Interest in phytochemicals has intensified because of their potential preventative effects on chronic diseases including CVD and cancer. A wealth of epidemiological and dietary intervention studies provides evidence indicating a protective effect of plant-based diets for CVD and cancer in adults( Reference Huang, Hirose and Wakai 1 – Reference Hooper, Kroon and Rimm 3 ). Although the mechanisms underlying the potential health benefits remain unclear, phytochemicals are thought to possess antioxidant and anti-inflammatory effects, act as cell signalling molecules, arrest the cell cycle, and most importantly manipulate detoxification phase I and phase II enzymes( Reference Lotito and Frei 4 – Reference Chen, Hurh and Na 6 ). Phase I enzymes mainly consist of cytochrome enzymes responsible for mixed-function oxidase activity, whereas phase II enzymes are frequently conjugation reactions necessary for drug metabolism or further metabolism of phase I enzyme products. Phase II metabolising enzymes such as glutathione S transferases (GST), UDP-glucuronosyltransferases (UGT) and NAD(P)H:quinone oxidoreductase 1 (NQO1) can increase the excretion rate of harmful xenobiotics in the adult( Reference Jancova, Anzenbacher and Anzenbacherova 7 ), but to date it is unclear whether dietary phytochemicals modulate the expression of these key protective phase II enzymes at different developmental stages with metabolic programming effects.
The concept of metabolic programming (imprinting) has gained widespread acceptance over the last two decades; however, many of the studies have concentrated on primary metabolic events leading to later-stage obesity, and other metabolic disorders( Reference Hanley, Dijane and Fewtrell 8 ). The potential effect of early exposure to dietary components of phytochemicals has been comparatively little studied. The question that arises is ‘whether living in a given nutritional environment conditions the genome or predisposes it to better or faster adjustment to a new or similar nutritional situation later in one's lifetime’( Reference Daniel, Drevon and Klein 9 ). In other words, can phase I or phase II response be ‘programmed’ by early exposure to phytochemicals and will this have a beneficial consequential effect on subsequent exposures?
The effect of lifetime exposure to soya protein has been shown to result in alterations in the expression of a number of cytochrome P450 enzymes in rats( Reference Ronis, Rowlands and Hakkak 10 ). Early soya exposure in rats has a differential effect on the constitutive expression of certain cytochrome P450 including CYP3A( Reference Ronis, Chen and Jo 11 ). However, up-regulation of CYP3A expression in rats fed soya for a short period post-weaning and the lack of induction in rats fed soya during gestation and lactation suggest that the effect is a direct consequence of exposure to components in soya protein isolate rather than any imprinting or programming effect( Reference Ronis, Chen and Badeaux 12 ).
Despite the known potential health benefits of fruit and vegetable consumption such as the ‘5-a-day’ message, dietary levels in adults and children are often sub-optimal( Reference Gidding, Dennison and Birch 13 ). Consumption in infants is currently unknown and is an area that warrants further investigation. Infants are not challenged with the majority of plant bioactive compounds until they are weaned, when non-milk-based foods are introduced. The infant body, specifically the liver and kidneys, needs to be able to express phase I and phase II metabolising enzymes to prevent the accumulation of potential toxins. A recent study showed that a functional polymorphism of a phase II NQO1 gene Pro187Ser; rs1800566 was significantly associated with fetal growth, possession of the fetal Ser allele protected against fetal growth retardation in the offspring of moderate smokers but not heavy smokers( Reference Price, Grosser and Plomin 14 ). If the effects of phytochemicals on phase II enzymes are positive, there is the potential to optimise infant nutrition at an early stage of development via the inclusion of bioactive phytochemicals in the diet, which may exert protective effects including the detoxification of xenobiotics. Liver cells from different ages would be the ideal models for this type of study, but they are not commercially available. Therefore, in the present study, an in vitro human skin cell model, representative of early- and late-stage infancy, in addition to a comparative adult model, was used to investigate the expression of protective phase II enzymes in response to key phytochemicals found in appreciable levels in the early infant diet. We focused on quercetin and catechin, since these polyphenols are present in many fruits and vegetables in addition to sulphoraphane, an isothiocyanate found in cruciferous vegetables such as broccoli, cauliflower, brussels sprouts and cabbages( Reference Scalbert and Williamson 15 , Reference Clarke, Dashwood and Ho 16 ). Plasma levels of quercetin and tea catechins in humans are predominantly in the low-μm concentration range although it is likely that some of their metabolites are present in the systemic circulation at levels much higher than those of their parent compounds( Reference Clarke, Dashwood and Ho 16 – Reference Graefe, Wittig and Mueller 19 ). It is estimated that the average intake of polyphenols through supplementation ranges from 50 to 300 mg/d, which is approximately 100 times higher than the intake traditionally obtained through the diet( Reference Scalbert and Williamson 15 , Reference Tanigawa, Fujii and Hou 20 ). From previous human studies, available data suggest maximal plasma sulphoraphane levels of 7·4 μm ( Reference Gasper, Al-Janobi and Smith 21 ). Therefore, in our cell culture experiments, we employed a range of phytochemical concentrations from 5 to 20 μm.
