Overall, 10 % of women have high blood pressure during pregnancy and 2–5 % will develop pre-eclampsia, a serious hypertensive condition associated with high maternal and fetal morbidity and mortality( Reference Duley 1 ). Furthermore, women who have had pre-eclampsia have a greater risk of developing hypertension, stroke and IHD in later life( Reference Wilson, Watson and Prescott 2 – Reference Levine, Vatten and Horowitz 4 ). Numerous strategies to prevent pre-eclampsia have been investigated, but, so far, all have failed to reach effectiveness.
Deficient placentation occurring during the first half of pregnancy is a frequent forerunner of the development of pre-eclampsia( Reference Redman and Sargent 5 ). Shallow trophoblast invasion and inadequate spiral arteriole remodelling result in a placenta that is not adequately perfused( Reference Redman and Sargent 5 , Reference Hubel 6 ). Thus, localised areas of ischaemia/reperfusion associated with placental oxidative and endoplasmic reticulum stress are established( Reference Hubel 6 – Reference Burton, Yung and Cindrova-Davies 9 ). This results in increased apoptosis and necrosis of the syncytiotrophoblast layer lining the intervillous space( Reference Hung, Skepper and Charnock-Jones 10 ). The oxidatively stressed syncytiotrophoblast responds by the increased release of two anti-angiogenic factors – soluble vascular endothelial growth factor receptor-1 (sVEGFR-1, also called sFlt-1) and soluble endoglin – into the maternal circulation that oppose the actions of vascular endothelial growth factor and placental growth factor (PlGF)( Reference Maynard, Min and Merchan 11 – Reference Venkatesha, Toporsian and Lam 13 ), leading to endothelial dysfunction, hypertension and proteinuria. Other characteristics of the condition include higher circulating levels of soluble adhesion molecules produced by the activated endothelium( Reference Daniel, Kupferminc and Baram 14 , Reference Austgulen, Lien and Vince 15 ), and of the potent inflammatory mediator peroxynitrite that causes vasoconstriction, platelet aggregation and thrombus formation( Reference Arteel, Briviba and Sies 16 – Reference Roggensack, Zhang and Davidge 18 ). In addition, placental microvesicles are released into the maternal circulation where they stimulate a maternal systemic inflammatory response and endothelial activation, the hallmark of pre-eclampsia( Reference Redman and Sargent 5 , Reference Cockell, Learmont and Smárason 19 – Reference Roberts, Taylor and Musci 22 ).
The essential trace mineral Se acting through selenoproteins/selenoenzymes has the capacity to reduce the risk of pre-eclampsia( Reference Rayman, Hatfield, Berry and Gladyshev 23 – Reference Trigona, Mullarky and Cao 29 ). A positive correlation between Se status and the incidence of pre-eclampsia has been shown in an epidemiological study of forty-five countries( Reference Vanderlelie and Perkins 30 ). Supplementation of Chinese women deemed to be at risk of pregnancy-induced hypertension (PIH) with Se has been shown to prevent PIH and gestational oedema, two of the signs of pre-eclampsia( Reference Han and Zhou 31 ). Serum/plasma Se and plasma glutathione peroxidase (GPx) concentrations have been found to be significantly lower in pre-eclamptic than in normal pregnancies( Reference Atamer, Koçyigit and Yokus 32 – Reference Ghaemi, Forouhari and Dabbaghmanesh 36 ), while significantly lower levels of the selenoenzymes, GPx and thioredoxin reductase have been found in placentae from pre-eclamptic women than from matched healthy controls( Reference Vanderlelie, Venardos and Clifton 8 , Reference Atamer, Koçyigit and Yokus 32 , Reference Mistry, Wilson and Ramsay 34 , Reference Walsh and Wang 37 ). In a retrospective study in a large Norwegian case–control cohort, women with pre-eclampsia were significantly more likely to carry the A allele of the G105A promoter polymorphism in the anti-inflammatory selenoprotein S (SEPS1)( Reference Moses, Johnson and Tømmerdal 38 ). In an interesting animal study, a Se-free diet caused a pre-eclampsia-like syndrome in pregnant rats with significantly increased blood pressure, proteinuria and placental oxidative stress and significantly lower pup weight compared with Se-adequate controls( Reference Vanderlelie, Venardos and Perkins 39 ).
In a previous study, we showed that Se status was low in Oxford women with normal (non-pre-eclamptic) pregnancy (median serum Se concentration 48·5 μg/l at 33 weeks)( Reference Rayman, Abou-Shakra and Ward 40 ). In a subsequent case–control study in the same location, we found that the concentration of Se in the toenails (laid down from 3 to 12 months previously) of women with pre-eclampsia was significantly lower than that of matched controls (P= 0·001)( Reference Rayman, Bode and Redman 41 ). Women in the bottom tertile of toenail Se were 4·4 (95 % CI 1·6, 14·9) times more likely to have pre-eclampsia. Within the pre-eclamptic group, lower Se status was significantly associated (P= 0·029) with more severe expression of disease, as measured by delivery before 32 weeks( Reference Rayman, Bode and Redman 41 ). Following on from that study, we decided to set up a randomised, placebo-controlled trial, SPRINT (Se in PRegnancy INTervention), to measure the effect of supplementation with dietary levels of Se (i.e. 60 μg/d, the UK-recommended intake for women)( 42 ), on the markers of pre-eclamptic risk, inflammation, endothelial and placental function.
Our hypothesis was that a small increase in the intake of Se in pregnant women of inadequate Se status would protect against the risk of pre-eclampsia, as assessed by biomarkers of pre-eclampsia, by increasing Se status and selenoprotein concentration.
