CVD are the leading causes of death among non-communicable diseases, posing a significant health and economic burden worldwide(Reference Yusuf, Reddy and Ôunpuu1, Reference Leal, Luengo-Fernández and Gray2). The American Heart Association reported that 17·7 million people (representing 31 % of all global deaths) died from CVD in 2015, and this number is projected to rise to 23·6 million by 2030(Reference Mozaffarian, Benjamin and Go3). Dyslipidaemia has been identified as a major risk factor for CVD(Reference Rader4–Reference McPherson, Frohlich and Fodor6). Thus, regulating and maintaining an optimal lipid profile is critical for the prevention of CVD. In this regard, statin therapy and diet modification are two of the most commonly prescribed approaches(Reference Ito7, Reference Chang and Robidoux8). However, statins, among other commonly used lipid-lowering pharmacotherapies(Reference Björnsson9), have been established to pose some serious adverse effects, such as myopathies and hepatotoxicity(Reference Padala and Thompson10, Reference Chalasani11). Thus, there is a demand to identify viable, anti-lipid agents that are able to pose cardio-protective effects without inducing any side effects.
l-Arginine is a semi-essential amino acid which our body derives either from dietary sources or from endogenous metabolism(Reference McNeal, Meininger and Reddy12, Reference Ástvaldsdóttir, Naimi-Akbar and Davidson13). l-Arginine is involved in several biochemical processes, including polyamine synthesis, ammonia detoxification, immune modulation and secretion of hormones such as glucagon and growth hormone and insulin(Reference McRae14–Reference Popovic, Zeh and Ochoa16). What is more, this amino acid produces nitric oxide (NO), a key molecule involved in the regulation of cell metabolism, insulin signalling and secretion, neurotransmission and immune system function(Reference Rodrigues-Krause, Krause and Rocha17, Reference Suliburska, Bogdanski and Szulinska18). It is suggested that l-arginine can be useful in improving lipid profile, due to its potential to increase NO production. Therefore, l-arginine has been investigated as a potentially cardio-protective compound, and seven meta-analyses concluded that it can be an effective tool in blood pressure management(Reference McRae19). Several trials investigated the potential of l-arginine supplementation for the treatment of abnormal lipid profile; however, the results are inconsistent. For instance, some trials report that l-arginine supplementation induced a reduction in circulating concentrations of lipid parameters(Reference Dashtabi, Mazloom and Fararouei20–Reference Bahrami, Mozaffari-Khosravi and Zavar-Reza23), while others report no significant effect(Reference Suliburska, Bogdanski and Szulinska18, Reference Bogdanski, Suliburska and Grabanska24–Reference Clarkson, Adams and Powe30). Discrepancies in the findings may conceivably be attributed to the differences in study designs, characteristics of study participants, duration and the supplementation dosage applied in the trials. Therefore, we conducted a meta-analysis of those randomised controlled trials (RCT) to examine the efficacy of l-arginine supplementation as a lipid-lowering agent.
Methods
We conducted and reported the present systematic review and meta-analysis following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (Supplementary Table S1)(Reference Moher, Liberati and Tetzlaff31) for identification, screening, eligibility and inclusion of articles. The present study was not prospectively registered. Participants, interventions, comparisons, outcomes and study design are shown in Table 1.
Table 1. PICOS criteria used to perform the systematic review and meta-analysis
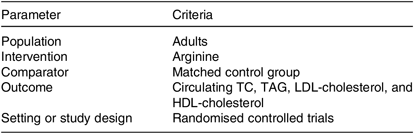
PICOS, participants, interventions, comparisons, outcomes and study design; TC, total cholesterol.
Search strategy
Two independent investigators (A. H. and E. G.) performed a systematic search of all articles published until April 2019 in the following online databases: PubMed, Scopus, Cochrane Library, ISI Web of Science and Google Scholar. We used the following Medical Subject Headings and corresponding keywords: (‘arginine’ OR ‘l-arginine’) AND (‘lipid’ OR ‘hyperlipidaemia’ OR ‘dyslipidemia’ OR ‘cholesterol’ OR ‘CHOL’ OR ‘hypercholesterolaemia’ OR ‘lipoprotein’ OR ‘hyperlipoproteinemia’ OR ‘high density lipoprotein’ OR ‘HDL’ OR ‘low density lipoprotein’ OR ‘LDL’ OR ‘triglyceride’ OR ‘TG’) AND (‘Intervention Studies’ OR ‘intervention’ OR ‘controlled trial’ OR ‘randomized’ OR ‘randomised’ OR ‘random’ OR ‘randomly’ OR ‘placebo’ OR ‘assignment’). The two reviewers also performed screening of the reference lists of relevant review articles and original papers that were selected for full-text review to identify potential eligible studies. Additionally, an email alert service was used to avoid missing any relevant articles. The language of the retrieved papers was restricted to English, while there were no restrictions regarding the year of publication.
Study selection
All studies retrieved from the electronic databases and reference lists were entered into endnote software (EndNote X6; Thomson Corporation) and duplicate studies were removed. In the next step, the titles and abstracts of the papers were examined by two independent reviewers (A. A. and E. G.) to exclude irrelevant articles. Afterwards, the full texts of the remaining publications were read and assessed according to the following four items: study design, participants, interventions and outcome measures. Finally, the studies were retained if they met the following inclusion criteria: (1) a randomised controlled design, (2) reporting the effect of l-arginine on at least one of the lipid profile parameters including total cholesterol (TC), TAG, LDL-cholesterol, and HDL-cholesterol and (3) intervention for more than 4 weeks. Studies were excluded if they (1) involved l-arginine supplementation in combination with some drugs or other types of supplements (minerals, vitamins or herbal supplements, unless a separate arm controlled the effect of the mixed substance), (2) reported duplicate data (in this case, the data with complete follow-up and outcome measures were included), (3) included adolescents as the population and (4) were not peer-reviewed articles (protocol or conference proceeding). Any disagreements regarding the process of study selection were resolved in consultation with the principal investigator (A. H.).
Data extraction
The major demographic and clinical data from each of the selected studies were screened and extracted independently by two investigators (A. H. and E. G.) using a predesigned Excel sheet. Any controversy was solved via discussion with a third, independent researcher to reach a consensus. The extracted information was as follows: the first author’s last name, publication year, study design, country, sample size, participants mean age, sex, baseline BMI, follow-up duration, intervention duration (in weeks), type of intervention, dose of l-arginine (g/d), type of control, health status of the participants and main results. Corresponding authors were contacted in case of any missing data.
Quality assessment
The Cochrane Collaboration tool(Reference Higgins and Green32) was used for quality assessment, and it includes seven items, namely, randomisation sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting and other biases. Each domain was classified into three categories: low risk of bias, high risk of bias and unclear risk of bias. Finally, the overall quality of the studies was categorised into weak, fair or good, if < 3, 3 or ≥4 domains were rated as low risk, respectively. Quality assessment was performed independently by two reviewers (A. A. and E. G.), while any disagreements were resolved by consulting the third reviewer (A. H.).