Materials and methods
Materials
Sulphoraphane (4-methylsulphinylbutyl isothiocyanate; purity, 98 %) was purchased from LKT laboratories (Alexis Biochemicals), while catechin and quercetin were purchased from Sigma. For cell cytotoxicity assays, water-soluble tetrazolium salt (WST-1) reagent was obtained from Roche, while for quantitative PCR, Bioscript RT kit, random hexamers, RNase out inhibitor and master mix reagent kit were purchased from Bioline, Promega, Invitrogen and PrimerDesign, respectively. Rabbit polyclonal GSTA1 was obtained from Calbiochem and goat polyclonal NQO1 and UGT1A were obtained from Santa Cruz. All other materials and reagents, unless otherwise specified, were purchased from Sigma Aldrich.
Cell culture
For the purpose of the present study, 1-month-old (CCD-32sk), 2-year-old (CCD-1092sk) and adult (142Br) normal primary human skin fibroblast cells were obtained from the ATCC (32sk and 1092sk) and ECACC (142Br). All cells were cultured in minimum essential medium with GluteMAX-1 (GIBCO) media supplemented with 10 % fetal bovine serum (V/V), 1 % Pen/Strep antibiotics and 1 % non-essential amino acids (GIBCO) kept at 37°C in a humidified atmosphere with 5 % CO2. Cells were media-changed every 48 h or subcultured as appropriate and used within ten passages.
Cytotoxicity assay
Cell cytotoxicity following phytochemical treatment was evaluated by the WST-1 assay which measures the activity of mitochondrial dehydrogenases. Tetrazolium salts are cleaved by the dehydrogenases of viable cells to produce formazan and the change of absorbance is detected. Briefly, cells were seeded at 2 × 104/well onto a ninety-six-well plate and allowed to adhere overnight. Cells were then treated for 24 h (time point previously determined to obtain maximal signal-to-background ratio; data not shown) with 5, 10, 25, 50 or 100 μm-sulphoraphane, catechin or quercetin plus no treatment control. At the end of the treatment period, 10 μl of WST-1 reagent were added to each well, and the plate was incubated for 2 h at 37°C in a humidified atmosphere of 95 % air, 5 % CO2. The absorbance was measured at 450 nm using a Plate Reader (BMG Labtech) and the average of three blank wells containing medium and WST-1 reagent alone was subtracted from each absorbance reading, and the resulting values were used for data analysis.