The biomarkers that we chose to measure were serum or plasma markers known to be significantly associated with the development of pre-eclampsia. The primary outcome measure of the present study was sFlt-1, a marker of placental dysfunction that blocks the actions of vascular endothelial growth factor and PlGF and impedes the maintenance of endothelial integrity( Reference Maynard, Min and Merchan 11 , Reference Levine, Maynard and Qian 12 ). Secondary outcome measures were as follows: PlGF, an angiogenic factor the levels of which are significantly lower in pregnancies where pre-eclampsia subsequently develops( Reference Tidwell, Ho and Chiu 43 ); soluble endoglin, an anti-angiogenic protein that inhibits the formation of capillary tubes and induces vascular permeability and hypertension( Reference Levine, Lam and Qian 44 ); activin A and inhibin A, placental endocrine factors that, when increased, give early warning of pre-eclampsia( Reference Muttukrishna, North and Morris 45 ); vascular cell adhesion molecule-1 and E-selectin, cell adhesion molecules that are indices of endothelial activation( Reference Daniel, Kupferminc and Baram 14 , Reference Austgulen, Lien and Vince 15 , Reference Anim-Nyame, Gamble and Sooranna 46 ); plasma nitrotyrosine, an indicator of peroxynitrite exposure observed in placental vascular endothelium in pre-eclampsia( Reference Myatt, Rosenfield and Eis 17 , Reference Roggensack, Zhang and Davidge 18 ); C-reactive protein (CRP), a measure of inflammation raised in early gestation in advance of pre-eclampsia( Reference García, Celedón and Sierra-Laguado 47 ); pentraxin-3, an inflammatory marker thought to be more specific to vascular inflammation than CRP( Reference Dubin, Li and Ix 48 ).
Methods
Design
The present double-blind, placebo-controlled, two-group study was registered at the International Standard Randomised Controlled Trial Number Registry (registration no. ISRCTN37927591; http://controlled-trials.com/ISRCTN37927591).
Ethics statement
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Milton Keynes Research Ethics Committee (REC reference no. 08/H0603/46). Written informed consent was obtained from all subjects.
Participants
We chose to study primiparous women because they have a higher risk of pre-eclampsia. Between 14 July 2009 and 6 June 2011, primiparous women attending the antenatal clinic at the John Radcliffe Hospital, Oxford, UK, for an ultrasound scan at 12 weeks of gestation were invited to join the trial.
Eligibility criteria
We initially planned to recruit women only in the bottom half of Se status in line with our hypothesis that a treatment effect would only be seen in women of low Se status. However, reviewers of our proposal recommended that we should recruit all women, regardless of Se status, and specify that the primary data analysis would be on women of low Se status. We accepted that modification to our protocol which had the added advantage of avoiding a time lag due to the screening process which would have resulted in our randomising women in pregnancy than we wanted.
The inclusion criteria were first pregnancy and 12–14 weeks of gestation at randomisation.
The exclusion criteria were as follows: under 18 years old; current smokers (who have a lower risk of pre-eclampsia); taking any supplement containing Se; taking thyroid medication; multiple pregnancy; abnormal fetal anomaly scan; chronic proteinuria; heparin treatment; HIV, Hep-B or Hep-C positive; yeast intolerance (supplement contains yeast); inability or refusal to give informed consent (the genetic component of the trial was omitted in those who did not give explicit consent to this aspect of the trial).
Intervention
Women were randomly allocated to one of two treatment groups: 60 μg/d of Se, as Se-enriched yeast (SelenoPreciseTM), or placebo yeast, both supplied by Pharma Nord (60 μg/d is the recommended intake for UK women)( 42 ). The treatment period was from randomisation (12 up to a maximum of 14 weeks) until delivery.
A blood sample (10 ml) was taken from all recruits at trial entry. Whole blood was used to measure Se concentration, while the remainder was separated into plasma and serum and banked. If separate, explicit, consent had been given, an additional 5 ml of blood were taken as part of the same procedure for potential genetic analysis. A urine sample was taken for possible future use.
The following information was recorded by the trial midwife: blood pressure; date of the last menstrual period/gestational age; weight; height; haematocrit; ethnicity; family history of pre-eclampsia; chronic illness, e.g. chronic hypertension, diabetes, thyroid disease; current medication; presence of severe morning sickness; alcohol use; vegetarian or not. Women were given a simple FFQ to complete and a further questionnaire about dietary supplements taken.
Women were asked to allow their toenails to grow for 4–6 weeks (for possible later analysis), cut them, place the clippings in the plastic envelope provided to return to the research midwife.
At 20 weeks (when attending for a scan) and 35 weeks, women were again seen at the John Radcliffe Hospital by the research midwife. Blood and urine samples were taken again, separated as appropriate into serum and plasma, and banked for the measurement of different components related to the risk of pre-eclampsia.
Data from the analysis of urine, toenails and FFQ will be the subject of subsequent publications.
Outcomes
The specified primary outcome measure of the present study was serum concentration of sFlt-1( Reference Maynard, Min and Merchan 11 , Reference Levine, Maynard and Qian 12 ). Specified secondary outcome measures were as follows: serum PlGF, soluble endoglin, activin A and inhibin A; plasma VCAM-1, E-selectin, 3-nitrotyrosine and CRP.
An additional outcome introduced because of a concurrent funded study being carried out at the John Radcliffe Hospital was plasma pentraxin-3( Reference Dubin, Li and Ix 48 ).
Se status was measured by whole-blood Se concentration and plasma concentration of selenoprotein P (SEPP1), the carrier of Se in the plasma. Whole-blood concentration of Se, a longer-term measure of Se status than plasma Se, was determined at baseline and 35 weeks. Plasma concentration of SEPP1, a major component of plasma Se and an important parameter of functional Se status, was measured at 35 weeks( Reference Burk and Hill 49 ).
