Reference Dontas, Lelovas and Kourkoulis37). The most refined method of ad libitum drinking from their cage bottles was selected as a non-stress-producing administration method over oral gavage administration.
Bone mineral density measurements
Dual-energy X-ray absorptiometry measurements were conducted at baseline (at 9 months of age) and at 3 and 6 months post-OVX. At the beginning of the procedure, the system was calibrated before every group’s measurement(Reference Jee and Yao38). A GE Lunar Prodigy Densitometer machine, equipped with small animal software, was used. For this purpose, all rats were anaesthetised in order to remain immobile during the scan by ketamine 50 mg/kg (Ketaset, Zoetis) and dexmedetomidine 0·25 mg/kg (Dexdomitor, Zoetis) intramuscularly; anaesthesia was reversed with atipamezole 1 mg/kg (Antisedan, Zoetis) intramuscularly. For analysis of the scans, the operator determined two different regions of interest(Reference Dontas, Lelovas and Kourkoulis37). One region of interest tool covering the whole tibia was properly placed for the calculation of the total tibial BMD; another (2 × 2 mm2) region of interest defining the proximal tibial metaphysis was placed 3 mm distal to the tibial plateau and was used for the calculation of the proximal tibial BMD, which is rich in trabecular bone. The in vitro precision (CV) of the system was 0·5 %, and placement of region of interest tools was conducted after the end of the protocol, by the same operator for all rats at every time point.
Ovariectomy
After the baseline BMD measurement, groups OVX and OVX + OE were ovariectomised(Reference Sophocleous and Idris39). The rats were submitted to general anaesthesia by ketamine (Ketaset, Zoetis) 50 mg/kg and dexmedetomidine (Dexdomitor, Zoetis) 0·25 mg/kg both intramuscularly; pre-operative analgesia by carprofen (Rimadyl, Zoetis) at a dose of 4 mg/kg and chemoprophylaxis by enrofloxacin (Baytril, Bayer) 10 mg/kg were administered both subcutaneously. Bilateral ovariectomy was preceded by a midline ventral incision under aseptic procedures. The layers of the peritoneum and skin were sutured by single interrupted sutures. Anaesthesia was reversed for quicker recovery with atipamezole 1 mg/kg (Antisedan, Zoetis) intramuscularly.
Euthanasia, specimen collection and examination
At the time of euthanasia, the animals were anaesthetised as previously described and their vena cava was exposed from which blood was collected into EDTA-coated tubes; they were then euthanised with overdose of anaesthesia (sodium thiopental). The blood was centrifuged, and the plasma was stored under −80 °C in Eppendorf tubes for further biochemical analysis. Plasma parameters measured were: alanine aminotransferase (ALT), gamma-glutamyltransferase (γ-GT), total cholesterol, HDL-cholesterol, LDL-cholesterol, Ca and P. Post-mortem necropsy was performed for possible pathological findings or malignancies and in order to evaluate the OVX operation conducted. Lack of ovarian tissue and atrophy of the uteri confirmed successful OVX. The uterus, abdominal fat, gastrocnemius muscle, heart, kidneys, brain and liver were carefully removed and immediately weighed. For these procedures, the users were blinded and did not know the group of each animal.
Biomechanical testing
This variable was assessed with the ex vivo three-point bending technique(Reference Steiner, Volkheimer and Meyers40). Both femurs of each animal, right and left, were placed in gauze soaked in saline and kept at −20 °C on the day of tissue collection. They were removed from the freezer and left in their package, in room temperature to thaw on the day of testing(Reference Kaye41). All assessments were performed with the use of the MTS 858 Mini Bionix frame which was connected to a computer, and the equipment was calibrated before the beginning of the first test. All femurs were horizontally positioned in the same way, and a vertical load was applied in the middle of the diaphysis up to the point of fracture of the bone at a displacement rate of 1 mm/minute. At the end of the procedure, the maximal load applied at the fracture was used by the software (TestWorks programmes 4) which created a graph exhibiting the relation between load and displacement variables.
Power analysis – sample size estimation
It was calculated that a sample size of ten rats per group was required in order to have an 80 % probability of demonstrating a difference between groups of >15 % (sd 10) in % change from baseline to 6th month of proximal BMD (OVX: −25 (sd 10) %, OVX + OE: −10 (sd 10) %) with a significance of <1·7 % (two-tailed test with Bonferroni correction) according to Galanis(Reference Galanis, Soultanis and Lelovas42). Sample size estimation was performed using G*Power 3.1.9.2 programme.