RNA extraction and analysis by TaqMan real-time PCR
Total cellular RNA was isolated using a Genelute Mammalian Total RNA Kit (Sigma-Aldrich) according to the manufacturer's instructions. Total RNA was quantified (260:280 nm ratio) using a NanoDrop spectrophotometer (Labtech International) and up to 1 μg RNA was reverse-transcribed using a Bioscript RT kit plus random hexamers and RNase out inhibitor expression of mRNA was determined by TaqMan real-time PCR using the ABI prism 7500 Sequence Detection System (Applied Biosystems). PCR were carried out in a ninety-six-well plate using the master mix reagent kit in a total volume of 25 μl/well consisting of 1 or 5 ng of sample as appropriate, 100 nmol/l probe labelled with 5′ reporter dye 6-carboxyfluoroscein and 3′ quencher 6-carboxytetramethylrhodamine and 200 nmol/l forward and reverse primers (Table 1). Standard curves were constructed with serial dilutions of control sample and analysed using ABI software 1.3.1. Data were normalised against a housekeeping gene, 18S ribosomal RNA. Gene expression was quantified by the − ΔΔ
C
t
method(
Reference Livak and Schmittgen
22
) where fold of induction =
![]() $2^{ - \Delta \Delta C _{ t }\ (control) - C _{ t }\ (treatment)}$
.
$2^{ - \Delta \Delta C _{ t }\ (control) - C _{ t }\ (treatment)}$
.
Table 1 Reference and target primer probe sequences

NQO1, NAD(P)H:quinone oxidoreductase 1; FAM, 6-carboxyfluorescein; TAMRA, tetramethylrhodamine; GSTA1, glutathione S-transferase A1; UGT1A1, UDP-glucuronosyltransferase 1A1; 18S rRNA, 18S ribosomal RNA.
Preparation of protein extracts and immunoblotting
Treated and control cells were washed twice with ice-cold PBS and then incubated for 30 min in the Nonidet P-40 buffer (20 mm-Tris–HCl, pH 8, 150 mm-NaCl, 10 % glycerol, 1 % Nonidet P-40) containing one tablet of complete mini-EDTA-free protease inhibitor cocktail (Roche) in 10 ml buffer. Cells were harvested by scraping and the homogenate was centrifuged at 13 684 g at 4°C for 15 min. The supernatants were collected and frozen at − 80°C. The protein concentrations were determined using Bradford reagent (Sigma) according to the manufacturer's instructions. Then, 20 μg of protein lysate was resolved by 10 % SDS-PAGE and transferred onto polyvinylidenedifluoride membranes (Bio-Rad) with a semi-dry transfer cell (Trans-Blot; Bio-Rad). Membranes were blocked for 1 h at room temperature or overnight at 4°C with Marvel fat-free milk powder (5 % w/v), Tween 20 (0·05 %, v/v) in PBS. Proteins of interest were visualised by exposing the membranes to primary antibodies in milk for 2 h at room temperature. Dilutions of antibodies were rabbit polyclonal GSTA1, 1:2000, goat polyclonal NQO1 1:1000 and goat polyclonal UGT1A 1:1000. Following primary antibody incubation, the membranes were incubated with suitable horseradish peroxidase-conjugated secondary antibody and signals were detected using an enhanced chemiluminescence kit (GE Healthcare) according to the manufacturer's instructions. β-Actin level was determined as the loading control and bands were visualised using Fujifilm LAS3000 Imager.
Statistical analyses
Statistical analyses were performed using SPSS (version 13.1). To assess the effects of various treatments, the data were first examined for normal distribution using the Shapiro–Wilk test (P < 0·05). Following this, one-way ANOVA with post hoc Dunnett's t test was employed to assess the significant interaction of cell type with treatment. If data were deemed non-normally distributed, the Kruskal–Wallis non-parametric test was used to examine significant interactions. Differences were considered significant if P < 0·05. Results are expressed as means with standard errors of three separate experiments, unless otherwise stated.
Results
Cytotoxicity of quercetin, catechin and sulphoraphane
In this in vitro study, we examined a range of phytochemical concentrations (1–100 μm) in cell models to examine dose–response and determine optimal concentrations for future experiments. WST-1 cell viability experiments demonstrated that cells isolated from 1-month-old (Fig. 1(a)) and adult (Fig. 1(c)) donors tolerate up to 50 μm concentrations of all candidate phytochemicals with at least 80 % viability. Cells isolated from the 2-year-old donor (Fig. 1(b)) tolerate up to 50 μm concentrations of quercetin and catechin with at least 80 % viability. However, incubation with sulphoraphane induced significant cell death at the 50 and 100 μm concentration range. On the basis of these data, further experiments were conducted at 5, 10 and 20 μm to avoid the deleterious effects of high phytochemical doses on cells.
