Marine algae have traditionally been consumed in great quantity in Asia and to a lesser degree in other parts of the world(Reference Nisizawa, Noda and Kikuchi1). In recent years, these marine vegetables have gained popularity in Western countries due to the introduction of authentic Asian cuisine and their use in vegetarian diets. Common edible algae belong to the genera Porphyra and Laminaria(Reference Mabeau, Vallat and Brault2–Reference McHuhh4). Edible seaweeds are rich in non-digestible polysaccharides (fibre), minerals and vitamins, and contain relatively high percentages of certain PUFA(Reference Bocanegra, Bastida and Benedí5).
Although consensus has not yet been reached, various authors have reported that seaweeds, their water-soluble fraction or isolated algal polysaccharides exhibit hypocholesterolaemic(Reference Bocanegra, Bastida and Benedí5, Reference Jiménez-Escrig and Sánchez-Muniz6) effects in experimental animals. However, data regarding their antioxidant effects are controversial(Reference Bocanegra, Bastida and Benedí5, Reference Bocanegra, Benedí and Sánchez-Muniz7). Several studies have shown that atherosclerosis risk is not only attributed to total serum cholesterol, but also to the distribution of cholesterol among the different lipoproteins(Reference Castelli8, Reference Barter and Rye9). Moreover, postprandial lipaemia/lipoproteinaemia has been considered a risk factor for CHD(Reference Alipour, Elte and van Zaanen10). Dietary fibre that contains viscous polysaccharides can affect digestion, absorption and subsequent metabolism of dietary fat and cholesterol by slowing gastric emptying(Reference Bocanegra, Benedí and Sánchez-Muniz7, Reference Olson and Schneeman11), and may thus modify postprandial lipaemia(Reference Lairon, Play and Jourdheuil-Rahmani12). Various soluble fibres delay the absorption of dietary fat in the rat(Reference Ebihara and Schneeman13). Nonetheless, test results are controversial because pre-adaptation of rats to cellulose, oat bran or psyllium did not alter postprandial lipid levels when a fibre-free, high fat test meal was fed(Reference Redard, Davis and Middleton14). According to Olson & Schneeman(Reference Olson and Schneeman11), plasma TAG levels were higher in a group of rats on an oat bran diet than in a cellulose diet group after 4·5 h. To the best of our knowledge, the effect of consumption of these algae on postprandial lipaemia in rats fed cholesterol-enriched diets has not been studied to date.
Arylesterase (AE) activity is one of the three major activities of the paraoxonase 1 enzyme(Reference Nus, Sánchez-Muniz and Sinisterra Gago15, Reference Canales and Sánchez-Muniz16), which is bound to the HDL particle and inhibits lipid peroxide generation in LDL(Reference Canales and Sánchez-Muniz16). AE activity in serum or plasma is inversely related to CHD(Reference Gur, Aslan and Yildiz17). Modifications of AE activity have been observed under different dietary conditions(Reference López-Oliva, Nus and Agis-Torres18). Antioxidants such as certain pomegranate polyphenols have been found to increase or maintain high AE activity levels(Reference Rock, Rosenblat and Miller-Lotan19). Consumption of wonderful variety pomegranate juice and extract increases paraoxonase 1 association with HDL and stimulates its catalytic activity in diabetic patients(Reference Rock, Rosenblat and Miller-Lotan19). To our knowledge, the effect of alga consumption on AE activity has not yet been tested.
The hypothesis of the present paper is that the effect of different types of algae on postprandial lipaemia, lipoproteinaemia and AE activity of rats varies between rats fed cholesterol-enriched diets and those given non-cholesterol-enriched diets. Thus, the aim of the present study was to compare the effects of dietary seaweed (Konbu or Nori) supplements on postprandial lipaemia and lipoproteinaemia and AE activity in growing male Wistar rats fed cholesterol- or non-cholesterol-enriched diets.