Statistical analysis
Data were expressed as mean and sd, and the Shapiro–Wilks test examined the normal distribution of the parameters.
At the beginning of the study (at 3 months of age), the animals were allocated into three groups with the basic criterion for the mean body weights of the groups to have no significant differences. In spite of this effort, the absolute values (at 9 months of age) of the total tibial BMD of the Control group were statistically significantly lower compared with the OVX and OVX + OE groups. For that reason, we decided that the analysis of the percentage change from baseline values was necessary in order to objectively compare the differences within and between the groups.
We used the two-way mixed ANOVA model using as factors ‘the intervention’ (between group) and ‘time’ (within group) for the analysis of BMD measurements. Since there was statistically significant interaction between these factors, we used univariate analysis, for example, the comparison between groups for each time point separately and comparison of time points for each group separately, making the appropriate adjustment of the P-values based on Bonferroni correction. More specifically, one-factor repeated measures ANOVA model was used for the comparison of different time measurements of BMD parameters for each group, and the one-way ANOVA model was used for the between groups comparison at each time point separately making all adjustments of P-values. The efficacy of the treatment during the observation period was evaluated by calculating the mean percentage changes from baseline after 3 and 6 months, respectively. Comparison of percentage change from baseline of BMD parameters during the observation period between the three groups was analysed using the one-way ANOVA model, and pairwise comparisons were performed using the Bonferroni test. The Kruskal–Wallis and Mann–Whitney tests were used in case of violation of normality.
The comparison of three-point bending measurements and ratio of organ weight:body weight were performed using the one-way ANOVA model. Pairwise comparisons were performed using the Bonferroni test. All tests were two-sided, and statistical significance was set at P < 0·05. All analyses were carried out using the statistical package SPSS version 17.00 (Statistical Package for the Social Sciences, SPSS Inc.).
Results
The dual-energy X-ray absorptiometry scan results (before OVX, 3 and 6 months post-OVX) are displayed in Tables 1 and 2.
Table 1. Comparison of total tibial bone mineral density (BMD) (absolute values and mean percentage changes from baseline, which was 1 month before ovariectomy (OVX), 3 and 6 months after OVX) between groups during the observation period of 6 months
(Mean values and standard deviations)

* P < 0·05 v. OVX.
† P < 0·001 v. OVX.
‡ P < 0·05 v. OVX + OE.
§ P < 0·001 v. OVX + OE.
|| P < 0·05 v. baseline.
¶ P < 0·001 v. baseline.
Table 2. Comparison of proximal tibial bone mineral density (BMD) (absolute values and mean percentage changes from baseline, which was 1 month before ovariectomy (OVX), 3 and 6 months after OVX) between groups during the observation period of 6 months
(Mean values and standard deviations)
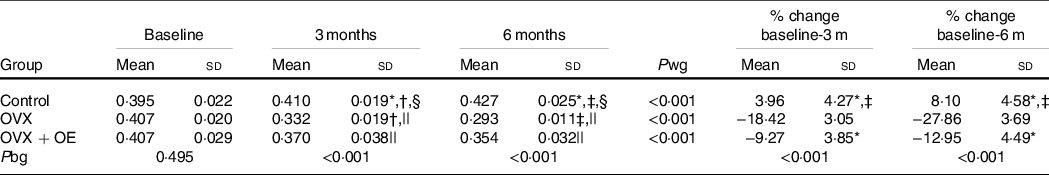
OE, table olive wastewater extract; P bg, P between groups; P wg, P within groups.
* P < 0·001 v. OVX.
† P < 0·05 v. OVX + OE.
‡ P < 0·001 v. OVX + OE.
§ P < 0·05 v. baseline.
|| P < 0·001 v. baseline.