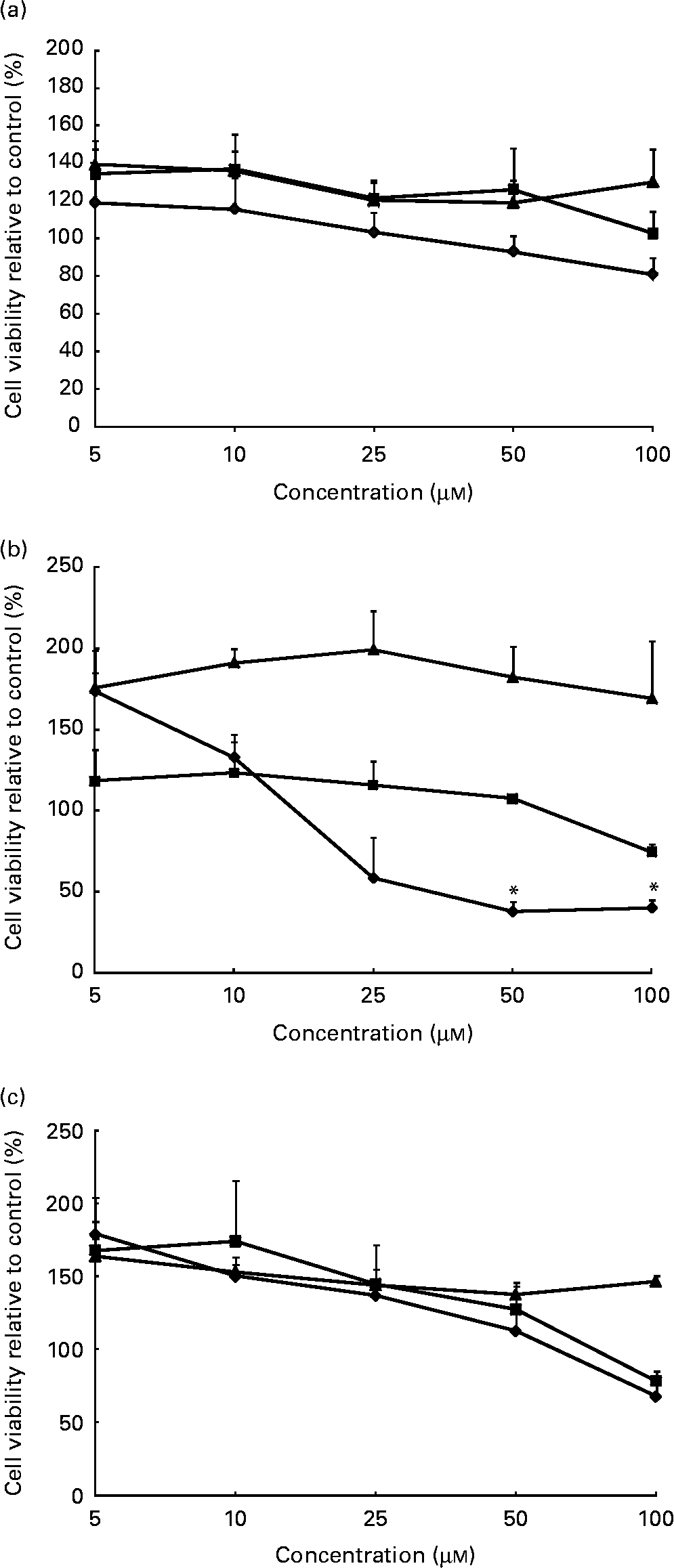
Fig. 1 Cell viability in (a) 1-month-old, (b) 2-year-old and (c) adult cell models following phytochemical treatments. Following treatment with sulphoraphane (
![]() ) significant loss of cell viability was observed in the 2-year-old cell model compared to control cells. No other significant changes in cell viability were observed in response to treatment with catechin (
) significant loss of cell viability was observed in the 2-year-old cell model compared to control cells. No other significant changes in cell viability were observed in response to treatment with catechin (
![]() ) or quercetin (
) or quercetin (
![]() ). Values are means (percentage of control) of at least three individual experiments, with their standard errors represented by vertical bars. *Mean values indicate significantly decreased cell viability when compared with control (P < 0·05; one-way ANOVA and post hoc Dunnett's t test).