Materials and methods
Materials
The edible red and brown marine seaweeds Nori (Porphyra tenera, class Rhodophyceae) and Konbu (Laminaria digitata, class Phaeophyceae) were obtained from a local supplier (Algamar C.B., Redondela, Pontevedra, Spain). These seaweeds were freeze-dried and grounded, using a cyclotic mill (Tecator 1093; Foss Tecator, Hoeganaes, Sweden), to a particle size of < 1·0 mm before use. The composition of the seaweeds employed in the present study has been reported previously(Reference Rupérez and Saura-Calixto20). Both algae contain a matrix of soluble, insoluble and total dietary fibre (g/100 g dry weight): 9·15; 26·98; 36·12, respectively, in Konbu and 14·56; 19·22; 33·78, respectively, in Nori(Reference Rupérez and Saura-Calixto20). Other compounds (g/100 g dry weight) found in Konbu and Nori included protein (10·7 and 28·3, respectively) and fat (1·83 and 1·64, respectively). Although the fat content of seaweeds is low, these vegetables have a high proportion of SFA and unsaturated fatty acids(Reference Colombo, Risè and Giavarini21). In fact, 20–50 % of their total fatty acid content consists of n-3 fatty acids(Reference Jeong, Cho and Moon22). Both algae are rich in minerals, but Nori contains higher amounts of Fe, Mn, Cu and Na, while Konbu is richer in Ca and K and has higher Zn/Cu ratio(Reference Bocanegra, Bastida and Benedí5). Konbu also contains higher amounts of some heavy metals such as arsenic(Reference Bocanegra, Bastida and Benedí5). Moreover, algae contain different antioxidant compounds such as catechins and phlorotannins(Reference Yoshie, Wang and Petillo23, Reference Nakamura24), whose antioxidant activity is similar to that of α-tocopherol(Reference Pavia and Aberg25).
Diet preparation and experimental design
Ninety-three percentage of each of the three homogeneous, experimental and semi-synthetic diets without supplementary cholesterol consisted of a commercial rodent diet (AIN-93M Purified Rodent Diet; DYETS, Inc., Bethlehem, Pennsylvania PA, USA), while the remaining 7 % consisted of a cellulose–wheat starch mix (35:65, w/w; non-cholesterol-enriched control diet, NChol-C) or 7 % freeze-dried algae (non-cholesterol-enriched Nori diet, NChol-N, or non-cholesterol-enriched Konbu diet, NChol-K). The three cholesterol-enriched diets (Chol-C, Chol-N and Chol-K) were identical to those previously described, but with 2 % cholesterol and 0·4 % Na cholate instead of 2·4 % maize starch (Table 1).
Table 1 Composition of the control, Nori and Konbu experimental diets with and without supplementary cholesterol

* Dyetrose (carbohydrate composition) (% by weight): monosaccharides 1; disaccharides 4; trisaccharides 5; tetrasaccharides and higher 90.
† Mineral mix contained AIN-93M Mineral Mix (g/kg): calcium carbonate 357·00; potassium phosphate monobasic 250·00; potassium citrate H2O 28·00; sodium chloride 74·00; potassium sulphate 46·60; magnesium oxide 24·00; ferric citrate U.S.P. 6·06; zinc carbonate 1·65; manganous carbonate 0·63; cupric carbonate 0·30; potassium iodate 0·01; sodium selenate 0·01 025; ammonium paramolybdate 4 H2O 0·00 795; sodium metasilicate 9H2O 1·45; chromium potassium sulphate 12H2O 0·275; lithium chloride 0·0174; boric acid 0·0815; sodium fluoride 0·0635; nickel carbonate 0·0318; ammonium vanadate 0·0066; sucrose finely powdered 209·806.
‡ AIN-93VX Vitamin Mixture (g/kg): niacin 3·00, calcium pantothenate 1·60, pyridoxine HCl 0·70, thiamine HCl 0·60, riboflavin 0·60, folic acid 0·20, biotin 0·02, vitamin E acetate (335·57 mg/g) 15·00, vitamin B12 (0·1 %) 2·50, vitamin A palmitate (150 000 μg retinol/g) 0·80, vitamin D3 (10 000 μg/g) 0·25, vitamin K1/dextrose mix (10 mg/g) 7·50, sucrose 967·23.
Animals and maintenance
Sixty growing male Wistar rats with a body weight of approximately 127 g at the outset were randomly divided into six groups of ten animals each, according to mean body weight. The rats were obtained from the breeding centre at the Facultad de Farmacia, Universidad Complutense de Madrid, Spain (Spanish Government Licence: ES 280790000085), and handled according to the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Research Council(26). Animals were housed individually in metabolic cells in a temperature-controlled room (22·3 ± 1·8 °C) with a 12 h light/dark cycle and fed one of six experimental diets for 3 weeks. The study was approved by the Spanish Science and Technology Advisory Committee and by an ethics committee of the Facultad de Farmacia of the Universidad Complutense de Madrid (Spain).