Statistical analysis
For carrying out the meta-analysis, we used STATA software (version 11; StataCorp). If outcome measures were reported in mmol/l, they were converted to mg/dl. The reviewers then extracted the mean difference between the baseline and endpoint data and the corresponding standard deviations in both intervention and control groups. If such data were not available, the mean difference was obtained by subtracting the mean value of the baseline point from that of the endpoint. If sd of the mean difference was not reported, it was calculated using the following formula: sd = square root ((sd pretreatment)2 + (sd post-treatment)2 – (2 × R × sd pretreatment × sd post-treatment)). To ensure meta-analysis was not sensitive to the selected correlation coefficient (R 0·5), all analyses were repeated using correlation coefficients of 0·2 and 0·8. Where only a standard error was reported, sd was estimated using the following formula: sd = se × √n (n being the number of subjects in each group). Using random-effects model developed by DerSimonian & Laird(Reference DerSimonian and Laird33), the summary estimate was pooled as weighted mean difference (WMD) and 95 % CI. The inconsistency index (I 2) was used to quantify statistical heterogeneity in the meta-analyses, and values greater than 50 % were considered indicative of high heterogeneity. To identify the source of heterogeneity, subgroup analysis was conducted focusing on mean age, baseline BMI, dose of l-arginine supplementation, study duration and participant’s health status. Sensitivity analysis was also performed to explore the extent to which inferences might depend on a particular study using the leave-one-out method (i.e. deleting one trail at a time and re-calculating the effect size). To assess publication bias, Begg(Reference Begg and Mazumdar34) and Egger’s(Reference Egger, Smith and Schneider35) regression tests were performed. In all statistical analyses, the level of significance was set at P < 0·05.
Ethical considerations
Ethical issues (including plagiarism, misconduct, data fabrication, falsification, double publication or submission, redundancy) have been completely observed by the authors. There was no human or animal involvement in the present study.
Results
Flow of study selection
A total of 2216 publications were identified after the search of the electronic databases, out of which 755 were removed as being duplicate (Fig. 1). By reviewing the title and abstracts of the remaining articles, 1438 publications not meeting the inclusion criteria were excluded. Subsequently, twenty-three full-text articles were carefully reviewed and eleven clinical trials were excluded because of the following reasons: five studies had a duration of supplementation period of less than 4 weeks; one study enrolled adolescents; two studies involved interventions that were a combination of other components together with l-arginine and the design did not enable evaluating l-arginine effect only; one study enrolled less than ten participants; and two articles reported the results from a same population. Finally, twelve trials(Reference Suliburska, Bogdanski and Szulinska18, Reference Dashtabi, Mazloom and Fararouei20–Reference Clarkson, Adams and Powe30) including fifteen treatment arms were considered eligible for the systematic review. However, one of the included articles(Reference Blum, Cannon and Costello28) did not report the data required for the meta-analysis. We contacted the corresponding author of the study twice but did not receive any response, and therefore it was subsequently, excluded, leaving eleven studies(Reference Suliburska, Bogdanski and Szulinska18, Reference Dashtabi, Mazloom and Fararouei20–Reference Monti, Galluccio and Villa27, Reference Pourghassem Gargari, Alizadeh and Safaeiyan29, Reference Clarkson, Adams and Powe30) for inclusion in the meta-analysis.
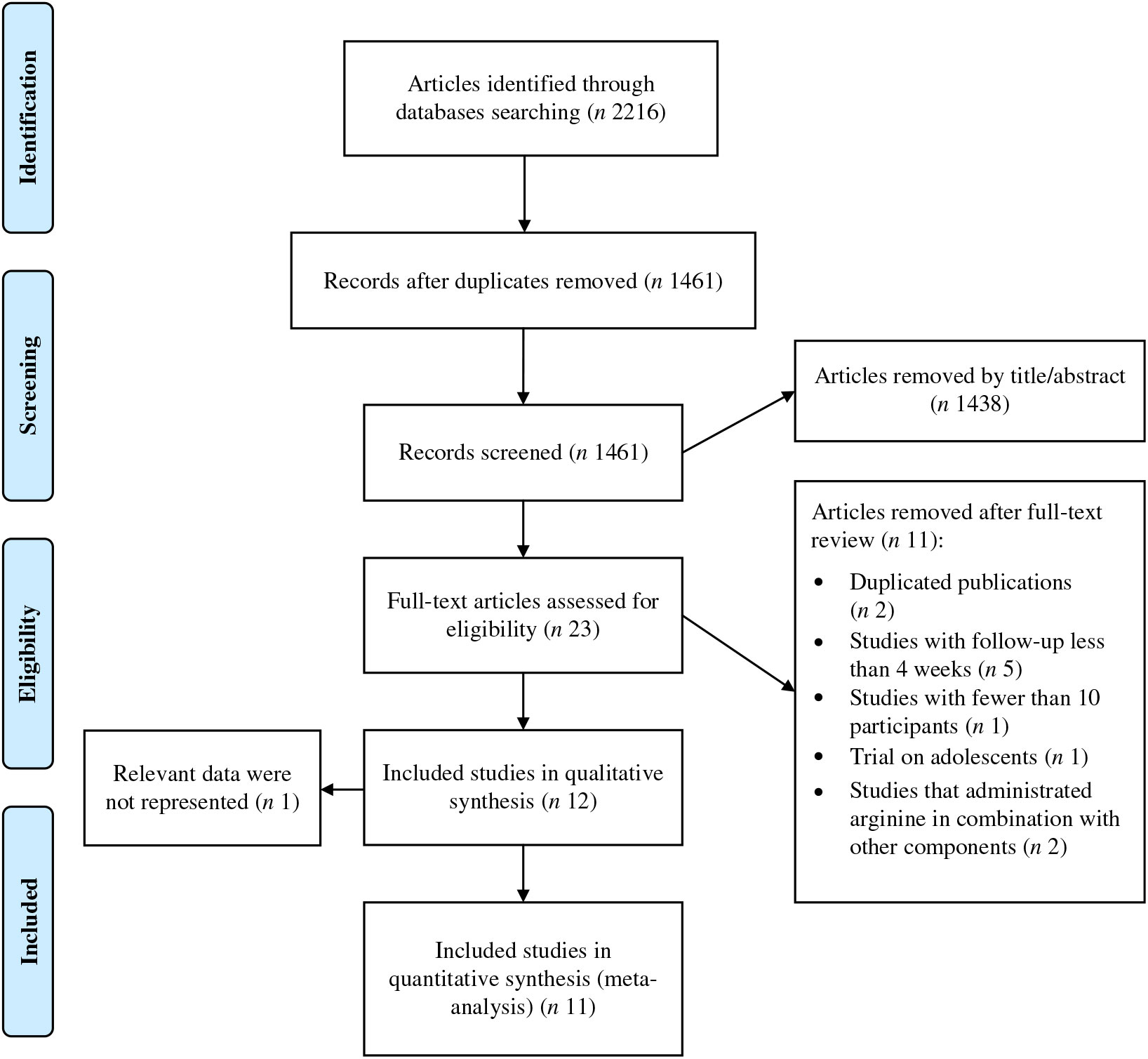
Fig. 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of study selection process.