). Values are means (percentage of control) of at least three individual experiments, with their standard errors represented by vertical bars. *Mean values indicate significantly decreased cell viability when compared with control (P < 0·05; one-way ANOVA and post hoc Dunnett's t test).
Quercetin differentially affects mRNA phase II enzymes in 1-month-old cell model
Cells from the 1-month-old model incubated with quercetin (Fig. 2(a), 1 month) demonstrated significant dose–response up-regulation of GST (3·9-fold) compared to control (P = 0·004). Similarly, and at the highest concentration of quercetin (20 μm), cells from the adult cell model also demonstrated up-regulation of GST (7·4-fold) and UGT (5·5-fold), P = 0·001 and 0·006, respectively (Fig. 2(a), adult). In contrast, cells obtained from the 2-year-old cell model did not demonstrate any significant changes in GST, UGT or NQO1 mRNA expression at all quercetin concentrations tested (Fig. 2(a), 2 year).
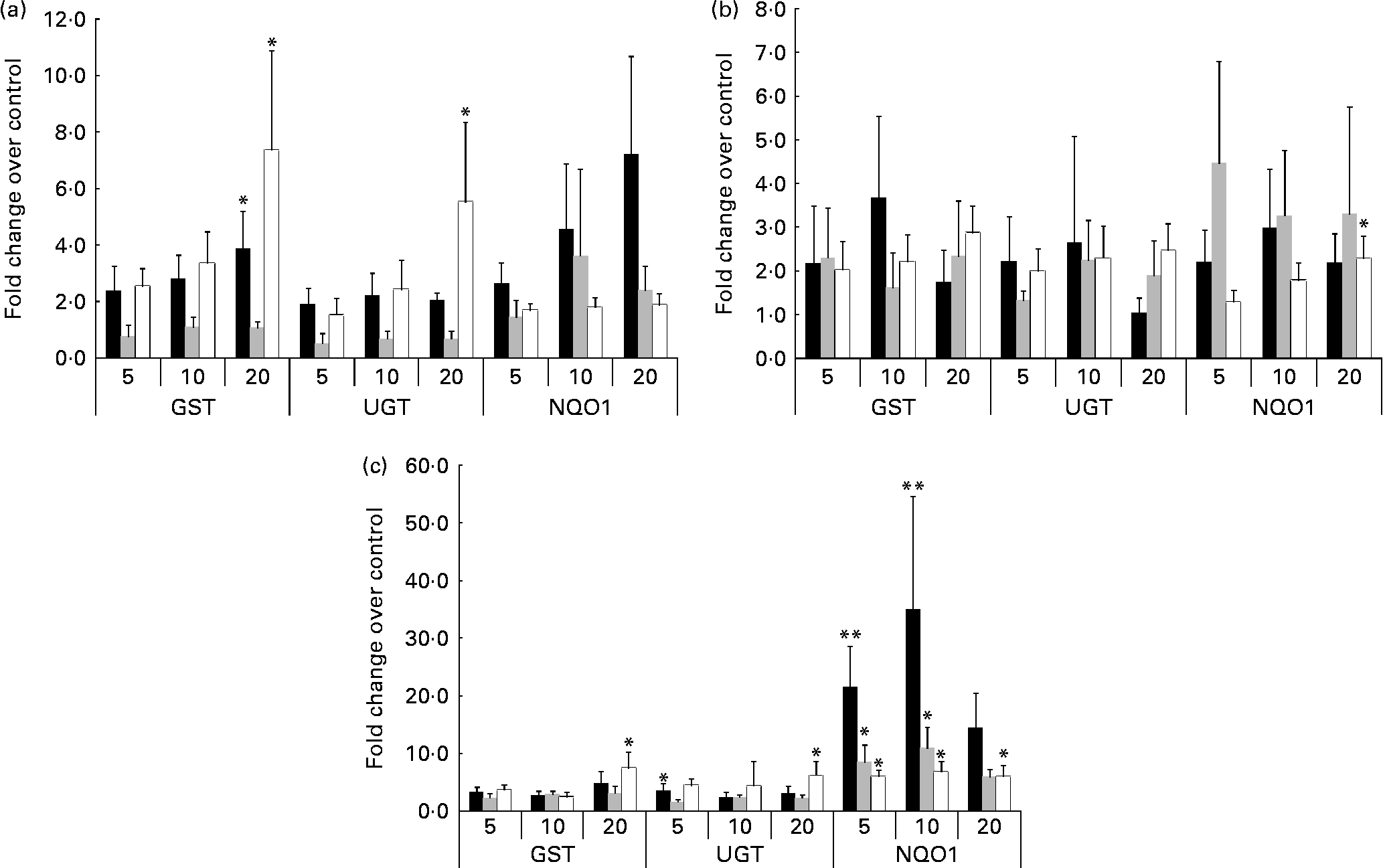
Fig. 2 Expression of mRNA in response to phytochemical treatment. Treatment with (a) quercetin: the adult cell model demonstrated a significant dose-dependent increase in both glutathione S-transferases (GST) and UDP-glucuronosyltransferases (UGT) mRNA expression (P = 0·001 and 0·006, respectively). Expression of GST and NAD(P)H:quinone oxidoreductase 1 (NQO1) mRNA was significantly increased within the 1-month-old (■) cell model but not the 2-year-old (
![]() ) cell model. Following (b) catechin treatment, the infant cell models demonstrated significant increases in GST and NQO1 mRNA (P = 0·019). (c) Sulphoraphane treatment: in the adult (□) cell line, a significant increase in GST, UGT and NQO1 mRNA was observed (P = 0·022), in addition to significant increases in GST and NQO1 in the infant cell models (P < 0·05). Values are means of at least three individual experiments, and normalised against control, with their standard errors represented by vertical bars. Mean values indicate significantly increased expression relative to control: *P < 0·05, **P < 0·01; one-way ANOVA and post hoc Dunnett's t test.