Dietary treatments
After weaning, rats were fed commercial rat pellets (Panlab, Barcelona, Spain) during a 1-week period of adaptation to environmental conditions and then switched to the experimental diets. All diets contained approximately 13 % protein, 4 % fat and 7 % total dietary fibre (Table 1). Water and food were provided ad libitum over the 3-week-long experiment that was considered adequate to observe significant results on lipoproteinaemia(Reference Sánchez-Muniz, García-Linares and García-Arias27). Food intake was checked daily and body weight variations were measured on alternate days. The dietary efficiency ratio was used to determine the relationship between body weight gain (g) and food intake (g), while apparent diet digestibility, i.e. the percentage of food digested and absorbed, was calculated using the formula (100 × (Food intake − faecal weight/food intake)). Both parameters are frequently employed in animal nutrition studies to evaluate food digestibility and utilisation.
To have an adequate postprandial lipaemia or lipoproteinaemia curve, a minimum of six points are needed. As lipoprotein determination requires a relatively large amount (1–2 ml) of serum or plasma(Reference Terpstra, Woodward and Sanchez-Muniz28), we were unable to obtain the area under the curve for rat postprandial lipoproteinaemia. Thus, the 3 h time point, frequently used to determine postprandial lipaemia, was chosen for the present study, as this is the point at which lipaemia values are usually the highest(Reference Olson and Schneeman11, Reference Daher, Slaiby and Haddad29).
At the end of the trial, rats had free access to food until between 05.00 hours and 06:30 hours and were euthanatised, after a 3 h diet withdrawal, between 08.00 hours and 09.30 hours. One animal at a time was taken at random from each of the six groups, anesthetised with an intraperitoneal injection of sodium pentobarbital (45 mg/kg body weight) and euthanatised by extracting blood from the descending aorta with a syringe.
Lipoprotein isolation
Blood from the descending aorta was collected into heparinised tubes. Plasma was separated from whole blood within 30 min of collection by centrifugation at 2500 rpm (1500 g) for 20 min and kept at 4°C until lipoprotein isolation.
Lipoproteins (chylomicrons, VLDL, LDL and HDL fractions) were isolated from plasma by density gradient ultracentrifugation. Chylomicrons were obtained by aspiration after 27 min at 30 000 rpm (153 000 g) and 4°C ultracentrifugation in a Beckman L8-70M ultracentrifuge, SW-40.1 rotor (Palo Alto, CA, USA), following a slight modification of the Terpstra method(Reference Terpstra30) by using quick acceleration and deceleration rates. After chylomicrons were isolated, tubes were filled with distilled water and ultracentrifuged for 21 h 40 min at 40 000 rpm (272 000 g) and 4°C in a Beckman L8-70M ultracentrifuge, SW-40.1 rotor, following a slight modification of the method of Terpstra et al. (Reference Terpstra, Woodward and Sanchez-Muniz28), without staining lipoproteins. Tubes were volume calibrated(Reference Terpstra, Woodward and Sanchez-Muniz28, Reference Terpstra30) and lipoproteins were separated by tube slicing at the density range of VLDL (ρ20 < 1·0063 g/ml), LDL (1·0063 < ρ20 < 1·057 g/ml) and HDL (1·057 < ρ20 < 1·21 g/ml).
Plasma lipid and lipoprotein lipid analyses
Total cholesterol, TAG and phospholipid contents were determined in plasma, chylomicrons and VLDL, LDL and HDL fractions by standard enzymatic analysis (Boehringer, Manheim, Germany). All intra-assay and inter-assay coefficients of variation were < 5·5 %. Protein content in the isolated lipoproteins was determined by the Lowry et al. (Reference Lowry, Rosebrough and Farr31) method. Total lipids were calculated as the sum of cholesterol, TAG and phospholipids. A given lipoprotein mass was calculated as the sum of lipids and proteins (both in mg/l) in this lipoprotein.
Arylesterase activity measurement
Rat plasma AE activity was measured following the Nus et al. (Reference Nus, Sánchez-Muniz and Sinisterra Gago15, Reference Nus, Sánchez-Muniz and Sánchez-Montero32) method. Reaction rates were monitored at 270 nm in thermostated quartz cuvettes with a 10 mm light path using a Shimadzu UV-2401 PC (Tokyo, Japan) spectrophotometer. Blanks without plasma samples were used to correct for the spontaneous hydrolysis of phenylacetate in the buffer. Each measurement was performed in duplicate.