Study and participant characteristics
Characteristics of the included trials are outlined in Table 2. In total, 631 participants were enrolled in the selected articles, out of which 359 individuals were allocated to l-arginine supplementation group and 272 subjects to the control group. These studies were published between 1996 and 2019 and were carried out in the Iran(Reference Dashtabi, Mazloom and Fararouei20–Reference Bahrami, Mozaffari-Khosravi and Zavar-Reza23, Reference Pourghassem Gargari, Alizadeh and Safaeiyan29), Italy(Reference Monti, Galluccio and Villa27), Poland(Reference Suliburska, Bogdanski and Szulinska18, Reference Bogdanski, Suliburska and Grabanska24, Reference Lucotti, Monti and Setola26), Israel(Reference Blum, Cannon and Costello28), UK(Reference Clarkson, Adams and Powe30) and Germany(Reference Schulze, Glos and Petruschka25). All studies except two(Reference Blum, Cannon and Costello28, Reference Clarkson, Adams and Powe30) adopted a parallel study design. The mean age of the participants ranged from 20·86 to 64·5 years, and the mean baseline BMI varied from 23·67 to 38·35 kg/m2. Only one(Reference Pahlavani, Jafari and Sadeghi21) of included studies involved exclusively male population, two(Reference Blum, Cannon and Costello28, Reference Pourghassem Gargari, Alizadeh and Safaeiyan29) involved women and the other trials involved populations of mixed sex. The follow-up period ranged from 4 to 77 weeks. In these studies, the daily supplementation dosage of l-arginine varied between 1 and 21 g/d. The health status of the included participants was mixed and included type 2 diabetes patients(Reference Rahimi and Naghizadeh22), postmeno-pausal women(Reference Blum, Cannon and Costello28), patients with CVD(Reference Lucotti, Monti and Setola26), subjects with hyper-cholesterolaemia(Reference Clarkson, Adams and Powe30), obese individuals(Reference Suliburska, Bogdanski and Szulinska18, Reference Dashtabi, Mazloom and Fararouei20, Reference Bogdanski, Suliburska and Grabanska24, Reference Pourghassem Gargari, Alizadeh and Safaeiyan29), subjects with hypertriacylglycerolaemia(Reference Schulze, Glos and Petruschka25), individuals with the metabolic syndrome(Reference Bahrami, Mozaffari-Khosravi and Zavar-Reza23), healthy subjects(Reference Pahlavani, Jafari and Sadeghi21) and those with impaired glucose tolerance and the metabolic syndrome(Reference Monti, Galluccio and Villa27). No major adverse effects attributable to intervention or control were reported in RCT.
Table 2. Characteristics of included studies
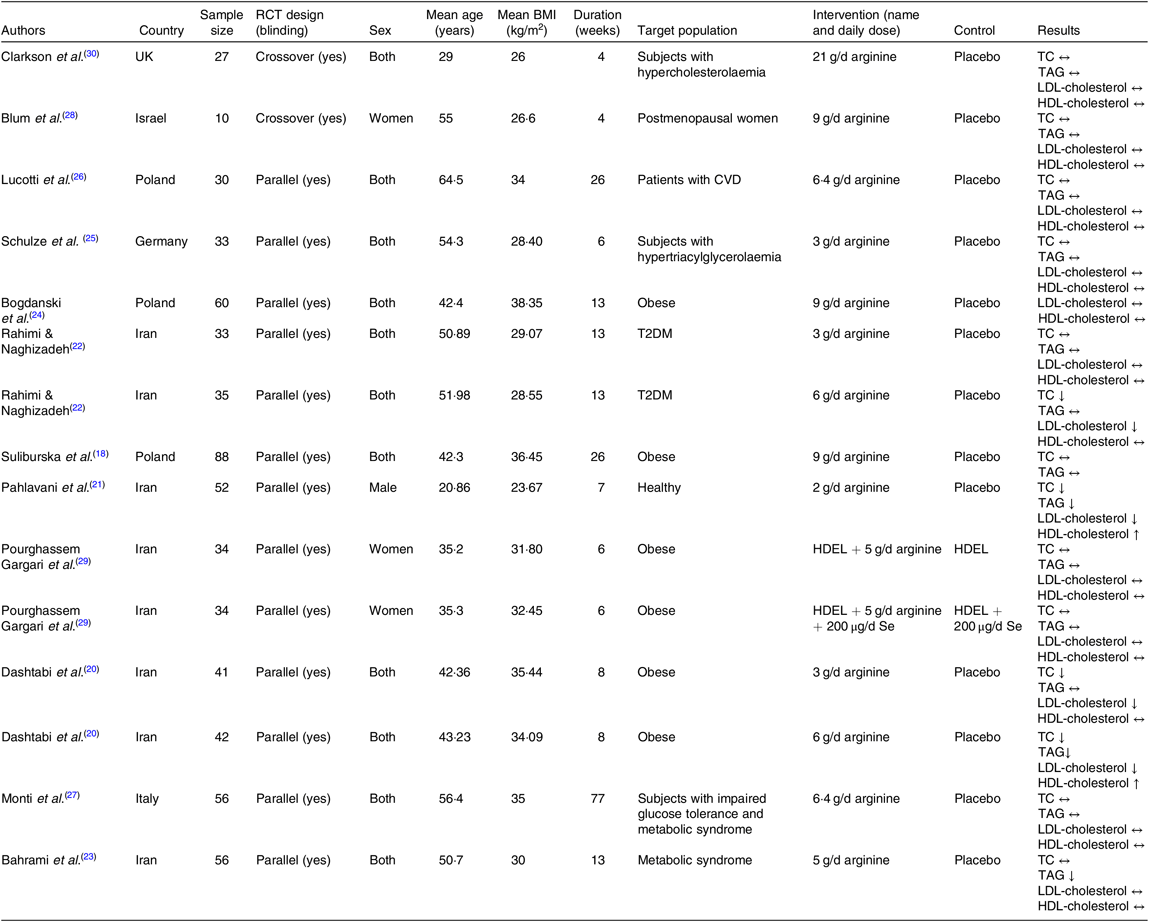
RCT, randomised controlled trial; TC, total cholesterol; T2DM, type 2 diabetes mellitus; HDEL, hypoenergetic diet enriched in legumes.
In a study by Pourghassem Gargari et al.(Reference Pourghassem Gargari, Alizadeh and Safaeiyan29), there were three intervention groups (arginine + hypoenergetic diet enriched in legumes (HDEL), arginine + HDEL + Se and HDEL + Se) and one control group (HDEL). We considered the result of the arginine + HDEL and HDEL groups as one arm and the result of the arginine + HDEL + Se and HDEL + Se groups as another arm. Furthermore, Rahimi & Naghizadeh(Reference Rahimi and Naghizadeh22) and Dashtabi et al.(Reference Dashtabi, Mazloom and Fararouei20) included two different arginine doses in their trials (3 or 6 g/d); therefore, we considered these imputations as four different arms.