Total tibial bone mineral density absolute values
There was statistically significant interaction between the factors ‘intervention group’ and ‘time’ (P < 0·005) for total tibial BMD measurement. Although the animals showed no difference in body weight at the time of allocation (at 3 months of age) to groups, the absolute values of total tibial BMD (g/cm2) of the Control group at baseline (at 9 months of age) (Table 1) were statistically significantly lower compared with OVX and OVX + OE groups before their ovariectomy. The Control group BMD statistically significantly increased from baseline measurement (0·204 (sd 0·009)) to 3 (0·215 (sd 0·010)) (P = 0·019) and 6 (0·228 (sd 0·015)) (P = 0·016) months. In contrast, the OVX group BMD statistically significantly decreased from baseline (0·225 (sd 0·015)) to 3 (0·204 (sd 0·007)) (P < 0·001) and 6 (0·195 (sd 0·009)) (P < 0·001) months. The OVX + OE group BMD also statistically significantly decreased from baseline (0·220 (sd 0·009)) at 3 (0·212 (sd 0·006)) (P = 0·006) and 6 (0·211 (sd 0·006)) (P = 0·031) months. A noteworthy finding for the OVX + OE group was that no significant difference was observed (P > 0·05) at the comparison between the 3- and 6-month measurements.
Proximal tibial bone mineral density absolute values
There was statistically significant interaction between the factors ‘intervention group’ and ‘time’ (P < 0·005) for proximal tibial BMD measurement. At the baseline measurement of the proximal tibia, the three groups had no statistically significant difference (Table 2). The Control group BMD statistically significantly increased from baseline (0·395 (sd 0·022)) to 3 (0·410 (sd 0·019)) (P = 0·041) and 6 (0·427 (sd 0·025)) (P = 0·004) months. The OVX group BMD statistically significantly decreased from baseline (0·407 (sd 0·020)) to 3 (0·332 (sd 0·019)) (P < 0·001) and 6 (0·293 (sd 0·011)) (P < 0·001) months. The same was found for the OVX + OE group BMD which statistically significantly decreased from baseline (0·407 (sd 0·029)) to 3 (0·370 (sd 0·038)) (P < 0·001) and 6 (0·354 (sd 0·032)) (P < 0·001) months.