Statistical analyses
Statistical analyses were performed using SPSS version 15.0 statistical analysis packages (SPSS Inc., Chicago, IL, USA). Results were expressed as mean values and standard deviation. Two-way ANOVA (type of diet, control, Nori and Konbu; and cholesterol-enriched or non-cholesterol-enriched diet) was used to compare responses to diet in the six groups. Pairwise comparisons of diet responses between groups were made employing the Bonferroni test. The effect of cholesterol consumption was evaluated using an unpaired Student's t test. Differences were accepted as significant when P < 0·05.
Results
Total food intake
In the present study, rats in the six experimental groups consumed similar amounts of food. Thus, total food intake was not significantly affected by dietary cholesterol or alga content (Table 2).
Table 2 Total food intake, body weight gain, dietary efficiency ratio, fecal weight and apparent digestibility in rats fed the Control, Nori and Konbu experimental diets with and without supplementary cholesterol
(Mean values and standard deviations for ten animals)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05, Bonferroni test).
Mean values were significantly different from supplementary cholesterol v. no supplementary cholesterol: *P < 0·05, **P < 0·01, ***P < 0·001.
† Weight gain/food intake.
‡ 100 × (Food intake − faecal weight/food intake).
Body weight gain, dietary efficiency ratio, faecal weight and apparent diet digestibility
Body weight gain ranged between 125·2 and 158·0 g. Weight gain was affected by the cholesterol supplement (P < 0·05), but not by the diet type (P < 0·1). The lowest weight gain was displayed by the Chol-C group, followed by the Chol-K group (Table 2).
Dietary efficiency ratio, faecal weight and apparent diet digestibility were significantly affected (P < 0·01, P < 0·01 and P < 0·05, respectively) by the cholesterol diet-type interaction (Table 2). Dietary efficiency ratio was significantly decreased (P < 0·001) by cholesterol consumption. However, this effect was not observed in NCholK v. Chol-K animals. Inclusion of seaweeds in both the NChol and Chol diets significantly increased (P < 0·001) faecal weights. The highest faecal weight values among NChol animals were seen in the NChol-K group, while the highest values among Chol rats were those of the Chol-N group. NChol-N and NChol-K rats displayed lower apparent diet digestibility values than animals fed the NChol-C diet. Dietary cholesterol supplementation decreased (at least P < 0·05) apparent diet digestibility in all groups (Table 2).
Lipaemia
Data of postprandial plasma lipids for the different groups are shown in Table 3. TAG levels were significantly affected (P < 0·05) by the cholesterol diet-type interaction. Supplementary cholesterol (P < 0·001) and diet type (at least P < 0·05) significantly modified plasma cholesterol and total lipid values, while diet type (P < 0·01) significantly influenced phospholipid concentrations. The cholesterol/phospholipid ratio was significantly affected by dietary cholesterol (P < 0·001). NChol-K rats presented significantly higher (at least P < 0·05) plasma cholesterol, TAG, phospholipid and total lipid values than NChol-C animals, while concentrations of these parameters of Chol-N and Chol-K rats were not significantly different than those of Chol-C animals. The postprandial triacylglycerolaemia was significantly decreased (P < 0·001) by supplementary cholesterol in Chol-K and Chol-N groups. Chol-N rats presented significantly lower (P < 0·05) plasma cholesterol levels than Chol-K animals.
Table 3 Plasma cholesterol, TAG, phospholipids, total lipid and cholesterol/phospholipid ratio in rats fed control, Nori and Konbu experimental diets with and without supplementary cholesterol
(Mean values and standard deviations for ten animals)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05, Bonferroni test).
Mean values were significantly different from those of the no supplementary cholesterol counterparts: *P < 0·05, **P < 0·01, *** P < 0·001.
† Total lipid: cholesterol plus phospholipids plus TAG.
Lipoproteinaemia
Table 4 shows the lipid and protein contents of the different lipoprotein fractions. A significant cholesterol diet-type interaction was observed for cholesterol of chylomicrons (P < 0·05); TAG of chylomicrons (P < 0·001), VLDL (P < 0·01) and LDL (P < 0·001); phospholipids of chylomicrons (P < 0·05), VLDL (P < 0·01), LDL (P < 0·001) and HDL (P < 0·05); total lipids of chylomicrons (P < 0·001), LDL (P < 0·001) and HDL (P < 0·05); proteins of chylomicrons (P < 0·001) and HDL (P < 0·001); and total mass of chylomicrons (P < 0·001), LDL (P < 0·001) and HDL (P < 0·01).
