Osteoporosis is a major health concern for postmenopausal women. Oestrogen reduction following menopause leads to enhanced osteoclast activity and decreased osteoblast activity resulting in bone loss( Reference Weitzmann and Pacifici 1 ). Compliance with osteoporosis medications reduces the risk of fracture( Reference Weycker, Macarios and Edelsberg 2 ); however, adherence is suboptimal( Reference Gogakos, Cheung and Bassett 3 , Reference Huybrechts, Ishak and Caro 4 ). Natural food products that promote bone health may be cost-effective alternatives to pharmacological treatment that could improve compliance.
Among fruits and vegetables tested, dried plum (DP) has shown promising results in the prevention and reversal of bone loss. Deyhim et al. ( Reference Deyhim, Stoecker and Brusewitz 5 ) demonstrated that a diet with 5 % of DP by weight could restore bone mass in ovariectomised female rats. When Bu et al. ( Reference Bu, Lucas and Franklin 6 ) compared supplementation with DP to treatment with parathyroid hormone in male rats, similar improvements in bone microarchitecture and bone mineral density (BMD) were observed in both groups. Additionally, the bone-protective effects of DP are not limited to hormone deficiency-induced bone loss; one animal study has shown that DP (25 % of diet) could also reduce bone loss due to ageing( Reference Halloran, Wronski and VonHerzen 7 ). In two short- and long-term clinical trials, the effects of DP on bone biomarkers and BMD were measured in postmenopausal women. Arjmandi et al. ( Reference Arjmandi, Khalil and Lucas 8 ) found that supplementation with 100 g DP over 3 months improved the markers of bone formation, and Hooshmand et al. ( Reference Hooshmand, Chai and Saadat 9 ) showed that supplementation with 100 g DP over 1 year significantly improved the BMD of the ulna and spine compared with 75 g dried apple used as a comparative control.
Various biomarkers have been measured to clarify the mechanism of action of DP in bone health. Studies have shown elevations in the levels of bone-specific alkaline phosphatase( Reference Arjmandi, Khalil and Lucas 8 , Reference Franklin, Bu and Lerner 10 ) and insulin-like growth factor-I( Reference Deyhim, Stoecker and Brusewitz 5 , Reference Arjmandi, Khalil and Lucas 8 , Reference Franklin, Bu and Lerner 10 ), as well as reductions in the levels of urinary deoxypyridinoline( Reference Deyhim, Stoecker and Brusewitz 5 , Reference Bu, Lucas and Franklin 6 , Reference Franklin, Bu and Lerner 10 ) and tartrate-resistant acid phosphatase-5b (TRAP-5b)( Reference Hooshmand, Chai and Saadat 9 ), suggesting that DP influences bone formation and resorption. Although several bone biomarkers have been assessed, its exact mechanism of action remains unclear.
Bone remodelling is a balance between bone resorption coupled with bone formation due to the activity of osteoblasts and osteoclasts. It is now understood that osteocytes can regulate this process by controlling osteoblast and osteoclast functioning through the production of the receptor activator of NF-κB ligand (RANKL) and sclerostin( Reference Galli, Passeri and Macaluso 11 , Reference Xiong and O'Brien 12 ). The RANKL is a component of the RANKL/receptor activator of NF-κB (RANK)/osteoprotegerin (OPG) axis that has been identified as the primary modulator of bone resorption. The RANK is a receptor found on osteoclast precursors, and the RANKL binds with the RANK to induce osteoclast formation and activation( Reference Boyle, Simonet and Lacey 13 , Reference Kostenuik 14 ). OPG inhibits this process by binding with the RANKL before it can interact with the RANK to stimulate osteoclastogenesis( Reference Boyle, Simonet and Lacey 13 , Reference Kostenuik 14 ). The RANKL:OPG ratio is considered as a marker of bone resorption( Reference Kostenuik 14 ), and Jabbar et al. ( Reference Jabbar, Drury and Fordham 15 ) demonstrated that elevated levels of the RANKL, OPG and RANKL:OPG ratio were associated with lower BMD in postmenopausal women. To date, only one animal study( Reference Franklin, Bu and Lerner 10 ) has measured the effects of DP treatment on the RANKL/RANK/OPG axis. Franklin et al. ( Reference Franklin, Bu and Lerner 10 ) found that medium (15 %) and high (25 %) doses of DP reduced the gene expression levels of RANKL and OPG in bone, which decreased the RANKL:OPG ratio. However, these regulatory markers and ratios have not been evaluated in a clinical study involving DP supplementation.