Total tibial bone mineral density percentage changes
Statistically significant differences in the percentage change of total tibial BMD from baseline to 3 months were detected between Control (5·65 (sd 4·27) %) and OVX (−8·87 (sd 3·80) %; P < 0·001), OVX + OE (−3·35 (sd 2·36) %; P < 0·001) and between OVX + OE v. OVX (P = 0·005).
The same differences were detected from baseline to 6 months: Control (11·96 (sd 8·83) %) v. OVX (−13·03 (sd 5·11) %; P < 0·001) and OVX + OE (−3·68 (sd 3·56) %; P < 0·001) and between OVX v. OVX + OE (P = 0·005) (Table 1, Fig. 2).
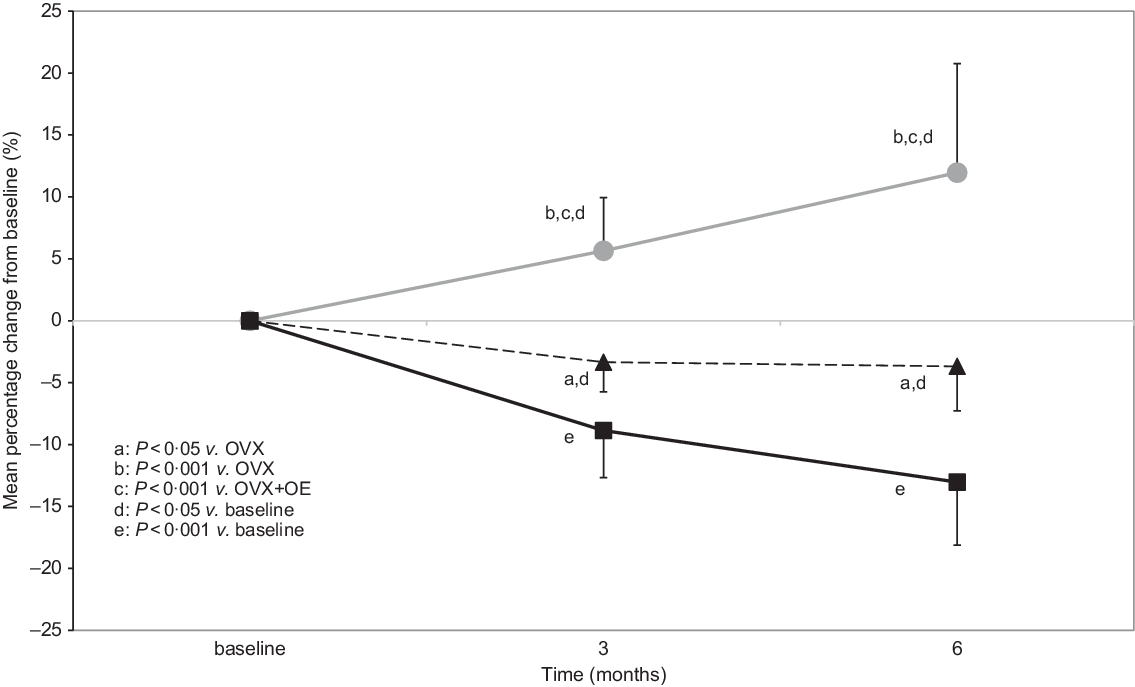
Fig. 2. Comparison of total tibial bone mineral density (BMD) (mean percentage changes from baseline, which was 1 month before ovariectomy (OVX), 3 and 6 months after OVX) between groups during the observation period of 6 months (all values are presented as mean and sd). OE, table olive wastewater extract. ![]() , control;
, control; ![]() , OVX;
, OVX; ![]() , OVX + OE.
, OVX + OE.
Proximal tibial bone mineral density percentage changes
Similarly, statistically significant differences were detected between the groups in the percentage change of proximal tibial BMD from baseline to 3 months between Control (3·96 (sd 3·57) %) v. OVX (−18·42 (sd 3·05) %; P < 0·001) and OVX + OE (−9·27 (sd 3·85) %; P < 0·001) and between OVX + OE v. OVX (P < 0·001).
The same differences were detected from baseline to 6 months: Control (8·1 (sd 4·58) %) v. OVX (−27·86 (sd 3·69) %; P < 0·001) and OVX + OE (−12·95 (sd 4·49) %; P < 0·001) and between OVX v. OVX + OE (P < 0·001) (Table 2, Fig. 3).
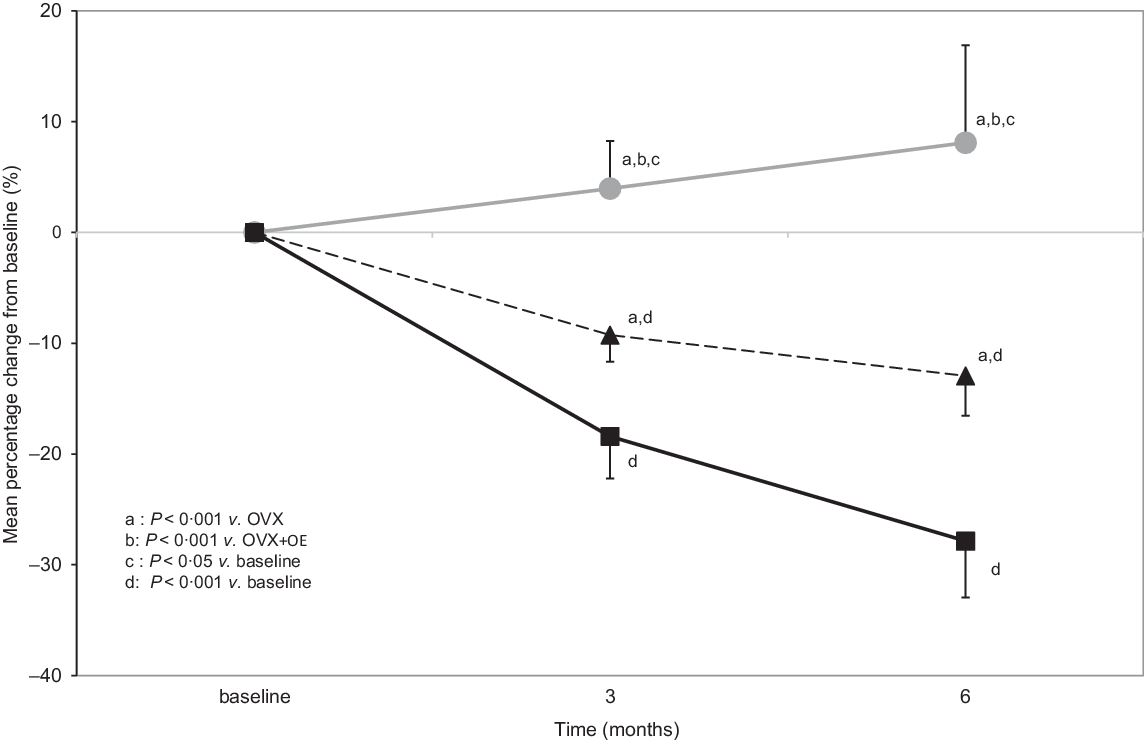
Fig. 3. Comparison of proximal tibial bone mineral density (BMD) (mean percentage changes from baseline, which was 1 month before ovariectomy (OVX), 3 and 6 months after OVX) between groups during the observation period of 6 months (all values are presented as mean and sd). OE, table olive wastewater extract. ![]() , control;
, control; ![]() , OVX;
, OVX; ![]() , OVX + OE.