Table 4 Postprandial lipoprotein lipid concentrations in rats fed the control, Nori and Konbu experimental diets with and without a cholesterol supplement
(Mean values and standard deviations of ten animals)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05, Bonferroni test).
Mean values were significantly different from those of the no supplementary cholesterol counterparts: *P < 0·05, **P < 0·01, ***P < 0·001.
† Total lipid: cholesterol plus phospholipids plus TAG.
‡ Lipoprotein mass: total lipids plus protein.
When dietary effect was separately studied in NChol and Chol animals, levels of VLDL cholesterol and LDL cholesterol; TAG of chylomicrons and LDL; phospholipids of chylomicrons; total lipids of chylomicrons and LDL; protein of chylomicrons, VLDL and HDL; and total lipoprotein mass of chylomicrons, VLDL, LDL and HDL were observed to be significantly higher (at least P < 0·05) in NChol-K rats than in NChol-C ones. Very few significant changes were induced by the NChol-N diet. Due to the type of diet, levels of cholesterol in chylomicrons; TAG in VLDL and LDL; phospholipids in VLDL and LDL; total lipids in LDL and HDL; proteins in chylomicrons and LDL; and lipoprotein mass in LDL differed significantly between Chol-N and Chol-C animals. Phospholipids of chylomicrons and proteins of LDL were significantly higher (at least P < 0·05) in Chol-K than in Chol-C rats.
The addition of cholesterol to the diet significantly affected (P < 0·001) the concentrations of lipids (except phospholipids, total lipids and lipoprotein mass in the chylomicrons) and proteins in the various lipoprotein fractions. The amount of cholesterol in the VLDL and chylomicron fractions increased severalfold with the cholesterol-enriched diets (Table 4).
Because the principal objective of the present study was to determine the effect of algae on postprandial lipoproteinaemia, which is mainly affected by chylomicrons and VLDL, only the composition of chylomicrons and VLDL (given as percentage contribution of the different lipids and proteins) is presented in Table 5. The percentage contributions of all components of chylomicrons and those of TAG and proteins of VLDL were significantly affected by the cholesterol diet-type interaction. When NChol and Chol rats were separately studied, chylomicrons of NChol-K rats presented lower cholesterol but higher protein percentages than chylomicrons of NChol-C animals, while VLDL of NChol-K rats had lower phospholipid and higher protein percentages than VLDL of NChol-C animals. Chylomicrons of NChol-N rats had higher TAG and lower protein percentages than chylomicrons of NChol-C, while chylomicrons of NChol-N had lower protein percentages than chylomicrons of NChol-K; VLDL of NChol-N had lower phospholipid and higher protein percentages than VLDL of NChol-C. With regard to the Chol animals, chylomicrons of Chol-N rats had lower TAG levels but higher cholesterol and protein percentages than those of Chol-C rats, while VLDL of Chol-N animals had percentually more proteins than VLDL of Chol-C rats. Chylomicrons of Chol-K rats had lower TAG but higher phospholipid percentages (both P < 0·05) than those of their Chol-C counterparts, while VLDL of Chol-K animals had higher protein percentages (P < 0·05) than VLDL of Chol-C rats. Cholesterol and TAG protein percentage contributions to chylomicrons and VLDL were significantly affected by cholesterol supplementation (at least P < 0·01) in all groups (Table 5).
Table 5 Percentage contribution of the different lipids and proteins to the total mass of the different lipoproteins in rats fed the control, Nori and Konbu experimental diets with and without supplementary cholesterol
(Mean values and standard deviations for ten animals)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05, Bonferroni test).
Mean values were significantly different from those of the no supplementary cholesterol counterparts: *P < 0·05, **P < 0·01, ***P < 0·001.
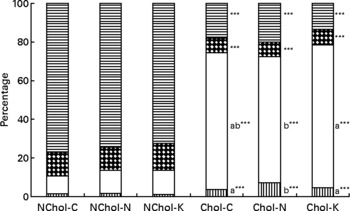
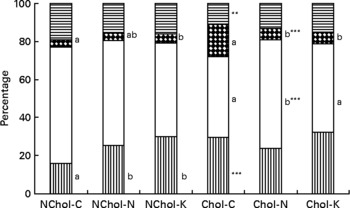
A comparison of cholesterol and TAG transport by the different lipoproteins is presented in Figs 1 and 2, respectively. In general terms, transport of cholesterol and TAG was significantly affected (at least P < 0·05) by the dietary cholesterol supplement and the type of diet. While HDL was the main cholesterol carrier in NChol rats, VLDL was the major cholesterol carrier in Chol rats.