) cell model. Following (b) catechin treatment, the infant cell models demonstrated significant increases in GST and NQO1 mRNA (P = 0·019). (c) Sulphoraphane treatment: in the adult (□) cell line, a significant increase in GST, UGT and NQO1 mRNA was observed (P = 0·022), in addition to significant increases in GST and NQO1 in the infant cell models (P < 0·05). Values are means of at least three individual experiments, and normalised against control, with their standard errors represented by vertical bars. Mean values indicate significantly increased expression relative to control: *P < 0·05, **P < 0·01; one-way ANOVA and post hoc Dunnett's t test.
NAD(P)H:quinone oxidoreductase 1 mRNA expression in adult cell lines is significantly up-regulated in response to catechin
Cells from the adult cell model (Fig. 2(b), adult) demonstrated dose–response up-regulation of NQO1 mRNA in response to catechin treatment (P = 0·001, with UGT also exhibiting a trend towards an increase. The 1-month-old cell model and 2-year-old cell model mRNA expression was not significantly affected.
Infant cell lines exhibit significantly increased expression of NAD(P)H:quinone oxidoreductase 1 mRNA following sulphoraphane treatment
Cells from the adult cell model (Fig. 2(c), adult) demonstrated a significant increase in GST, UGT and NQO1 mRNA levels; in the infant cell models in particular, significant increases in NQO1 (6–35-fold increases) were observed following sulphoraphane treatment (Fig. 2(c), 1 month and 2 year) compared to control. However, GST mRNA expression was also significantly increased in response to sulphoraphane in infant cell models, but not to the same extent as NQO1.