Measurement of outcomes
Whole-blood selenium
The analysis was carried out at the SAS Trace Element Unit, Southampton General Hospital by dynamic reaction cell-inductively coupled plasma MS on an Elan 6100 DRC plus (SCIEX Perkin-Elmer). Se concentration was measured using methane as the dynamic reaction cell gas to remove the argon dimer background( Reference Sloth and Larsen 50 ). Samples were run against a bovine blood calibration curve spiked with Se (Se standard solution 1000 ppm; Fisher Chemical). To increase signal sensitivity, a sample diluent containing 0·5 % butanol( Reference Sieniawska, Meniskov and Delves 51 ) was used for calibration, testing and quality control. Rhodium was used as the internal standard. A certified reference material (Trace Elements Whole Blood: Seronorm; Sero) assured accuracy: observed values 1·44 (sd 0·25) μmol/l (n 42) for lot number 1103129 (target value 1·42 μmol/l) and 3·02 (sd 0·19) μmol/l (n 32) for lot number 1003193 (target value 3·29 μmol/l).
Selenoprotein P
Frozen plasma samples were shipped to the laboratory of Raymond Burk. Immediately before the analysis, samples were thawed and SEPP1 concentrations were measured using an ELISA as described previously( Reference Burk, Norsworthy and Hill 52 ).
Soluble vascular endothelial growth factor receptor-1, placental growth factor and soluble endoglin
Concentrations of sFlt-1, PlGF and soluble endoglin were measured using commercially available Duoset kits for the development of a sandwich ELISA kit (R&D systems), according to the manufacturer's instructions. The intra- and inter-assay CV were 5·2 and 10·5 % for sFlt-1, 5·2 and 8·3 % for PlGF, and 4·9 and 11·6 % for soluble endoglin, respectively.
Inhibin A and activin A
The levels of inhibin A and activin A were measured using a two-site ELISA as described previously( Reference Tannetta, Muttukrishna and Groome 53 ). Affinity-purified human activin A was used as the assay standard for the measurement of total activin A. The intra- and inter-assay CV for inhibin A were 5·6 and 12·2 % and for activin A were 6·8 and 10·5 %, respectively.
E-selectin and vascular cell adhesion molecule-1
The circulating levels of soluble E-selectin and vascular cell adhesion molecule-1 were measured using Quantikine ELISA Kits (R&D Systems, Inc.), according to the manufacturer's instructions. The intra- and inter-assay CV were 5·8 and 7·9 % for E-selectin and 3·1 and 7·0 % for vascular cell adhesion molecule-1, respectively.
3-Nitrotyrosine
Circulating plasma levels of 3-nitrotyrosine were measured using a Hycult-Biotech ELISA kit (Hycult-Biotech), according to the manufacturer's instructions. The inter- and intra-assay CV were < 15 %.
C-reactive protein
Circulating levels of CRP in plasma samples were detected using an in-house, high-sensitivity ELISA adapted from a previously described competition ELISA( Reference Gillespie, Dow and Raynes 54 ) by comparing the direct detection of bound CRP by horseradish-peroxidase-conjugated rabbit anti-human CRP (DakoCytomation) and visualisation using Tetra Methyl Benzidine (Sigma-Aldrich). The intra- and inter-assay CV were 5 and 8 %, respectively.
Pentraxin-3
Plasma samples were analysed using a pentraxin 3 ELISA kit (DIESSE Diagnostica Senese SpA), according to the manufacturer's instructions. The between-batch CV was 6·55 % at a pentraxin-3 concentration of 1·92 ng/ml, 7·58 % at 8·48 ng/ml and 8·45 % at 13·34 ng/ml.
Sample size
We knew that our trial was not powered to look at pre-eclampsia risk per se, but believed that we had adequate power to observe a significant difference in our chosen primary end-point of sFlt-1, a recognised marker of pre-eclampsia risk. We calculated sample size using graphical data on sFlt-1 in normal pregnant women( Reference Maynard, Min and Merchan 11 ). Taking the standard deviation as 0·2 ng/ml, twenty-nine subjects per group gave us 80 % power to detect a difference of 1·5 ng/ml in the mean sFlt-1 values between the placebo- and Se-treated groups at a two-sided 5 % significance level. As our hypothesis was that an effect of Se supplementation would only be seen in women of inadequate Se status (e.g. in the bottom tertile)( Reference Rayman, Bode and Redman 41 ), we increased the sample size to 100 per group to include a sufficient number of such women. To allow for a 15 % dropout, we planned to recruit 115 subjects per group, making a total of 230 subjects. It subsequently came to light that an error had been made, in that the standard deviation of 0·2 ng/ml was actually the standard error, resulting in the present trial being underpowered to detect a difference in the sFlt-1 end-point.
Using the observed difference in means between the Se-treated and placebo groups and the measured standard deviation in both groups, we have now calculated the true power of the present study to achieve two-sided significance at the 5 % level when comparing the means of sFlt-1 in the two arms of the study to be 10 % overall and 36·8 and 54·5 % for women in the bottom tertile and quartile of Se status, respectively. As neither power level is adequate to achieve a significant effect of treatment, we have redefined the present study as a pilot trial.
Randomisation
Centralised randomisation was performed by the National Perinatal Epidemiology Unit Clinical Trials Unit, University of Oxford. The randomisation schema was created using the subroutine ralloc.ado (version 3.5.2) in Stata software (version 10.1). Treatments were allocated in the ratio of 1:1 using permuted block randomisation with variable block sizes of 2, 4 and 6 (seed used: 221010). Block sizes were allocated proportional to the elements of Pascal's triangle.
A third party, Pharma Nord, who were not involved in any way in the recruitment of participants for the trial, provided the treatment tablets. Pharma Nord was notified of the allocation sequence by an independent statistician at the National Perinatal Epidemiology Unit, and packed eight blister strips of twenty-eight tablets into plain white boxes.