Fig. 1 Relative cholesterol transport by different lipoproteins in rats consuming control, Nori and Konbu diets with and without supplementary cholesterol. NChol-C, rats fed the control diet without supplementary cholesterol; NChol-N, rats fed the Nori diet without supplementary cholesterol; NChol-K, rats fed the Konbu diet without supplementary cholesterol; Chol-C, rats fed the control diet with supplementary cholesterol; Chol-N, rats fed the Nori diet with supplementary cholesterol; Chol-K, rats fed the Konbu diet with supplementary cholesterol. ![]() , chylomicrons, □, VLDL;
, chylomicrons, □, VLDL; ![]() , LDL;
, LDL; ![]() , HDL. ***P < 0·001, supplementary cholesterol v. no supplementary cholesterol. Values for the same lipoprotein within the groups fed the supplementary cholesterol diets bearing a different letter were significantly different (P < 0·05).
, HDL. ***P < 0·001, supplementary cholesterol v. no supplementary cholesterol. Values for the same lipoprotein within the groups fed the supplementary cholesterol diets bearing a different letter were significantly different (P < 0·05).

Fig. 2 Relative TAG transport by different lipoproteins in rats consuming control, Nori and Konbu diets with and without supplementary cholesterol. NChol-C, rats fed the control diet without supplementary cholesterol; NChol-N, rats fed the Nori diet without supplementary cholesterol; NChol-K, rats fed the Konbu diet without supplementary cholesterol; Chol-C, rats fed the control diet with supplementary cholesterol; Chol-N, rats fed the Nori diet with supplementary cholesterol; Chol-K, rats fed the Konbu diet with supplementary cholesterol. **P < 0·01; ***P < 0·001, supplementary cholesterol v. no supplementary cholesterol. Values for the same lipoprotein within the groups fed the supplementary cholesterol diets or the groups fed the non-supplementary cholesterol diets bearing a different letter were significantly different (P < 0·05). ![]() , chylomicrons; □, VLDL;
, chylomicrons; □, VLDL; ![]() , LDL;
, LDL; ![]() , HDL.
, HDL.
Arylesterase activity
A significant cholesterol diet-type interaction (at least P < 0·05) was found when AE activity was normalised adjusted for HDL cholesterol, HDL lipids, HDL phospholipids and HDL mass. NChol-N and NChol-K rats presented significantly higher AE activity levels (P < 0·05) than NChol-C animals. AE activity decreased significantly (at least P < 0·05) in all groups receiving supplementary cholesterol. Similar effects were observed when data were adjusted for cholesterol and VLDL mass contents (Table 6).
Table 6 Arylesterase activity in rats fed the Control, Nori and Konbu experimental diets with and without supplementary cholesterol
(Mean values and standard deviations for ten animals)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05, Bonferroni test).
Mean values were significantly different from those of the no supplementary cholesterol counterparts: *P < 0·05, **P < 0·01, ***P < 0·001.
Discussion
Food consumption and body weight changes
Diets containing Konbu and Nori were well accepted, and consumed at a rate similar to that of control diets(Reference Wong, Sam and Cheung33). Results on body weight changes in the present study are comparable with those reported by Wong et al. (Reference Wong, Sam and Cheung33) and Sánchez-Muniz et al. (Reference Sánchez-Muniz, García-Linares and García-Arias27) in rats.
Lipidemia and lipoproteinaemia
Postprandial lipaemia and triacylglycerolaemia values of NChol-C rats were similar to those found in other studies(Reference Olson and Schneeman11, Reference Vázquez and Sánchez-Muniz34), but elevated with respect to others performed in comparison with those observed in fasted rats(Reference Sánchez-Muniz, García-Linares and García-Arias27, Reference Vázquez and Sánchez-Muniz34). Nonetheless, cholesterolaemia and phospholipaemia values in the normocholesterolaemic NChol-C rats of the present study were similar to those reported in fasted animals(Reference Sánchez-Muniz, García-Linares and García-Arias27) with all NChol-C rats being normocholesterolaemics(Reference Sanchez-Muniz and Bastida35). Thus, the non-fasting condition mainly affects TAG-rich lipoproteins. The absolute content and composition of LDL and HDL in the NChol-C group were comparable with those reported in other studies(Reference Garrido-Polonio, García-Linares and García-Arias36). HDL cholesterol and HDL lipids account for 70 % or more of total cholesterol and lipaemia. Cholesterol ester transfer protein activity is very low in rats(Reference Hogarth, Roy and Ebert37), explaining, at least in part, the present results. Rats, moreover, display a very effective uptake of VLDL and a low transference of apo B from VLDL to LDL(Reference Sigurdsson, Nicoli and Lewis38), which explains the low levels of LDL found in the present study and in previous ones(Reference Sánchez-Muniz, García-Linares and García-Arias27, Reference Wong, Sam and Cheung33, Reference Sanchez-Muniz and Bastida35, Reference Garrido-Polonio, García-Linares and García-Arias36).