Sclerostin, a product of the sclerostin (SOST) gene, is also produced by osteocytes to alter the rate of bone remodelling( Reference Galli, Passeri and Macaluso 11 ). Sclerostin interferes with canonical Wnt signalling, which results in reduced bone formation by the suppression of osteoblast activation and the promotion of apoptosis( Reference Moester, Papapoulos and Lowik 16 ). To date, there have been no studies analysing the effects of DP on the biomarker sclerostin.
To better understand the mechanism of action of DP in reversing bone loss, the present study measured the effect of daily consumption of DP on bone biomarkers in postmenopausal women. The primary aim of the present study was to assess the effect of DP on bone metabolism by measuring the serum levels of RANKL and OPG, and to measure the effect on the circulating levels of sclerostin.
Experimental methods
Participants
A total of 236 women who were 1–10 years postmenopausal were recruited. Of the volunteers, 160 women, with mild bone loss (those with a BMD t-score >2·5 sd were excluded), met inclusion and exclusion criteria, and were randomised to either the treatment (100 g DP/d; California Dried Plum Board) or control (75 g dried apple/d, Atwater Foods; LLC) group and were provided with 500 mg Ca plus 400 IU (10 μg) vitamin D daily for 1 year. Women taking hormone replacement therapy, bisphosphonates, calcitonin, raloxifene and anabolic drugs within 3 months before the study were excluded from participating. Any other medications or supplements that could influence bone mass were also not permitted. Women with chronic diseases of the bone, liver, gastrointestinal tract, respiratory tract, kidneys or cardiovascular system, as well as those with diabetes or cancer were excluded from the study. Finally, women who drank prune juice or ate DP on a daily basis or who smoked more than twenty cigarettes/d were not included. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board at San Diego State University. Written informed consent was obtained from all subjects.
Anthropometric data
Anthropometric data, medical history, dietary intakes and physical activity patterns were measured at baseline, 3 months, 6 months and at the end of the study. The details of these measurements have been described elsewhere( Reference Hooshmand, Chai and Saadat 9 ).
Bone mineral density measurements and fracture risk
BMD of the ulna, spine, hip and whole body were measured by dual-energy X-ray absorptiometry (GE Healthcare Lunar). Before data collection, the equipment was calibrated according to the manufacturer's instructions. Measurements were taken at baseline and after 12 months. The 10-year risk of hip and other major osteoporotic fractures was calculated using the Fracture Risk Assessment Tool (FRAX®)( Reference Watts 17 ).
Laboratory analysis
Venous blood samples were obtained from the participants following an overnight fast. Serum was separated by centrifugation at 3500 g for 15 min at 4°C and then stored at − 80°C. All biomarkers were assessed using commercially available ELISA kits. Baseline levels of TRAP-5b, bone-specific alkaline phosphatase, deoxypyridinoline and osteocalcin were measured with ELISA kits from Quidel Biosystems. Intra-assay (within-run) and inter-assay (between-run) coefficients of variation were 1·9–2·2 and 2·0–3·0 % for TRAP-5b, 4·0–6·0 and 5·0–8·0 % for bone-specific alkaline phosphatase, 5·0–12·0 and 10–17 % for deoxypyridinoline and 5·0–10·0 and 5·0–10·0 % for osteocalcin.