The pharmacist at the John Radcliffe Hospital held and dispensed the boxes, each of which bore a label with a unique pack identification number. Each blister pack within each box also bore the randomisation number. The packs were collected by the research midwife from pharmacy as required. A log was kept which, on dispensing the packs, was signed by two people, the research midwife and pharmacist or nurse prescriber. The pack label was also signed before giving the trial box to each participant.
Blinding
Apart from the independent statistician at the National Perinatal Epidemiology Unit who generated the sequence, all study personnel and the participants were blinded to allocation.
Assessment of compliance
Compliance was assessed by tablet count. Stamped, addressed envelopes were provided to participants who were instructed to return empty/partially used/unused blister packs by post to the research midwife who recorded the number of tablets taken. The midwife contacted those who failed to return the packs from the previous 4 weeks within 10 d of the expected return date by telephone. To assess compliance, the total number of study tablets reported as having been taken was expressed as a percentage of the number of days between the study consent date and the date of giving birth. Participants were deemed to be compliant if they took 80 % of their study tablets.
Rationale for an approach to data analysis
We observed a large and significant reduction (12 %) in whole-blood Se concentration from 12 to 35 weeks in the placebo group in the present study, resulting, at least in part, from the increase in plasma volume that occurs over the course of normal pregnancy( Reference Ross and Idah 55 ). We found that we could mitigate this dilution effect by correcting Se concentration by haematocrit, which reduced the decline in the concentration of Se in the placebo group to 7 %. Therefore, this adjustment was made in some of the analyses as explained below.
As the earlier study on which the present pilot trial was based( Reference Rayman, Bode and Redman 41 ) showed a significantly higher incidence of pre-eclampsia in women in the bottom tertile of toenail Se than in the rest, analyses in subgroups with low baseline Se status were the pre-specified primary analyses. As the earlier study and this trial used different matrices (toenail Se and whole-blood Se, respectively) and toenail cuttings reflect Se status before pregnancy, we endeavoured to determine the equivalent baseline whole-blood Se cut-off point in pregnant women to that used for toenail Se (bottom tertile cut-off point 0·547 mg/kg) in the previous study. Using the data from Behne et al. ( Reference Behne, Alber and Kyriakopoulos 56 ) and taking the density of whole blood to be 1·06 kg/l, we calculated the equivalent whole-blood Se cut-off point to be 106·5 μg/l. As pregnancy causes an expansion of blood volume from the non-pregnant state, the equivalent whole-blood Se cut-off value would be 95·1 μg/l, assuming that the increase was the same as that observed in plasma volume at 12 weeks of gestation, i.e. 12 %( Reference Whittaker and Lind 57 ). This value represents a cut-off between the bottom third and the bottom quarter of baseline whole-blood Se. Therefore, we examined the treatment effect in both the bottom quartile and the bottom tertile of Se status by blood Se concentration at baseline.
Statistical analysis
Intention-to-treat analysis
None of the dependent variables was normally distributed, nor was baseline Se; therefore, they were log-transformed to facilitate parametric testing. Thus, medians (ranges) or geometric means and their 95 % CI, computed by back transformation of log values, are reported. All analyses of continuous data used one observation per subject in a general linear model (SAS PROC MIXED), with treatment (Se or placebo) as the independent variable. Additional covariates were included as appropriate. These covariates were log baseline blood Se (adjusted or unadjusted for baseline haematocrit) and log baseline haematocrit. The treatment effect was assessed using the LSMEANS and PDIFF options of this SAS statistical procedure.
The pre-specified primary data analysis of this study was carried out on the subgroup of women with low Se levels at recruitment (the lowest third or the lowest quarter). Subgroups with low Se status (quartiles and tertiles) were defined as blood Se concentration at baseline divided by haematocrit concentration at baseline.
Sensitivity analysis
Soluble vascular endothelial growth factor receptor-1 by compliance
The analysis was repeated including only those women who had taken 80 % or more of their trial tablets.
Exploratory subgroup analyses
Soluble vascular endothelial growth factor receptor-1 by Se quartile at baseline
Quartiles of baseline blood Se and treatment were simultaneously contrasted to determine whether a trend could be observed across quartiles. The quartile was an additional categorical independent variable in the model and the contrast was assessed using the ESTIMATE option of the PROC MIXED procedure.
Pre-eclampsia and pregnancy-induced hypertension
Pre-eclampsia and PIH were not pre-specified outcomes. PIH is defined as new hypertension appearing for the first time after 20 weeks of pregnancy. Hypertension is defined as diastolic blood pressure ≥ 90 mmHg on two occasions ( ≥ 4 h apart). Pre-eclampsia is defined as PIH and new-onset proteinuria after 20 weeks of pregnancy ( ≥ 300 mg/24 h or ≥ 2+ dipstick midstream specimen of urine/catheter specimen of urine). A protein:creatinine ratio of >30 mg/mmol of creatinine was accepted as confirmation of proteinuria. These criteria are an extension of those adopted by the International Society for the Study of Hypertension in Pregnancy( Reference Davey and MacGillivray 58 ). Women were dichotomised according to whether they had either pre-eclampsia/PIH, or neither. The relationship with study treatment (Se or placebo) was then analysed by logistic regression.
Associations with baseline selenium status
The correlation of whole-blood Se (log-transformed and haematocrit-corrected) at baseline with other parameters was investigated using appropriate statistical tests.
Results
Participants
A total of 230 participants were recruited between July 2009 and June 2011. The mean gestational age at recruitment was 12·3 weeks. One participant was recruited at 16 weeks; she was included in the data analysis. In each treatment group, eleven participants discontinued the treatment. Figure 1 shows the participant flow through the study.