Quality assessment
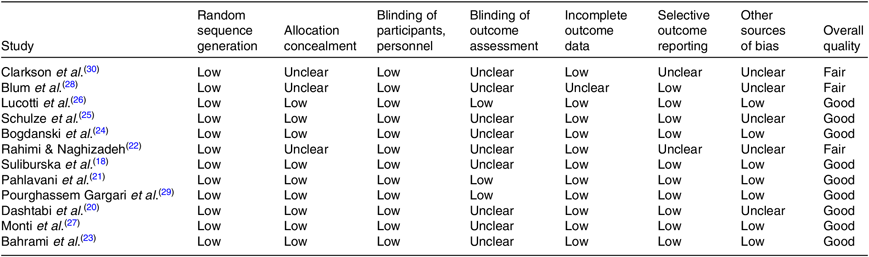
Among twelve studies included in the present review, nine trials(Reference Suliburska, Bogdanski and Szulinska18, Reference Dashtabi, Mazloom and Fararouei20, Reference Pahlavani, Jafari and Sadeghi21–Reference Pourghassem Gargari, Alizadeh and Safaeiyan29) were categorised as good quality and three trials(Reference Rahimi and Naghizadeh22, Reference Blum, Cannon and Costello28, Reference Clarkson, Adams and Powe30) as fair quality. The details of the risk of bias in individual studies according to the domains used by the Cochrane Collaborations tool are provided in Table 3.
Table 3. Quality assessment of included studies based on Cochrane guidelines
Findings from the systematic review
The present systematic review revealed that three trials(Reference Dashtabi, Mazloom and Fararouei20–Reference Rahimi and Naghizadeh22) reported that l-arginine supplementation managed to reduce TC levels, while eight studies(Reference Suliburska, Bogdanski and Szulinska18, Reference Bahrami, Mozaffari-Khosravi and Zavar-Reza23, Reference Schulze, Glos and Petruschka25–Reference Clarkson, Adams and Powe30) failed to find any significant effect on this parameter. In terms of changes in TAG levels, three trials observed a significant reduction after l-arginine supplementation in references (Reference Dashtabi, Mazloom and Fararouei20, Reference Pahlavani, Jafari and Sadeghi21, Reference Bahrami, Mozaffari-Khosravi and Zavar-Reza23), while eight studies(Reference Suliburska, Bogdanski and Szulinska18, Reference Rahimi and Naghizadeh22, Reference Schulze, Glos and Petruschka25–Reference Clarkson, Adams and Powe30) did not find such an effect. Evidence points out that arginine may pose favourable effects on LDL-cholesterol concentration as well – three trials(Reference Dashtabi, Mazloom and Fararouei20–Reference Rahimi and Naghizadeh22) found that supplementation induced a decrease in plasma LDL-cholesterol, while other trials report no significant changes in this outcome(Reference Bahrami, Mozaffari-Khosravi and Zavar-Reza23–Reference Clarkson, Adams and Powe30). Finally, l-arginine may induce favourable changes in HDL-cholesterol levels as confirmed in two trials;(Reference Dashtabi, Mazloom and Fararouei20, Reference Pahlavani, Jafari and Sadeghi21) however, the remaining studies(Reference Rahimi and Naghizadeh22–Reference Clarkson, Adams and Powe30) did not reach the same conclusion.
Findings from the meta-analysis
In total, we pooled the data from thirteen arms corresponding to ten studies(Reference Suliburska, Bogdanski and Szulinska18, Reference Dashtabi, Mazloom and Fararouei20–Reference Bahrami, Mozaffari-Khosravi and Zavar-Reza23, Reference Schulze, Glos and Petruschka25–Reference Monti, Galluccio and Villa27, Reference Pourghassem Gargari, Alizadeh and Safaeiyan29, Reference Clarkson, Adams and Powe30) which included 561 participants, to estimate the effect of l-arginine supplementation on plasma TC levels. After using a meta-analysis random-effects model, we found that l-arginine supplementation did not significantly affect the serum TC levels (WMD: –5·03 mg/dl; 95 % CI –10·78, 0·73; P = 0·08) (Fig. 2). The between-study heterogeneity was non-significant (P = 0·07, I 2 = 39·0 %). Subgroup analysis based on participants’ mean age, baseline BMI, study duration, participants health status and l-arginine dose confirmed that the effect is not statistically significant in any of the subgroups (Table 4). Findings from the sensitivity analysis revealed that the exclusion of any single study from the analysis did not alter the overall effect.
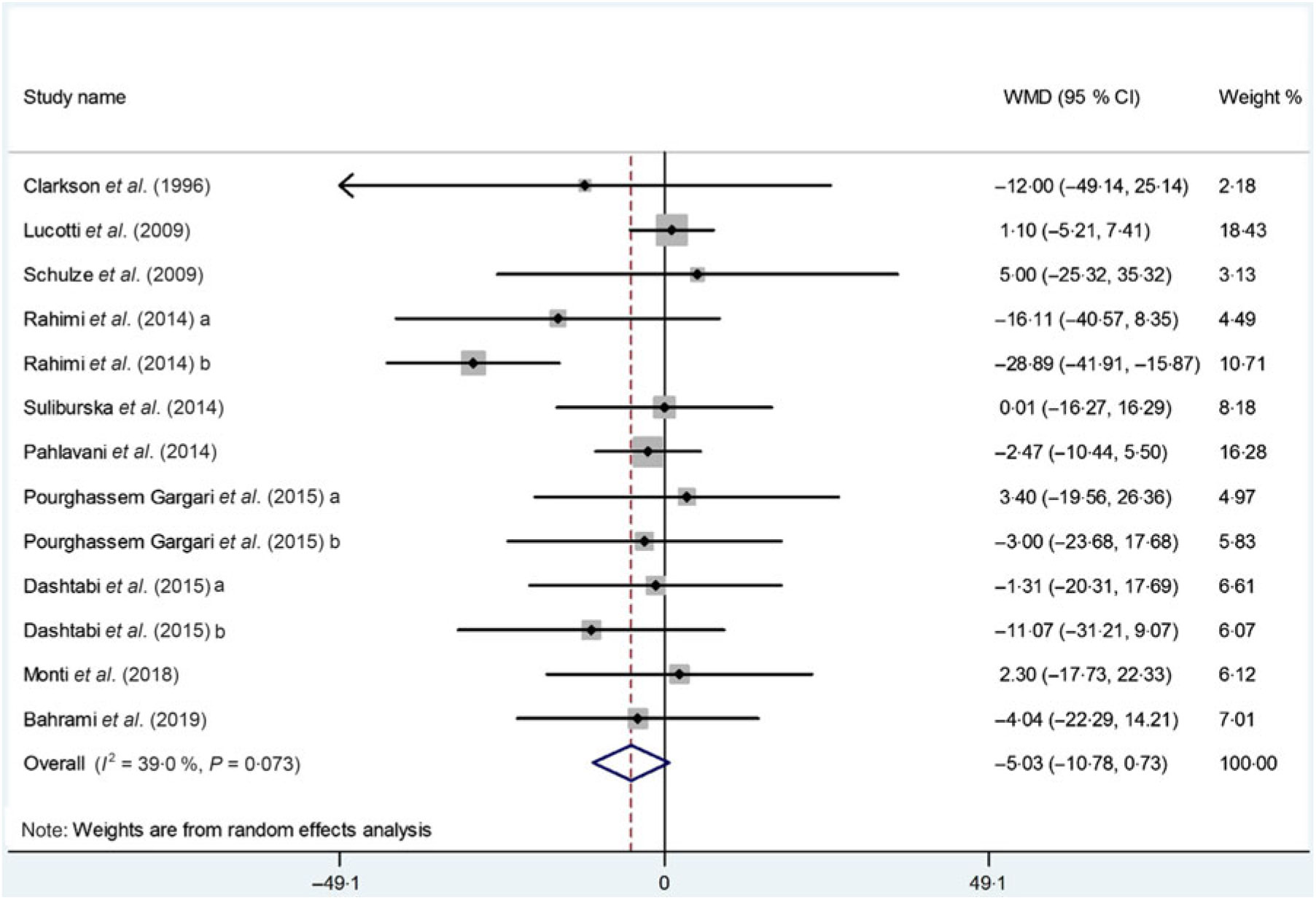
Fig. 2. Forest plot of the effect of l-arginine supplementation on total cholesterol. WMD, weighted mean difference.