, OVX + OE.
Biomechanical testing
The maximum load (N) did not reveal statistical differences between the groups neither for the left femur (Control: 122·47 (sd 18·09), OVX: 111·92 (sd 11·25), OVX + OE: 113·97 (sd 18·00); P = 0·361) nor for the right femur (Control: 116·18 (sd 18·30), OVX: 120·86 (sd 22·21), OVX + OE: 118·71 (sd 11·53); P = 0·860).
Extract consumption, organs, body weights and serum blood tests
Extract consumption was within normal limits (5–12 ml/100 g body weight per d)(Reference Suckow, Weisbroth and Franklin43,Reference Wolfensohn and Maggie44) during the study where the mean daily intake was 25·5 ml per OVX + OE animal. Organ and abdominal fat ratios to final body weight (rt%) were calculated and statistically analysed. The comparison of organ and abdominal fat ratios between OVX and OVX + OE groups did not show any statistical significance. All results are presented in Table 3 and body weight changes during the research project period in Fig. 4.
Table 3. Organ and abdominal fat ratios to final body weight (rt%)
(Mean values and standard deviation)

OVX, ovariectomy; OE, table olive wastewater extract.
Pairwise comparisons: *P < 0·05 v. Control. **P < 0·005 v. Control.
OVX v. OVX + OE: All P are non-significant (P > 0·05).

Fig. 4. Body weight changes during the observation period of 6 months (absolute values). OVX, ovariectomy; OE, table olive wastewater extract. ![]() , control;
, control; ![]() , OVX;
, OVX; ![]() , OVX + OE.
, OVX + OE.
The uterine weight of the OVX and OVX + OE groups was statistically significantly lower than those of the Control group, which confirmed the success of the surgical procedure of ovariectomy. The fact that the comparison of the uterine ratios between the OVX and OVX + OE groups was not significantly different shows that the extract did not have any effect on the weight and size of the uterus.
Results for the plasma parameters determined (ALT, γ-GT, total cholesterol, HDL-cholesterol, LDL-cholesterol, Ca and P) were all within the normal range for Wistar rats(Reference Suckow, Weisbroth and Franklin43,Reference Alemán, Más and Rodeiro45) and did not show significant differences between the groups.
Discussion
Nowadays, researchers and patients have shown an interest in alternative, natural treatments of postmenopausal osteoporosis in order to face the effects caused by the lack of oestrogens as well as the adverse effects of some pharmaceutical treatments. One category of these substances is phyto-oestrogens such as oleuropein, tyrosol and hydroxytyrosol, and the beneficial action of these phyto-oestrogens has been proved in vitro and in vivo (Reference Melguizo-Rodríguez, Ramos-Torrecillas and Manzano-Moreno46,Reference Chin and Ima-Nirwana30) . The OE used in this study is rich in these substances; therefore, we aimed to test their efficacy in the protection of the bone from the expected absorption and bone mass density loss in the rat model of osteoporosis. To this aim, the extract administration started immediately after OVX as in other studies with this animal model of postmenopausal osteoporosis and for a 6-month duration(Reference Dontas, Lelovas and Kourkoulis37,Reference Patsaki, Tchoumtchoua and Passali47,Reference Passali, Patsaki and Lelovas48,Reference Galanis, Soultanis and Lelovas42) .