Protein expression in infant cell models
In the 1-month-old cell model, the expression of NQO1, UGT and GST did not demonstrate significant responses to the three phytochemicals. In the 2-year-old cell model, protein expression of all enzymes was affected by phytochemical exposure. In addition, the expression of UGT, GST and NQO1 proteins were significantly up-regulated in response to higher doses of catechin and sulphoraphane P < 0·01 (Fig. 3(b)). In the adult cell model, significant up-regulation of NQO1 protein expression was observed in response to sulphoraphane, P = 0·03 (Fig. 3(c)). Expression of UGT was affected by sulphoraphane treatment, demonstrating a trend towards a dose–response, while GST expression increased in response to both quercetin and catechin (Fig. 3(c)).

Fig. 3 Effect of phytochemical treatment on phase II enzyme protein expression. Immunoblotting for effect of (a) 24 h phytochemical treatment (control, 5, 10 and 20 μm as indicated) and protein expression of phase II enzymes in the 1-month-old cell model. No significant effects of phytochemicals were observed in this cell model, immunoblotting for effect of (b) 24 h phytochemical treatment and protein expression of phase II enzymes in the 2-year-old cell model. Here, higher concentrations of catechin and sulphoraphane induce significant increases in protein expression (*P < 0·01), immunoblotting for effect of (c) 24 h phytochemical and protein expression of phase II enzymes in the adult cell model. Protein expression was significantly increased in response to sulphoraphane treatment alone (*P = 0·034). All membranes were stripped and re-probed for anti-β-actin antibody to ensure equal loading. Experiments were repeated at least three times and the results are expressed as the ratio of protein of interest relative to the expression of β-actin.
![]() , Glutathione S-transferases (GST);
, Glutathione S-transferases (GST);
![]() UDP-glucuronosyltransferases (UGT);
UDP-glucuronosyltransferases (UGT);
![]() , NAD(P)H:quinone oxidoreductase 1 (NQO1).
, NAD(P)H:quinone oxidoreductase 1 (NQO1).
Discussion
The mechanisms underlying the health benefits of phytochemicals are multifactoral with significant interest in their ability to influence the levels of detoxification enzymes( Reference Yu and Kong 23 ). However, the effect of phytochemicals such as polyphenols and isothiocyanates on the expression of common phase II enzymes in infants is unknown. We used primary skin fibroblast cells as a model for infant and adult phase II metabolism since an optimal model, such as a model based on liver cells, is not available. Skin fibroblasts are commonly used in studies of age-related differences( Reference Rolfe, Cambrey and Richardson 24 , Reference MacLean, Gonzales and Greenland 25 ). The skin, along with the liver, gut and lungs are constantly exposed to xenobiotics in the environment and therefore express (albeit at different levels) protective phase II enzymes to prevent the build-up of potentially harmful xenobiotics.
In the present study, following treatment with quercetin, cells isolated from adult and infant donors (1-month-old model) demonstrated significant up-regulation of NQO1 and GST mRNA compared to controls. However, the induction of these enzymes was not significant in the 2-year-old model, suggesting a difference in the age-related response to phytochemicals. In the adult cells, this increase in expression was also translated for NQO1 and GST protein (P = 0·034), with a similar trend demonstrated for the 1-month-old cell model. Tanigawa et al. ( Reference Tanigawa, Fujii and Hou 20 ) and Valerio et al. ( Reference Valerio, Kepa and Pickwell 26 ) also demonstrated the ability of quercetin to induce NQO1 mRNA in the hepatoma cell line HepG2 and breast carcinoma cell line Michigan Cancer Foundation-7 (MCF-7), respectively, which supports our present findings. Following treatment with catechin, significant increases in the levels of NQO1 mRNA were observed in the adult cell model. In addition, significant up-regulation in the expression of GST and NQO1 protein was observed in response to higher doses of catechin in the 2-year-old cell model. To our knowledge, no previous studies have used a similar comparative experimental approach, which emphasises the novelty of the present study. However, Muzolf-Panek et al. ( Reference Muzolf-Panek, Gliszczyńska-Swigło and de Haan 27 ) demonstrated the ability of green tea catechins, such as epigallocatechin gallate and gallocatechin gallate, to induce electrophile responsive element-mediated detoxifying gene expression of NQO1 in a mouse hepatoma cell line, supporting our findings.