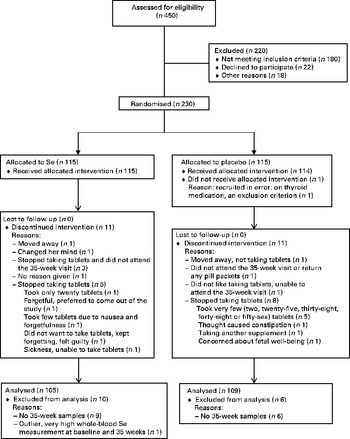
Fig. 1 Participant flow through the study.
The baseline characteristics of the participants are shown in Table 1 from which it can be seen that there were no significant differences between the groups at baseline.
Table 1 Baseline characteristics of the 229 participants, overall and by treatment group (Mean values and standard deviations; numbers and percentages; medians and ranges)
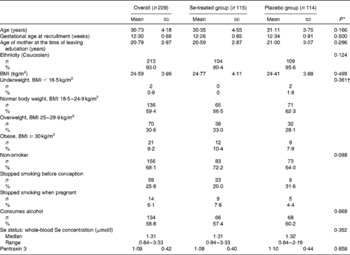
* P value for a comparison between the Se-treated and placebo groups.
† P value for the distribution of BMI categories between the Se-treated and placebo groups.
The mean gestational age at delivery was 39·65 (95 % CI 39·45, 39·86) weeks overall, 39·75 (95 % CI 39·50, 40·00) weeks in the Se-treated group and 39·56 (95 % CI 39·24, 39·88) weeks in the placebo group, with no significant difference being observed between the Se-treated and placebo groups.
Selenium status
Blood was successfully obtained from the 230 participants at baseline, though one was subsequently found to be ineligible for the trial, and from 215 participants at the 35-week visit. The overall median whole-blood Se concentration at baseline was 1·31 (range 0·84–3·33) μmol/l (Table 1). By 35 weeks, whole-blood Se concentration had increased significantly in the Se-treated group (see Table 2), whereas there was a significant reduction (12 %) in the placebo group. Whole-blood Se concentration and plasma SEPP1 concentration were significantly higher at 35 weeks in the Se-treated group than in the placebo group (Table 2). Figure 2 shows the difference in the effect of Se v. placebo treatment on plasma SEPP1 concentration and whole-blood Se concentration at 35 weeks.
Table 2 Effect of selenium supplementation on the parameters of selenium status (Median values and ranges)
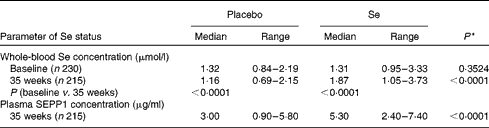
SEPP1, selenoprotein P.
* P value for a comparison between the placebo and Se-treated groups at 35 weeks.
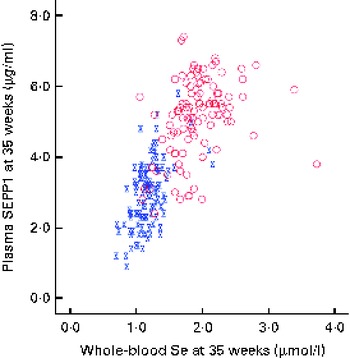
Fig. 2 Selenoprotein P (SEPP1) v. whole-blood selenium concentration at 35 weeks. ![]() , Placebo;
, Placebo; ![]() , selenium. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
, selenium. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Compliance with treatment
Of the participants, 22 % in the Se-treated group and 23 % in the placebo group were non-compliant, i.e. took less than 80 % of the trial tablets. However, 28 % of the participants failed to return some or all of the empty packets, though many claimed to have taken the tablets; compliance may therefore have been somewhat better than the estimates suggest.
In the placebo group, one participant took a pregnancy supplement containing 30 μg Se sporadically from 16 to 20 weeks of pregnancy along with the trial tablets. She was included in the data analysis.
Effect of treatment: primary outcome measure (serum soluble vascular endothelial growth factor receptor-1)
The intention-to-treat analysis included all participants (apart from one clear outlier) for whom we had an sFlt-1 measurement, regardless of compliance, i.e. 105 participants in the Se-treated group and 109 in the placebo group (see Fig. 1). Table 3 shows the effect of Se v. placebo supplementation on sFlt-1 concentration at 35 weeks in participants in the bottom quartile and tertile of whole-blood Se at baseline (primary data analysis of the present study) and in the whole dataset. Because the ratio of sFlt-1:PlGF is often taken to be a more sensitive indicator of the risk of pre-eclampsia as the fall in the concentration of PlGF amplifies the concurrent rise in the concentration of sFlt-1( Reference Levine, Maynard and Qian 12 , Reference Rana, Powe and Salahuddin 59 ), the data for PlGF and the ratio of sFlt-1:PlGF are also presented. In participants in the lowest quartile of Se status at baseline, treatment with Se significantly lowered the concentration of sFlt-1 (P= 0·039) and halved the ratio of sFlt-1:PlGF, though the latter effect did not quite reach significance (P= 0·066); however, in all participants taken together, Se treatment had no effect on sFlt-1 concentration, PlGF concentration or sFlt-1:PlGF ratio.
Table 3 Effect of selenium supplementation v. placebo on soluble vascular endothelial growth factor receptor-1 (sFlt-1) concentration, placental growth factor (PlGF) concentration and the ratio of sFlt-1:PlGF at 35 weeks in participants in the bottom quartile* (n 50) and tertile* (n 67) of selenium status at baseline and in all participants† ‡ (n 214) (Mean values and 95 % confidence intervals)
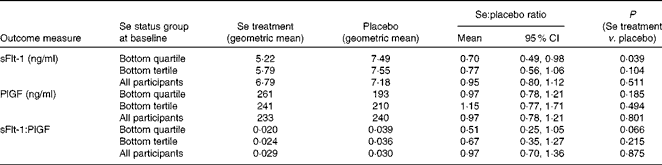
* For division into quartiles/tertiles, baseline Se concentration was adjusted for baseline haematocrit.