Table 4. Result of subgroup analysis of included studies in meta-analysis
(Effect sizes and 95 % confidence intervals; inconsistency indices (I 2); P values)
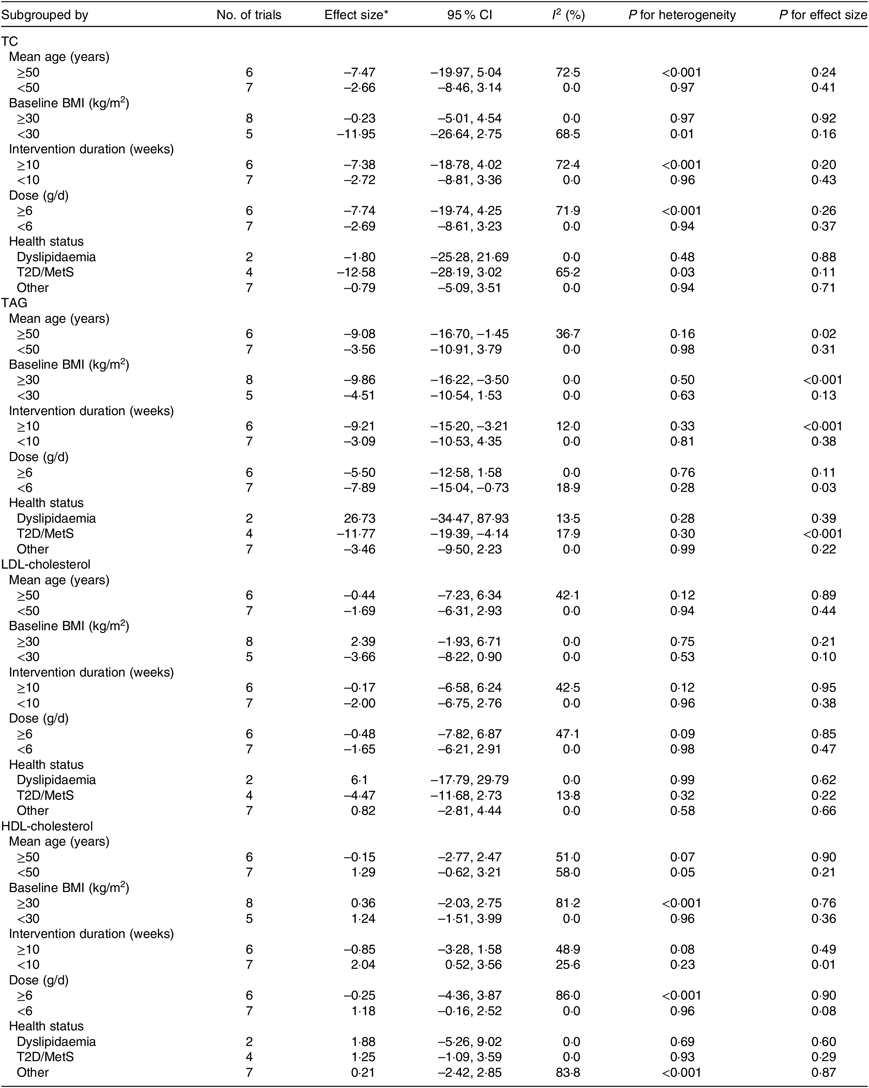
TC, total cholesterol; T2D, type 2 diabetes mellitus; MetS, metabolic syndrome.
* Calculated by random-effects model.
Thirteen arms from ten studies(Reference Suliburska, Bogdanski and Szulinska18, Reference Dashtabi, Mazloom and Fararouei20–Reference Bahrami, Mozaffari-Khosravi and Zavar-Reza23, Reference Schulze, Glos and Petruschka25–Reference Monti, Galluccio and Villa27, Reference Pourghassem Gargari, Alizadeh and Safaeiyan29, Reference Clarkson, Adams and Powe30) including 561 participants reported the effect of l-arginine on serum TAG concentration. The pooled effect demonstrated a significant decrease in TAG levels following l-arginine supplementation (WMD: –7·04 mg/dl; 95 % CI –11·42, –2·67; P < 0·001) with a non-significant heterogeneity among included studies (P = 0·59, I 2 = 0·0 %) (Fig. 3). Subgroup analysis based on participants’ mean age, baseline BMI, study duration, participants health status and l-arginine dose revealed that the effect was significant in studies that included participants with a mean age ≥50 years (WMD: –9·08 mg/dl; 95 % CI –16·70, –1·45; P = 0·02), a baseline BMI ≥30 kg/m2 (WMD: –9·86 mg/dl; 95 % CI –16·22, –3·50; P < 0·001), l-arginine dose < 6 g/d (WMD: –7·89 mg/dl; 95 % CI –15·04, –0·73; P = 0·03), type 2 diabetes/metabolic syndrome (WMD: –11·77 mg/dl; 95 % CI –19·39, –4·14; P < 0·001) and intervention duration ≥10 weeks (WMD: –9·21 mg/dl; 95 % CI –15·20, –3·21; P < 0·001) (Table 4). The sensitivity analysis demonstrated that by removing the study conducted by Bahrami et al.(Reference Bahrami, Mozaffari-Khosravi and Zavar-Reza23), the effect of l-arginine supplementation on TAG levels becomes non-significant (WMD: –4·65 mg/dl; 95 % CI –9·51, 0·19; P = 0·06).