At the time point of 3 months, the total tibial BMD percentage change of the OVX + OE group was −3·35 (sd 2·36) % (significantly higher than −8·87 (sd 3·80) % of the OVX group) and at the 6-month time point OVX + OE: −3·68 (sd 3·56) % (not quite changed compared with the 3-month value and significantly higher than −13·03 (sd 5·11) % of the OVX group). Similar findings highlighting the protective action of the OE can be seen at the proximal tibial BMD mean percentage change (baseline to 3 months: OVX + OE: −9·27 (sd 3·85) % when OVX: −18·42 (sd 3·05) % and baseline to 6 months: OVX + OE: −12·95 (sd 4·49) % when OVX: −27·86 (sd 3·69) %) with all P < 0·05. Previous studies with oral administration of phyto-oestrogenic substances such as Amphimas pterocarpoides, Sideritis euboea and red wine polyphenols extracts depict similar protective actions on the bone(Reference Dontas, Lelovas and Kourkoulis37,Reference Patsaki, Tchoumtchoua and Passali47,Reference Passali, Patsaki and Lelovas48) . What is quite interesting in our findings is that the OE importantly limits the bone loss more than these extracts at the 6-month measurement with a proximal tibial BMD percentage change of −12·95 %, when A. pterocarpoides percentage change was −22·41 %, S. euboea −16·57 % (median values used in this study) and red wine polyphenols −18·57 %. Puel et al. describe that in an 80-d study with younger Wistar rats than ours (6-month-old), diet supplemented with oleuropein or olive oil did not succeed in preventing bone loss of the femur in the OVX animals(Reference Puel, Quintin and Agalias29). Liu et al. showed that extra virgin olive oil in 6-month-old Sprague–Dawley rats managed to slow down the decrease of the bone density of lumbar spine and left femur compared with the OVX group after 3 months of administration, which is a shorter period than our study period(Reference Liu, Huang and Li49).
The biomechanical testing results revealed that the maximum load was higher for the Control group (119·32 (sd 4·62) N) compared with the OVX (116·39 (sd 4·13) N) and the OVX + OE group (116·34 (sd 4·13) N), but their comparisons were not statistically significant. This outcome could potentially be related to the fact that the midshaft of the femoral bone (used for this test) is a cortical-dominated bone site and as a result slower in showing the effect of the treatment. According to Jee and Yao after OVX in rats, the changes of cortical bone are slower than the ones of trabecular bone and can become evident on bone strength after 9 months(Reference Jee and Yao38), which may explain the lack of statistical differences in bone strength at the 6-month time point of our study. Similar findings to this are documented by Galanis et al., where the Glycyrrhiza glabra extract showed very good BMD results but did not reveal any significant differences in the biomechanical parameters evaluation also conducted at 6 months(Reference Galanis, Soultanis and Lelovas42). This pattern was also followed in studies with other phyto-oestrogens as well, such as Diarylheptanoid from Curcuma comosa Roxb., Drynariae rhizoma and a selective oestrogen receptor modulator, the CHF 4227.01(Reference Tantikanlayaporn, Wichit and Weerachayaphorn50–Reference Armamento-Villareal, Sheikh and Nawaz52). Prodinger et al. suggest that the noise of the results could potentially be reduced by adapting the biomechanical testing setup for each bone individually(Reference Prodinger, Bürklein and Foehr53). Puel et al. in two (80 and 84 d) studies with 6-month-old OVX Wistar rats receiving diet supplemented with oleuropein or olive oil and diet supplemented with either tyrosol or hydroxytyrosol or olive mill wastewater or olive mill water extract, respectively, describe that no significant changes were detected in the biomechanical parameters evaluation(Reference Puel, Mardon and Kati-Coulibaly54). On the other hand, A. pterocarpoides, S. euboea, red wine polyphenols and Pachyrhizus erosus effects were found to show significant results in the biomechanical testing, which might indicate faster effects of these extracts on bone strength than the effect of the OE(Reference Patsaki, Tchoumtchoua and Passali47,Reference Dontas, Lelovas and Kourkoulis37,Reference Passali, Patsaki and Lelovas48,Reference Nurrochmad, Leviana and Wulancarsari55) .