† In all participants, adjustment for blood Se and haematocrit covariates at baseline and for haematocrit at 35 weeks made no substantial difference to the results.
‡ Analysis on all participants was not part of the primary analysis.
Effect of treatment: secondary outcome measures
The effect of Se and placebo treatments on secondary outcome measures is shown in Table 4. None of the parameters was significantly affected by Se treatment either in the participants in the bottom tertile or quartile of Se status or in the whole group.
Table 4 Effect of selenium supplementation v. placebo on secondary outcome measures at 35 weeks in participants in the bottom quartile* and tertile* of selenium status at baseline and in all participants† ‡ (Mean values and 95 % confidence intervals)
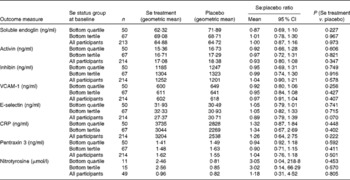
VCAM-1, vascular cell adhesion molecule-1; CRP, C-reactive protein.
* For division into quartiles/tertiles, baseline Se concentration was adjusted for baseline haematocrit.
† In all participants, adjustment for blood Se and haematocrit covariates at baseline and for haematocrit at 35 weeks made no substantial difference to the results.
‡ Analysis on all participants was not part of the primary analysis.
Subgroup analyses (exploratory)
Soluble vascular endothelial growth factor receptor-1 by compliance
Reanalysis of the effect of treatment on sFlt-1 concentration when only participants who reported taking 80 % of the trial tablets were included made no difference to the result (data not shown).
Soluble vascular endothelial growth factor receptor-1 by quartile of baseline selenium status
Given the significant effect of Se treatment on sFlt-1 concentration in women in the lowest quartile of Se status at baseline, we decided to determine whether the effect showed a trend by quartile. The ratio of sFlt-1 means shown in Table 5 illustrates that, rather than a trend, treatment with Se appears to reduce sFlt-1 concentration only in participants in the bottom quartile of Se status at baseline, with no effect being observed in those in the other quartiles. There is a near-significant difference between the effect in the bottom quartile and the rest (ratio for the lowest quartile compared with the other quartiles: 0·67, 95 % CI 0·45, 1·00; P= 0·051).
Table 5 Results for soluble vascular endothelial growth factor receptor-1 by quartile of whole-blood selenium concentration at baseline (Mean values and 95 % confidence intervals)
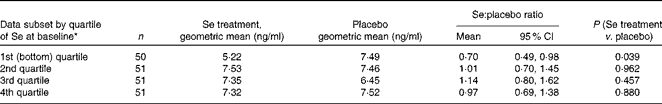
* Division into quartiles by baseline Se status corrected for haematocrit.
Pre-eclampsia and pregnancy-induced hypertension
Neither pre-eclampsia nor PIH was a designated outcome because of the known lack of power; there were eleven cases of pre-eclampsia (eight in the placebo group and three in the Se-treated group), and nine cases of PIH (six in the placebo group and three in the Se-treated group). As expected, the effect of Se treatment on the incidence of either outcome failed to reach significance (data not shown). However, when the two outcomes were combined on the basis that there may be some continuity between the conditions( Reference Saudan, Brown and Buddle 60 – Reference Davis, Mackenzie and Brown 63 ), and when the analyses were adjusted for baseline Se concentration and haematocrit, Se treatment significantly reduced the odds of having either pre-eclampsia or PIH in all participants (OR 0·350, 95 % CI 0·126, 0·974; P= 0·044) and with near significance in those in the bottom third of Se status at baseline (Table 6), with large treatment effects.
Table 6 Effect of selenium treatment v. placebo on the risk of pre-eclampsia (PE) and pregnancy-induced hypertension (PIH) combined, by treatment group, in all participants and in those in the bottom tertile* and quartile* (Odds ratios and 95 % confidence intervals)
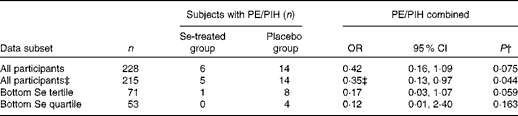
* Division into tertiles/quartiles by baseline Se concentration corrected for haematocrit.
† χ2 test.
‡ Adjusted for baseline Se concentration and baseline haematocrit.
Associations with selenium at baseline
At baseline (12 weeks), with adjustment for haematocrit, whole-blood Se was negatively correlated with BMI (Pearson's correlation (r) − 0·222; P= 0·001); there were no other significant correlations.
Adverse events
Overall two adverse events were reported: one participant had a rash on her legs below the knees, deemed to be mild, and possibly related to the study treatment. Another participant had spontaneous torsion of her right ovary that required right oophorectomy, a rare complication of pregnancy, not connected with the study supplement.
Discussion
Effect on selenium status
We used whole blood to measure Se concentration as it gives a longer-term measure than does plasma Se. Se supplementation from 12 to 35 weeks increased whole-blood Se concentration by 43 % in the Se-treated group, while there was a significant fall of 12 % in the placebo group (Table 2). Other studies that measured whole-blood Se in pregnancy have also found a substantial fall in its concentration with increasing gestational age( Reference Butler, Whanger and Tripp 64 , Reference Zachara, Wardak and Didkowski 65 ). Apart from plasma volume expansion, an additional cause of a fall in blood Se concentration throughout pregnancy may be the transfer of Se to the fetus by SEPP1, a major component of plasma Se that transports Se to other tissues( Reference Burk and Hill 49 ). This has been suggested by a recent study by Burk et al. ( Reference Burk, Olson and Hill 66 ) in pregnant mice that identified two mechanisms of Se transfer; from early to mid-gestation, plasma GPx and SEPP1 were transported via the uterine fluid, probably by pinocytosis, whereas in the latter half of gestation, placental transfer of maternal SEPP1 occurred through apoE receptor 2. Even in mice with normal Se status, maternal plasma SEPP1 concentration fell rather dramatically in late pregnancy with a sharp rebound the day after delivery. Therefore, pregnancy may be putting similar pressure on the Se stores of UK pregnant women with marginal Se status. Women supplemented with Se had at least partial correction of their Se deficiency, raising the SEPP1 concentration, though most participants did not reach the maximum value (see Fig. 2).