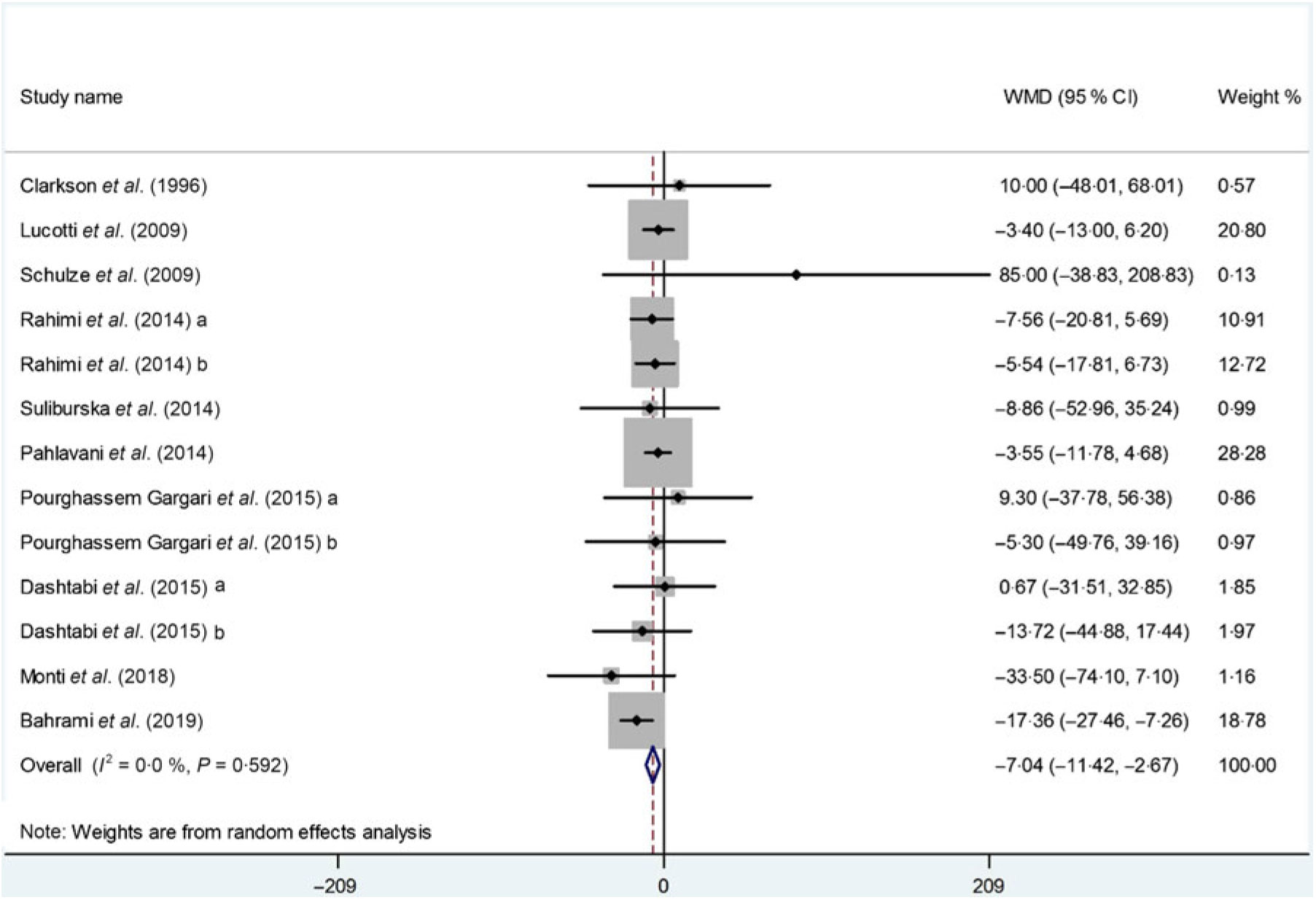
Fig. 3. Forest plot of the effect of l-arginine supplementation on TAG. WMD, weighted mean difference.
The impact of l-arginine supplementation on LDL-cholesterol levels was assessed in ten trials(Reference Dashtabi, Mazloom and Fararouei20–Reference Pourghassem Gargari, Alizadeh and Safaeiyan29, Reference Clarkson, Adams and Powe30), with thirteen treatment arms including 533 participants. The meta-analysis revealed that l-arginine supplementation did not significantly affect LDL-cholesterol levels (WMD: –0·47 mg/dl; 95 % CI –3·61, 2·66; P = 0·76), while the heterogeneity among the included studies was NS (P = 0·53, I 2 = 0·0 %) (Fig. 4). The subgroup analysis based on participants’ mean age, baseline BMI, study duration, participants health status and l-arginine dose also showed that the effect is not statistically significant in any of the subgroups (Table 4). Furthermore, removing each individual study by sensitivity analysis did not change the pooled effect size.
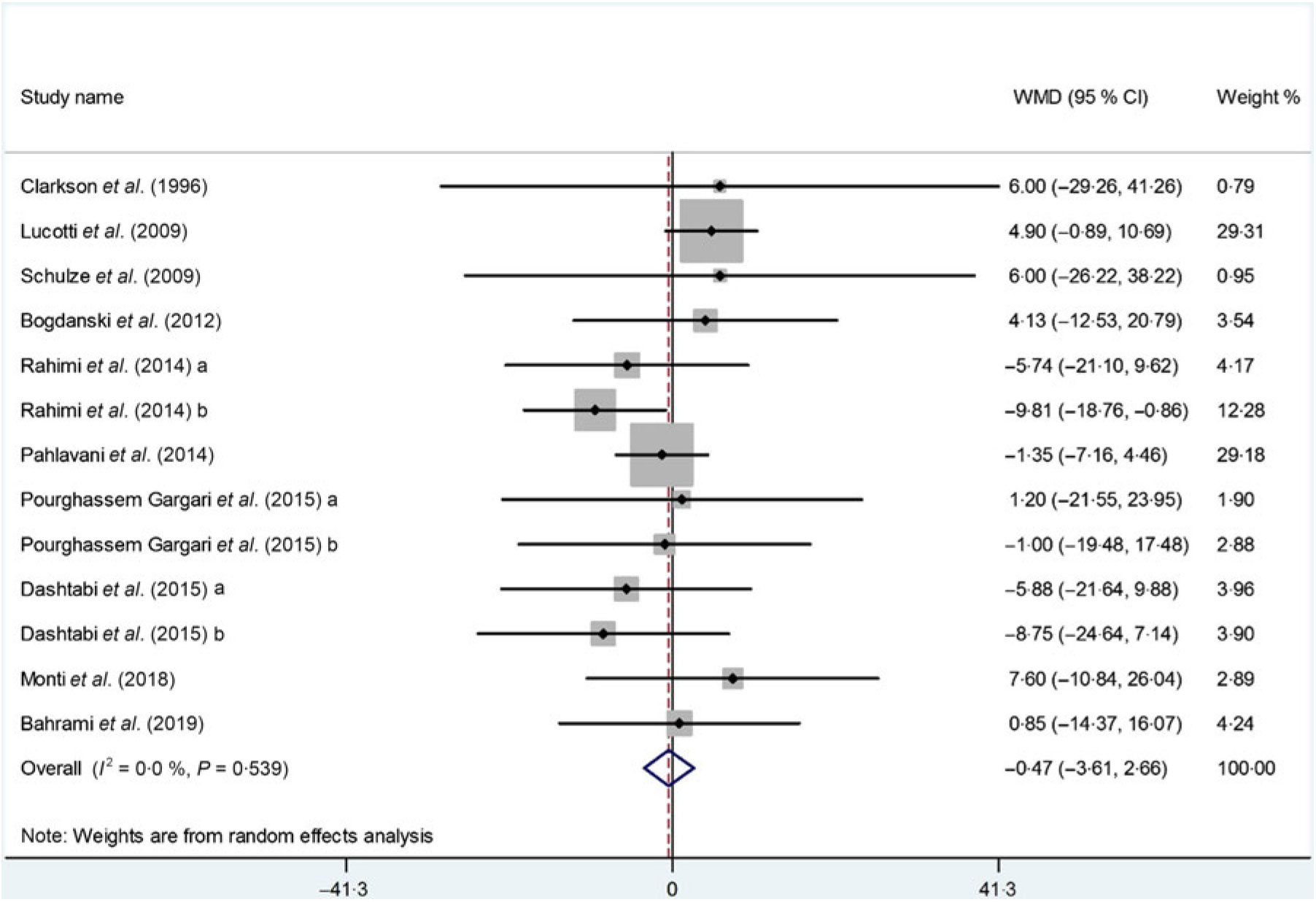
Fig. 4. Forest plot of the effect of l-arginine supplementation on LDL-cholesterol. WMD, weighted mean difference.