Consumption of the NChol-K diet increased all lipoprotein masses, suggesting increased production of all rat lipoproteins, while that of the NChol-N diet increased only chylomicron mass, suggesting that those chylomicrons metabolism were slowed down. Differences in lipid and lipoprotein levels between NChol groups must first be attributed to the different fibre composition of the diets and secondly to other compounds, such as minerals, that may also affect postprandial lipaemia(Reference Bocanegra, Bastida and Benedí5, Reference Gueux, Mazur and Rayssiguier39). A previous publication involving the rats of the present study(Reference Bocanegra, Nieto and Blas40) reported that alga remains were found in the cecum of NChol-K rats, but not in NChol-N animals, suggesting clear differences in gastric emptying time and digestion speed between both alga diets. Nori contains more viscous-soluble fibre than Konbu, partially explaining why the 3 h postprandial lipaemia values of NChol-K rats were higher than those of their NChol-N counterparts. However, the cellulose consumed by the NChol-C rats, in contrast to the algal fibre consumed by the NChol-N and NChol-K animals, accelerates the digestion process and the chylomicrons metabolisation. NChol-N chylomicrons presented higher TAG and lower protein levels than NChol-K and NChol-C chylomicrons, suggesting a lower degree of metabolisation due to their more recent presence in plasma. Olson & Schneeman(Reference Olson and Schneeman11) reported that after a 4·5-h period of food deprivation, rats fed oat bran displayed higher plasma TAG levels (in the form of TAG-rich lipoproteins) than rats given cellulose, but the fibre type did not affect postprandial plasma and lipoprotein cholesterol levels. Plasma TAG levels were lower after meals including fibre in the form of guar gum(Reference Gatti, Catenazzo and Camisasca41), oat bran, wheat fibre or wheat germ(Reference Cara, Dubois and Borel42) than after meals without fibre. Other investigators have reported that certain dietary fibres enhanced postprandial lipaemia(Reference Dubois, Armand and Senft43). The accumulation of TAG-rich lipoproteins after a meal is most likely due to delayed clearance(Reference Olson and Schneeman11, Reference Schneeman, Kotite and Todd44). The NChol-K diet contained higher amounts of arsenic and a higher Zn/Cu ratio than the NChol-N or NChol-C diets. According to States et al. (Reference States, Srivastava and Chen45), epidemiological studies have shown that chronic arsenic exposure is associated with increased CVD morbidity and mortality. Klevay(Reference Klevay46) found that the hypercholesterolaemic effect of diets is related to the Zn/Cu dietary ratio.
Postprandial results in rats fed cholesterol-enriched diets suggest that supplementary dietary cholesterol modified the effect of fibre on gastric emptying time. The inclusion of the hypercholesterolaemic agent together with Nori or Konbu partially but significantly blocked the postprandial hypertriglyceridaemic effects induced by these algae in the NChol animals. Moreover, chylomicrons of Chol-N animals contained more cholesterol and fewer TAG than chylomicrons of Chol-C rats. Phospholipid levels greatly increased in the chylomicron particles of Chol-K rats, suggesting the remodelling of these lipoproteins. Postprandial plasma cholesterol increased in Chol rats mainly as a result of the cholesterol enrichment of VLDL(Reference Sánchez-Muniz, García-Linares and García-Arias27, Reference Jacques, Sugano and Beynen47, Reference Chiang, Chen and Huang48). However, in comparison with the postprandial cholesterol and VLDL cholesterol levels of Chol-C rats, those of Chol-N animals were 25 and 21 % lower, respectively, while those of Chol-K rats were 15 and 18·6 % higher, respectively. Nori has more solubility than Konbu(Reference Jiménez-Escrig and Goñi3, Reference Bocanegra, Bastida and Benedí5), which would explain the differences found in plasma and lipoprotein cholesterol levels between these two dietary groups. Water-soluble fractions of seaweeds or isolated algal polysaccharides induce hypocholesterolaemic effects in experimental animals(Reference Bocanegra, Bastida and Benedí5, Reference Jiménez-Escrig and Sánchez-Muniz6). As previously commented, Konbu has a higher Zn/Cu ratio(Reference Bocanegra, Nieto and Bastida49), and higher concentrations of arsenic than Nori, which may explain why the Chol-K diet is less hypocholesterolaemic than the Chol-N diet(Reference Bocanegra, Nieto and Bastida49).