Per-protocol analyses
sFlt-1 was the primary outcome measure of the present study, and our hypothesis was that there would only be an effect of Se supplementation in pregnant women of inadequate Se status. Owing to an initial statistical error, the trial was powered only at 36·8 and 54·5 % to observe a significant difference between sFlt-1 means in the two arms of the study in women in the lowest third and lowest quarter of Se status, respectively. Therefore, it is important that we found a significant difference in participants in the lowest quartile of Se status at baseline, in whom Se treatment lowered the concentration of sFlt-1 by 30 % (ratio of means 0·70, 95 % CI 0·49, 0·98; P= 0·039; Table 3). When we examined the effect of Se supplementation on the ratio of sFlt-1:PlGF, a more sensitive indicator of risk of pre-eclampsia( Reference Levine, Maynard and Qian 12 , Reference Rana, Powe and Salahuddin 59 ), we found that it had decreased by 49 % in the bottom quartile, though the effect did not quite reach significance (P= 0·066). We observed no significant effect of treatment on any of the secondary outcome measures of the present study, perhaps because of the lack of power, so these will not be discussed further.
What are the mechanisms by which treatment with Se could cause a fall in sFlt-1 concentration, a primary marker of pre-eclampsia risk? Selenoproteins are able to reduce oxidative stress, endoplasmic reticulum stress and inflammation, characteristics of pre-eclampsia, while protecting endothelial cells and up-regulating the expression of the protective haem oxygenase 1 (HO-1)( Reference Rayman, Hatfield, Berry and Gladyshev 23 – Reference Lewin, Arthur and Riemersma 26 , Reference Ejima, Layne and Carvajal 28 , Reference Trigona, Mullarky and Cao 29 , Reference Vunta, Davis and Palempalli 67 , Reference Vunta, Belda and Arner 68 ). Se supplementation was found to decrease NF-κB activation and down-regulate the expression of inflammatory genes( Reference Vunta, Davis and Palempalli 67 , Reference Vunta, Belda and Arner 68 ). Both GPx and thioredoxin reductase have been shown to protect endothelial cells from oxidants including oxidised LDL( Reference Brigelius-Flohé, Banning and Schnurr 25 , Reference Lewin, Arthur and Riemersma 26 ). Thioredoxin reductase has been shown to up-regulate the expression of HO-1 by a number of pathways during a pro-oxidant challenge, resulting in the reduced expression of pro-inflammatory genes( Reference Ejima, Layne and Carvajal 28 , Reference Trigona, Mullarky and Cao 29 ); HO-1 has antioxidant effects and is linked to successful placentation, inhibition of sFlt-1 release, uterine quiescence, placental haemodynamic control and the regulation of apoptotic and inflammatory cascades in trophoblast cells( Reference Bainbridge and Smith 69 – Reference Ahmed, Rahman and Zhang 72 ). SEPP1, which is recruited to the endothelium in the areas of inflammation, scavenges the powerful inflammatory agent peroxynitrite, thereby reducing oxidative stress and inflammation and shielding endothelial membranes from its attack( Reference Arteel, Briviba and Sies 16 , Reference Traulsen, Steinbrenner and Buchczyk 73 ). Gene expression of SEPS1 is activated by endoplasmic reticulum stress, and binding sites exist for NF-κB in the human SEPS1 promoter; thus, SEPS1 plays a key role in the control of the inflammatory response( Reference Gao, Hannan and Wanyonyi 27 ).
The effects of Se outlined above can mostly be attributed to selenoproteins; therefore, it is entirely understandable that we only observed an effect in the bottom quartile of Se status in the present study and in the bottom tertile in our earlier study( Reference Rayman, Bode and Redman 41 ). Above a whole-blood Se concentration of 1·3 μmol/l, plasma GPx activity is maximised( Reference Thomson, Robinson and Butler 74 ). The mean whole-blood Se concentration in trial participants at baseline was 1·31 μmol/l, suggesting that approximately 50 % would not have had maximal plasma GPx activity. If the effects that we observed are attributable to GPx, then only a subgroup of women in the present study could have benefited from Se supplementation, as observed by our group. In contrast to plasma GPx, the concentration of SEPP1 does not plateau until a plasma Se concentration of 1·6 μmol/l is reached( Reference Hurst, Armah and Dainty 75 ). The results suggest that in whole blood, SEPP1 concentration may plateau at about 1·8 μmol/l (Fig. 2), which accords with the findings in plasma. Supplementation with Se from 12 to 35 weeks was insufficient to raise the concentration of SEPP1 to that plateau level in the majority of women in the Se-treatment group (Fig. 2). Therefore, if beneficial effects are related to the effect of SEPP1, a higher dose or an intervention earlier than 12 weeks of gestation would have been required to observe it.
Exploratory analyses
In addition to the pre-specified analyses described above, we carried out hypothesis-generating exploratory analyses in order to have some further insight into the effect of Se supplementation.
When we analysed sFlt-1 concentration by quartiles to determine whether a trend could be detected, we observed a reduction in the concentration of sFlt-1 only in participants in the bottom quartile of Se status at baseline, with those in the other quartiles showing no effect (Table 5). Thus, there was a near-significant difference between the effect in the bottom quartile and the rest (P= 0·051), suggesting a threshold effect that affected only participants in the bottom quartile of Se status.