Treatment with sulphoraphane in the infant cell models demonstrated a significant increase in both GST and UGT and a particularly strong response in NQO1 mRNA. The same pattern of expression was observed in the adult cell model, with significant increases in GST and UGT mRNA and a more pronounced increase in NQO1. These findings were translated into protein expression changes, with sulphoraphane treatment inducing significant up-regulation of NQO1 and UGT protein in the 2-year-old and adult cell line in particular. The phase II enzyme inducing effects of sulphoraphane have been well established in a variety of cell types including bladder cells( Reference Zhang, Munday and Jobson 28 ), colon cell lines( Reference Svehlíková, Wang and Jakubíková 29 ), prostate cells( Reference Brooks, Paton and Vidanes 30 ) and in vascular cells( Reference Zhu, Jia and Strobl 31 ). Marrot et al. ( Reference Marrot, Jones and Perez 32 ) used neonatal human skin keratinocytes and melanocytes to assess the induction of phase II genes. They found that sulphoraphane was the most potent inducer of NQO1. In addition, Dinkova-Kostova et al. ( Reference Dinkova-Kostova, Fahey and Wade 33 ) demonstrated in human skin punch samples as well as in mouse skin epidermis that sulphoraphane, in the form of broccoli sprout extract, induced NQO1 enzyme activity in a dose-dependent manner. The induction of phase II enzymes by sulphoraphane was mainly through the nuclear factor-E2-related factor 2-antioxidant responsive element pathway( Reference Lee and Surh 34 – Reference Keum 36 ). Although the mechanism by which polyphenols influence the phase II gene expression is not fully understood, the activation of nuclear factor-E2-related factor 2 may play an important role( Reference Tanigawa, Fujii and Hou 20 ).
Phase II metabolising enzymes include many families of enzymes such as GST, UGT, N-acetyltransferases and sulphotransferases. It is known that GST, UGT and NQO1 protect against potentially harmful xenobiotics in the adult, but to date it is unclear whether components in the diet, like phytochemicals, can aid in the rapid development of protective phase II enzymes in infants. Infants are not challenged with the majority of bioactive compounds in foods until they are weaned when non-milk-based foods are introduced. The infant body (liver and kidneys in particular) needs to be able to express metabolising enzymes to prevent the accumulation of potential toxins. The present study demonstrates that the expression of protective phase II enzymes can be induced by phytochemicals found commonly in the infant diet. Of particular importance seems to be the expression of NQO1 in response to sulphoraphane treatment. At the mRNA protein levels, the expression of NQO1 in the infant and adult cell models has been particularly inducible in comparison to other enzymes. The concentrations used (5–20 μm) in this study may be higher than the levels that could be achieved in human plasma through a normal diet. However, the human diet contains a large number of structurally different polyphenols, and their corresponding metabolites can attain high levels in plasma, which may also contribute to their bioactivities. Future studies are required to examine potential interactions between these phytochemicals in relation to phase II metabolism. In summary, there was a difference in the age-related response to phytochemical treatment in cell culture models, i.e. the response to phytochemicals at the mRNA level in the adult cell line was greater than that in either the 2-year-old or the 1-month-old cell model. The 1-month-old cell line responded to the phytochemical treatments especially at the mRNA level, although there was less of an effect at the protein expression level. These results suggest that the inclusion of dietary phytochemicals in the infant diet may help to induce the expression of key protective metabolic pathways such as phase II enzymes in the infants, thereby providing an avenue to increase protection from potentially harmful xenobiotics in adulthood.
Acknowledgements
The authors' contributions to the present study were as follows: A. C., B. H. and Y. B. designed the study. E. W. conducted the experiments and completed statistical analyses of the data. A. C., E. W. and Y. B. drafted the manuscript which B. H. and Z. E. J. reviewed. All authors read and approved the final version of the manuscript. A. C., E. W. and Y. B. have no conflicts of interest. B. H. and Z. E. J. were/are employed by Mead Johnson. This work was supported by Mead Johnson Nutrition (IN, USA).