Postprandial HDL cholesterol and HDL mass levels in the Chol-C group were lower than those of the NChol-C rats (Fig. 1). Lower HDL cholesterol levels have been previously described in rats given cholesterol supplements(Reference Sánchez-Muniz, García-Linares and García-Arias27, Reference Terpstra and Beynen50). Little is known about the mechanisms responsible for those results, although the reduction may be due to increased uptake of HDL by SR-B1 receptors(Reference Loison, Mendy and Serougne51). This lipoprotein cholesterol is used in the final step of the reverse cholesterol transport pathway for bile acid synthesis and for excretion as free cholesterol into the bile(Reference Bothan and Bravo52).
All LDL compounds decreased in Chol-N rats, suggesting that fewer LDL is formed in those rats; moreover, VLDL composition suggests that VLDL of Chol-N is larger than those of Chol-K or Chol-C rats. According to Havel(Reference Havel53), large VLDL contributes less to the formation of LDL than small VLDL in rats and other animals.
Arylesterase activity
AE is involved in lipoprotein metabolism and inhibits lipoperoxidation in LDL and HDL(Reference Aviram54). AE binds to HDL, but also to other lipoproteins(Reference Fuhrman, Volkova and Aviram55). AE activity increases in rats consuming pomegranate polyphenols(Reference Rock, Rosenblat and Miller-Lotan19). The presence of antioxidants and other phytochemicals in Nori and Konbu(Reference Bocanegra, Bastida and Benedí5) at least partially explains the greater absolute AE activity in NChol-N and NChol-K rats than that observed in NChol-C animals. The same effect or tendency was observed when data were adjusted for cholesterol, HDL cholesterol and HDL cholesterol mass.
AE activity decreased after the hypercholesterolaemic induction in all diets, but mainly in those containing algae. Hypercholesterolaemia may be associated with increased lipid peroxidation(Reference Reilly, Pratico and Delanty56). Low AE values in rats fed the Chol-C may be due to one or both of the following causes: (1) increased peroxidation(Reference Bhattacharyya, Nicholls and Topol57) and/or (2) increased hepatic uptake of HDL in the reverse cholesterol transport pathway, as AE is primarily bound to that molecule. However, the possible negative effect of some alga heavy metals (such as arsenic) on the AE activity should not be discarded because consumption of these algae has been found to negatively affect the glutathione system of the liver(Reference Bocanegra, Bastida and Benedí5, Reference Bocanegra, Benedí and Sánchez-Muniz7), and arsenic has been found to increase oxidative stress and morbidity and mortality from CVD(Reference States, Srivastava and Chen45).
In short, inclusion in the diet of 7 % dried Nori or Konbu in the absence of cholesterol increased postprandial AE activity in growing Wistar rats. Konbu slightly but significantly increased postprandial lipoprotein concentrations in these rats. Postprandial levels of plasma cholesterol were lower in rats given cholesterol-enriched diets including Nori, suggesting that Nori is the alga of choice in dietary treatment of hypercholesterolaemia.
Acknowledgements
The present work was supported by the Spanish Ministerio de Investigación y Ciencia projects AGL-2005-07 204-C02-01/ALI and AGL-2008 04 892-C03-02 and by Consolider-Ingenio 2010 project reference CSD2007-00 016.All authors have significantly contributed to the paper and agree with the present version of the manuscript. F. J. S.-M. is the corresponding author and Guarantor of the paper and has contributed to the study design, data discussion and writing of the paper. A. B., J. B. and S. B. have contributed to the data acquisition and analysis and writing of the paper, M. N. and J. M. S.-M. have contributed to arylesterase measurement, data analysis and writing of the paper. The rats were handled according to the Guide for the Care and Use of Laboratory Animals published by the National Research Council. The study was approved by the Spanish Science and Technology Advisory Committee and by an ethics committee of the Facultad de Farmacia of the Universidad Complutense de Madrid (Spain). The authors declare that there are not conflicts of interest.