The ad libitum consumption of the OE during the 6-month measurements was always within the normal limits of water consumption for adult rats(Reference Suckow, Weisbroth and Franklin43). The body weight of the OVX + OE group at the end of the study was significantly higher than the Controls (P = 0·003) and non-significantly higher than the OVX (P = 0·697). This body weight increase may be due to OVX-induced obesity of the ovariectomised groups, which has also been observed in other studies with ovariectomised rats (A. pterocarpoides, S. euboea and red wine polyphenols extracts)(Reference Patsaki, Tchoumtchoua and Passali47,Reference Dontas, Lelovas and Kourkoulis37,Reference Passali, Patsaki and Lelovas48) as well as due to the increased energetic intake of the OE. In accordance with the body weight findings, due to the effect of ovariectomy, the abdominal fat:gastrocnemius ratio was significantly higher in the OVX + OE group compared with the Control group, but not significantly higher than the OVX group (P = 0·688). These findings indicate that the administration of the high energetic OE(Reference Patsios, Papaioannou and Karabelas56–Reference Katsoni, Frontistis and Xekoukoulotakis58) did not contribute to additional obesity to rats, that were provided with controlled amount of daily food according to the Controls consumption.
Regarding the organ weights, the uterine:body weight ratio was significantly reduced in the OVX and OVX + OE groups, as expected with the conduct of ovariectomy. The kidney and brain ratios were also significantly lower in the OVX groups compared with the Control group (P = 0·011 and P = 0·046, respectively), due to the increased body weight of the OVX rats which is the denominator of the ratios and contributes to the ratios’ reduced value. However, no significant differences were detected between the OVX groups (P = 0·799 and P = 0·272, respectively). While the absolute values of the kidneys, heart, gastrocnemius, brain and liver did not reveal any significant differences, this significant increase of the ratio of the kidneys can be seen as the consequence of the significant body weight differences between both OVX groups v. Control. All other organ weight ratios were found to be similar between the groups. These results are in accordance with Patsaki et al. (Reference Patsaki, Tchoumtchoua and Passali47) and with Min Cheng et al. (Reference Cheng, Wang and Fan59) where the administration of a phyto-oestrogenic extract did not show any effect on organ weight. Of the plasma parameters examined, it is noteworthy that the lipid profile of OVX + OE rats, after 6 months of OE consumption, did not have significant differences from the other groups, indicating a neutral effect.
Limitations
Although the allocation to groups at the beginning of the study was based on having similar mean body weights in each group, the baseline BMD measurements were not similar between groups. This issue was resolved by studying the percentage change from baseline measurements.
In the future in similar studies, one extra later check point, that is, at 9 months post-OVX, might be useful in order to assess more safely whether the extract can still maintain the bone loss preventive effect and whether it shows a later beneficial effect on bone biomechanical strength as well. Additionally, the future inclusion of more dose groups may possibly indicate the maximum and minimum effective and safe doses.
It is acknowledged that additional parameters such as those resulting from bone biomarkers and bone histomorphometry, had they been possible to conduct in our laboratory, would have provided more information. These parameters may provide the necessary information to establish the extract’s mechanism of action.
Conclusion
Our study depicts that the consumption of OE provides protection from postmenopausal bone density loss, without causing negative effects on the blood parameters examined. Consumption of this extract as part of a balanced diet could enhance the prevention of the onset of osteoporosis, with concurrent attention to the total daily energetic intake of a person’s diet. Further studies examining the effect of this extract as treatment of bone loss already established are needed.
Acknowledgements
This work has been supported by the SYNERGASIA 2009 PROGRAMME co-funded by the European Regional Development Fund & National Resources Project code: OSTEOPRO 09SYN-13-1076.SYNERGASIA 2009 PROGRAMME had no role in the design, analysis or writing of this article.
The authorship of this manuscript received the following contributions: A. S. Z., P. L., I. D. and A. G. contributed to project design and data analysis; A. G. statistical analysis; A. S. Z., I. D., P. L., A. G., D. G., S. K. K., A. T., E. C. and A. L. S. final review, manuscript presentation and critical review of the manuscript for important intellectual content; A. S. Z., I. D., P. L., D. G., A. P., C. P. and M. M. with the conduct of experiments and laboratory tests; S. B. with extract preparation and with extract analysis.
The authors declare that there are no financial or non-financial conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521000465