Ten studies(Reference Dashtabi, Mazloom and Fararouei20–Reference Monti, Galluccio and Villa27, Reference Pourghassem Gargari, Alizadeh and Safaeiyan29, Reference Clarkson, Adams and Powe30) including 533 participants from thirteen intervention arms measured changes in serum HDL-cholesterol concentrations following l-arginine supplementation. Pooled results from the random-effects model revealed that l-arginine supplementation had no significant effect on serum HDL-cholesterol levels (WMD: 0·57 mg/dl; 95 % CI –1·28, 2·43; P = 0·54) (Fig. 5). There was a significant heterogeneity among the studies (P < 0·001, I 2 = 68·4 %), and the subgroup analysis showed that baseline BMI (< 30 kg/m2; P = 0·96; I 2 = 0·0 %), duration of follow-up (< 10 weeks: P = 0·23, I 2 = 25·6 %), participants health status (dyslipidaemia: P = 0·69, I 2 = 0·0 %) or (type 2 diabetes/metabolic syndrome: P = 0·93, I 2 = 0·0 %) and l-arginine dosage (< 6 g/d: P = 0·96; I 2 = 0·0 %) were significant contributors to the between-study heterogeneity. Besides, the subgroup analysis showed that l-arginine supplementation increases HDL levels in trials with a follow-up duration < 10 weeks (WMD: 2·04 mg/dl; 95 % CI 0·52, 3·56; P = 0·01) (Table 4). Findings from the sensitivity analysis revealed that the exclusion of any single study from the analysis did not alter the overall effect.
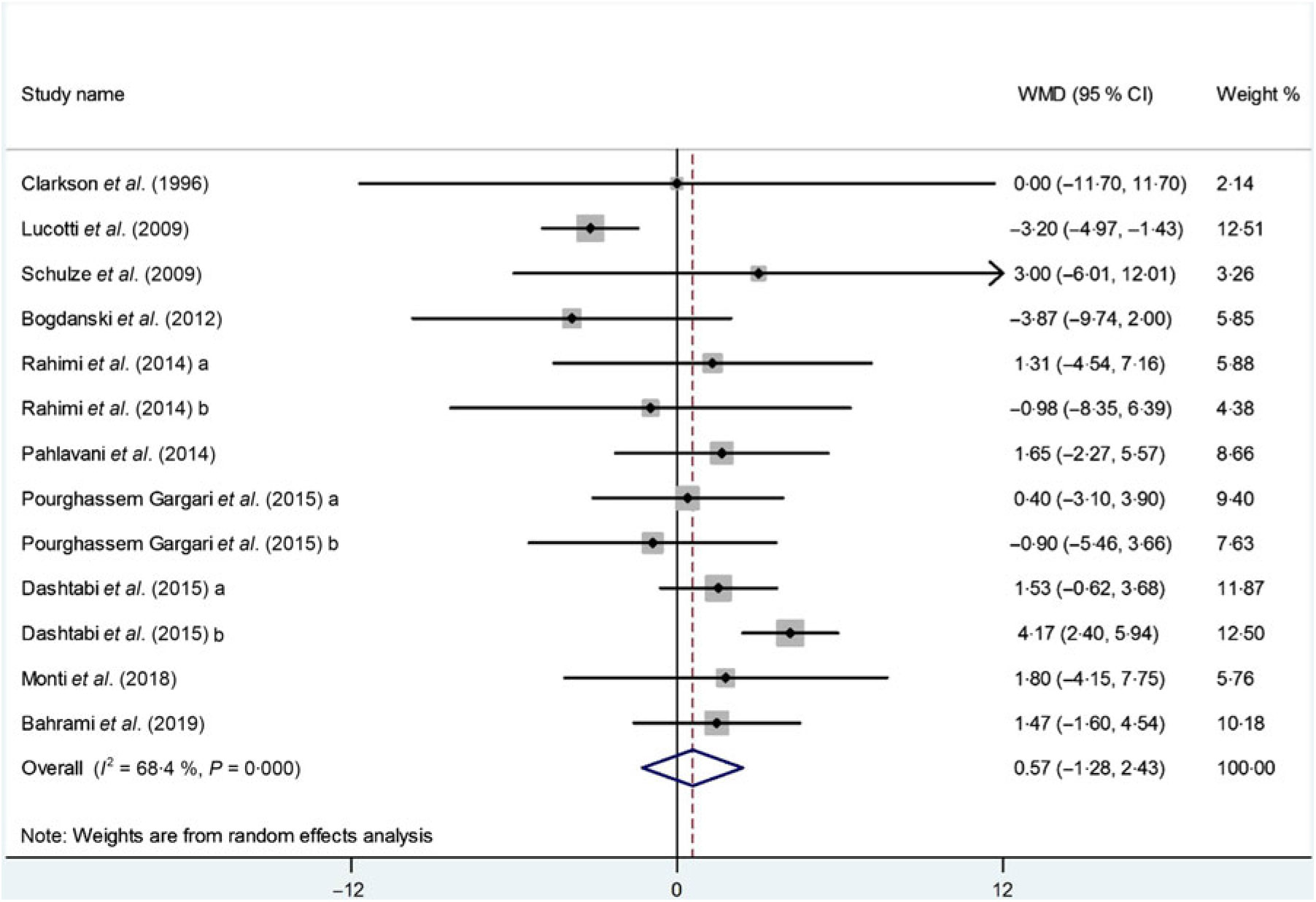
Fig. 5. Forest plot of the effect of l-arginine supplementation on HDL-cholesterol. WMD, weighted mean difference.
Publication bias
Although the visual inspection of funnel plots showed slight asymmetries, no significant publication bias was detected in the meta-analyses in the case of TC (Begg’s test, P = 0·54; Egger’s test, P = 0·45), TAG (Begg’s test, P = 0·54; Egger’s test, P = 0·56), LDL-cholesterol (Begg’s test, P = 0·39; Egger’s test, P = 0·80) or HDL-cholesterol (Begg’s test, P = 0·39; Egger’s test, P = 0·86).
Discussion
To the best of our knowledge, the present study is the first systematic review and meta-analysis that measured the effect of l-arginine supplementation on lipid profile by summarising the data from published RCT. Our results indicate that l-arginine supplementation was not able to induce changes in TC, LDL-cholesterol and HDL-cholesterol concentrations; however, it did induce a significant decrease in TAG levels. Subgroup analyses further confirmed that l-arginine supplementation imposed a significant TAG-lowering effect in studies that implemented long-term treatment (≥10 weeks) as well as in studies where participants’ baseline BMI was ≥30 kg/m2, mean age was ≥50 years, supplementation dosage was < 6 g/d and participants were type 2 diabetes or metabolic syndrome patients. Although the pooled effect size of l-arginine supplementation on HDL-cholesterol levels was NS, subgroup analysis revealed that this effect was significant only in studies that lasted longer than 10 weeks.