We had very small numbers of participants affected by pre-eclampsia( Reference Maynard, Min and Merchan 11 ) and PIH( Reference Burton, Yung and Cindrova-Davies 9 ), so we combined the two outcomes on the basis that there may be some continuity between the conditions( Reference Barton, O'Brien and Bergauer 62 , Reference Davis, Mackenzie and Brown 63 ). Se treatment significantly reduced the odds of the combination (pre-eclampsia/PIH) in all participants (OR 0·35, 95 % CI 0·13, 0·97; P= 0·044) and with near significance (P= 0·059) in those in the bottom third of Se status at baseline, with large treatment effects (eight women developed pre-eclampsia/PIH in the placebo group compared with one in the Se-treated group) (Table 6). Although pre-eclampsia and PIH are not synonymous, the distinction between the two is not always completely clear; 15–45 % of women with PIH will eventually develop pre-eclampsia( Reference Barton, O'Brien and Bergauer 62 , Reference Davis, Mackenzie and Brown 63 ). The earlier the time of presentation, the more likely is PIH to be a harbinger of pre-eclampsia( Reference Saudan, Brown and Buddle 60 ). If PIH is associated with some other feature of pre-eclampsia than proteinuria, such as hyperuricaemia or intra-uterine growth retardation, it will be now recognised as a possible atypical variant of pre-eclampsia( Reference Brown and de Swiet 61 ).
Baseline associations
The negative correlation (r − 0·222; P= 0·001) between baseline whole-blood Se and BMI is in line with other findings in the literature on the relationship between serum/plasma Se and BMI in non-pregnant groups( Reference Kimmons, Blanck and Tohill 76 – Reference Méplan, Crosley and Nicol 78 ).
Trial limitations
The major limitation of the present study was in having very limited power (only 54·5 % in the bottom quartile and 36·8 % in the bottom tertile of participants) to observe a difference in our primary endpoint of sFlt-1.
Intervention at 12 weeks of gestation may have been later than ideal. Our earlier study( Reference Rayman, Bode and Redman 41 ) that found a significant difference in Se status between pre-eclamptic and matched control pregnant women used toenail clippings that probably reflected Se status before conception, as toenails are laid down up to 12 months before clipping. We were always aware that if the effect of Se supplementation was primarily in the early stages of gestation, we would not be able to observe it with the present protocol. A number of processes that occur during the periconceptual period might benefit from protection by selenoproteins. Indeed, periconception ‘multivitamin’ (often including Se) use has been shown to reduce the risk of pre-eclampsia( Reference Bodnar, Tang and Ness 79 ).
We have observed an effect in the bottom quartile (upper cut-off value 1·18 μmol/l in whole blood) of Se status in UK pregnant women. This might roughly equate to 1·07 μmol/l (84·5 μg/l) in serum/plasma. These results may therefore only be generalisable to other population groups of similar Se status such as that likely to be found in other countries of Europe. Vanderlelie & Perkins( Reference Vanderlelie and Perkins 30 ) found a significant reduction (P= 0·0007) in the incidence of pre-eclampsia in countries with a reported serum/plasma Se concentration ≥ 95 μg/l (1·2 μmol/l). Our trial population falls within that range.
Suggestions for future work
To date, this is the only study to have investigated the effect of Se supplementation in a population with low Se status that examined an outcome related to the risk of pre-eclampsia. Given the present findings of significantly lower concentrations of sFlt-1 in women in the bottom quartile of Se status supplemented with Se, and the suggestion that Se supplementation may lower the incidence of PIH+pre-eclampsia, the trial should be repeated, properly powered for the outcome of pre-eclampsia (and PIH), in a population with a Se status as low, or lower, than that of the present study. Ideally, recruitment would start earlier than 12 weeks. On the basis of our inability to optimise SEPP1 during the course of the trial, we would recommend a higher, though safe, dose of Se-enriched yeast, i.e. 100 μg/d( Reference Rayman 80 ). A major difficulty in conducting such a trial in a Western country is recruiting sufficient women who are not taking a pregnancy supplement; such supplements are increasingly available at relatively low cost in supermarket chains.
Despite these difficulties, the potential benefits to be gained by conducting a properly powered trial would make such a demanding exercise worthwhile.
Acknowledgements
The authors thank Dr Christine Sieniawska of the Trace Element Unit, Southampton University Hospital NHS Trust for the analysis of Se in whole blood, the laboratory of Raymond Burk, University of Vanderbilt, for the analysis of SEPP1 (under grant NIH ES02497), and Alex Matthews of the Clinical Research Centre, University of Surrey for database management. The authors are extremely grateful to all the women who took part in the present study, and the team at the John Radcliffe Hospital including the midwives who helped recruit the women, the laboratory personnel and Ed Juszczak of the National Perinatal Epidemiology Unit who carried out the randomisation procedure. J. M. was supported by a fellowship from the Nestlé Nutrition Institute and S. C. B. by an MRC Population Health Scientist Fellowship.
The present study was financially supported by the Wellcome Trust (grant no. 083918/Z/07/Z). The funder had no role in the design, analysis or writing of this article.
The authors' contributions are as follows: M. P. R. and C. W. G. R. designed the study; E. S. was the research midwife responsible for recruiting the participants, supervised by C. W. G. R; L. K. and K. B.-S. carried out the ELISA measurements; S. J. carried out the data analysis; S. C. B. and J. M. advised on the data analysis. All authors contributed to the writing or revising of the paper. All authors had full access to all of the study data and took responsibility for the integrity of the data and the accuracy of the data analysis. M. P. R. was the principal investigator and drafted the manuscript. M. P. R. was the guarantor for the contents of the paper.
The full protocol can be obtained from Professor M. P. R.
There are no conflicts of interest.