In this meta-analysis, we concluded that l-arginine was able to induce favourable changes only on TAG levels, while this was not observed in the case of other lipid parameters. The possible explanation for the observed lack of significant effect for all parameters except TAG might be the fact that the included population had only TAG levels above the recommended upper value, according to the definition of the metabolic syndrome. Thus, this observation may imply that individuals with higher TAG levels are better respondents to l-arginine therapy.
The biological plausibility of lipid-associated l-arginine implications comes from the existing relationship between this amino acid and glucose metabolism. Human clinical trials have concluded that l-arginine can be an effective tool in reducing blood glucose levels in type 2 diabetes patients(Reference Wascher, Graier and Dittrich36), increasing insulin sensitivity(Reference Natarajan Sulochana, Lakshmi and Punitham37) and improving insulin resistance(Reference Grundy38). One of the proposed explanations for the observed results is that insulin resistance of the adipocytes can lead to an increased release of fatty acids into the circulation. Increased free fatty acid flux reaches the liver where it stimulates the assembly and secretion of VLDL, which results in hypertriacylglycerolaemia(Reference Grundy38, Reference Ginsberg39). Another possible mechanism might be related to the lowering effect of l-arginine on blood glucose, the decrease in blood glucose levels leads to an increase in the concentration of cyclic AMP which in turn decreases the TAG levels(Reference Wu, Satterfield and Bazer40, Reference Paolisso, Tagliamonte and Marfella41). Therefore, given the beneficial role of l-arginine in the glucose homeostasis, it is proposed that supplementation with this amino acid can lower serum TAG levels(Reference Paolisso, Tagliamonte and Marfella41, Reference Li, Bazer and Gao42).
Other putative mechanisms may be related to the arginine NO synthase pathway. All NO synthase isoforms utilise l-arginine as a substrate, which undergoes a two-step metabolic conversion, yielding at the end l-citrulline and NO(Reference Forstermann and Sessa43). Elevated NO production consequently increases lipoprotein lipase activity(Reference Ricart-Jane, Casanovas and Jane44); and finally by performing hydrolysis of TAG, NO reduces TAG concentration in the plasma(Reference Schulze, Glos and Petruschka25, Reference Mendez and Balderas45). Animal study data also confirm l-arginine to be an effective lipid-lowering agent by decreasing the white fat expansion and improving serum TAG levels in rats(Reference Wu, Satterfield and Bazer40, Reference de Castro Barbosa, Jiang and Zierath46).
A neutral effect of l-arginine supplementation on TC, LDL-cholesterol and HDL-cholesterol was observed in the present meta-analysis. Subgroup analysis of the effect of l-arginine supplementation on cholesterol or lipoprotein levels did not moderate the outcome. These findings are generally in line with the majority of individual studies selected for this review. Only three studies(Reference Dashtabi, Mazloom and Fararouei20–Reference Rahimi and Naghizadeh22) showed a significant change in TC, LDL-cholesterol and HDL-cholesterol levels and others failed to find such a relationship. Furthermore, not all animal studies reported consistent results; as reducing(Reference El-Kirsh, Abd El-Wahab and Abd-Ellah Sayed47, Reference Emadi, Jahanshiri and Kaveh48) or even increasing effect of l-arginine supplementation on cholesterol level has been reported(Reference Kumar, Kumar and Tiwari49). Madeira et al. reported that dietary l-arginine supplementation increases the concentration of total lipids, VLDL and TAG(Reference dos Santos Madeira, Rolo and Pires50). However, Hu et al.(Reference Hu, Li and Rezaei51) reported that l-arginine supplementation decreases TAG and cholesterol levels in the plasma. In addition, He et al.(Reference He, Kong and Wu52) showed that l-arginine supplementation reduces VLDL, lipids and TAG concentrations in piglets. However, some animal studies reported that l-arginine might reduce cholesterol or lipoprotein levels by these mechanisms: (1) decrease the expression of hepatic 3-hydroxyl-3-methylglutaryl-CoA reductase mRNA, which shows interaction of l-arginine with cholesterol metabolism(Reference Fouad, El-Senousey and Yang53); (2) increased the lipolysis as well as the oxidation of fatty acids and (3) increase plasma adiponectin levels which improved NEFA β-oxidation. On the other hand, others reported that possible TC increasing effect of l-arginine could be due to increased fat accretion in the carcass(Reference Madeira, Alfaia and Costa54). l-Arginine can also increase the level of HDL-cholesterol through its effect on inflammation(Reference de Castro Barbosa, Jiang and Zierath46, Reference Tan, Yin and Liu55, Reference Harisa56).
l-Arginine has generally been well tolerated when administered in small doses (≤30 g/d)(Reference Böger and Bode-Böger57). However, there were some reported benign side effects, which include abdominal pain, bloating, nausea and vomiting, airway inflammation, diarrhoea, hypotension, worsening of asthma and allergic reactions(Reference Böger and Bode-Böger57–Reference Utagawa59). Furthermore, given that a major part of l-arginine is metabolised to ornithine and urea(Reference Böger and Bode-Böger57, Reference Morris60), patients with gout or renal function impairment should pay special caution when consuming it. Finally, due to its vasodilating properties, l-arginine has also been shown to interfere with certain medications (including Viagra and blood pressure medications) thus imposing negative reactions(Reference Böger and Bode-Böger57).
This meta-analysis has certain limitations that should be noted. First, the included studies involved individuals with different health status, resulting in a heterogeneous sample. Second, the sample sizes of individual trials were small, thus our results might more easily suffer from sample imbalances and an influence of baseline confounding factors. Third, the influence of sex remains unknown, since there was just one article that involved men. Women have different sex hormones compared with men that may affect lipid profile, thus implying that l-arginine may impose sex-dependent effects. Lastly, most RCT were not primarily designed to assess the effects of l-arginine on lipid concentrations. In order to draw straightforward conclusions regarding recommending l-arginine supplementation as a lipid-lowering agent, we need more RCT designed to specifically address this issue in a target population of patients with abnormal lipid profile.
The present study also has its strengths. It is only a systematic review and meta-analysis study to investigate the effect of arginine supplementation on lipid profile. Our systematic search makes it unlikely that large reports were missed, and error and bias were minimised by independent, duplicate decisions on the whole process of review by adhering to the PRISMA guidelines. Also, subgroup analysis and assessment of mean age, baseline BMI, dose of l-arginine, study duration and health status were done on the overall effect sizes.
Conclusion
The present systematic review and meta-analysis demonstrated that l-arginine supplementation leads to a significant reduction in TAG levels. However, no significant effect was observed in the case of other lipid parameters including TC, LDL-cholesterol and HDL-cholesterol. In order to confirm the results of our study, further clinical trials that exclusively examine the effects of l-arginine on participants with dyslipidaemia are required.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
A. H. and E. G. wrote the concept, design and carried out drafting of the present study. A. A. and E. G. performed searches of the electronic databases, screened the articles and extracted the data. A. H. performed the acquisition, analysis and inter-pretation of data. A. H. and E. G. critically revised the manuscript. A. P. performed a final revision and proofread of the article. All authors approved the final version of the manuscript. A. H. and E. G. are the guarantors of the present study.
The authors declare no conflict of interest.