Baseline and 12-month samples were analysed for changes in the levels of RANKL, OPG and sclerostin. Serum total RANKL level was measured with an ELISA kit from ALPCO Diagnostics (intra-assay CV 0·9–3·5 %; inter-assay CV 7·1–9·3 %). Total OPG and sclerostin levels were measured using ELISA kits from Quidel Corporation. Intra-assay and inter-assay coefficients of variation were 2·1–3·5 and 4·2–6·1 % for OPG and 1·3–1·6 and 1·8–2·7 % for sclerostin.
Statistical analysis
Statistical analysis of data was conducted using SPSS for Microsoft Windows (version 21.0). ANOVA was used to assess the main effects of treatment group and time, as well as the interaction effects. The data were assessed with and without outliers. When appropriate, values >3·3 sd units from the mean were excluded from the analysis. However, if the removal of outliers did not affect the statistical results, they were included in the final analysis. Unless otherwise specified, results are expressed as means and standard deviations. An α-level of < 0·05 was considered to be statistically significant.
Results
Baseline characteristic data of the participants in the dried apple and DP groups are presented in Table 1. The mean age was significantly higher in the DP group; however, age since menopause was similar between the two groups. In women, most bone loss occurs within the first 10 years after menopause( Reference Finkelstein, Brockwell and Mehta 18 ); therefore, for the present study, we recruited women who were within 10 years of menopause and not necessarily within a certain age range. There were no significant differences in baseline BMI, biochemical markers, 10-year fracture risk and BMD of the hip or whole body between the two groups. However, spinal and ulna BMD were slightly higher in the dried apple group at baseline.
Table 1 Baseline characteristics of the study participants (Mean values and standard deviations)
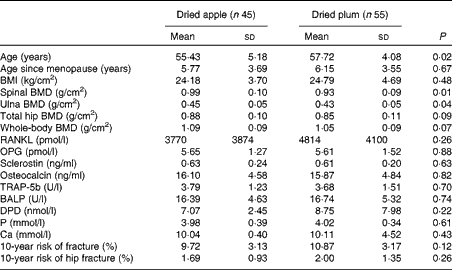
BMD, bone mineral density; RANKL, receptor activator of NF-κB ligand; OPG, osteoprotegerin; TRAP-5b, tartrate-resistant acid phosphatase-5b; BALP, bone-specific alkaline phosphatase; DPD, deoxypyridinoline.
Attrition rates were not significantly different between the two groups (37·5 %). Overall, the dried fruit regimens were well accepted and considered to be palatable. Compliance to the dried fruit regimens was on average 82 % in each group. BMD increased in both groups receiving the dried fruits, yet the participants in the DP group experienced greater increases in the BMD of the ulna and spine compared with those in the dried apple group. The results are displayed in Fig. 1.
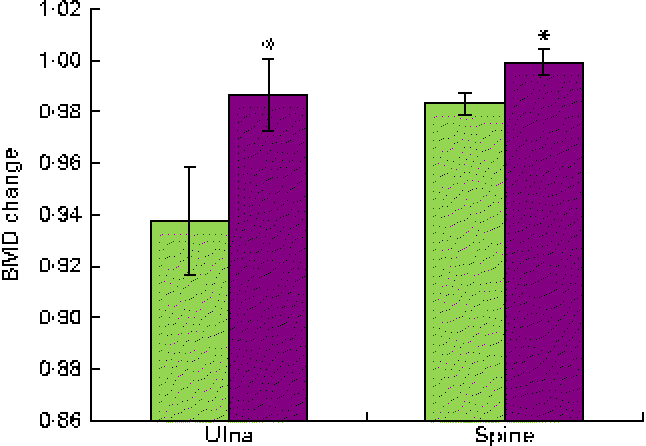
Fig. 1 Bone mineral density (BMD) changes from baseline in the ulna and spine after a 1-year consumption of dried apple (![]() ) or dried plum (
) or dried plum (![]() ). Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the dried apple group (P< 0·05). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
). Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the dried apple group (P< 0·05). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Serum levels of RANKL, a promoter of osteoclastogenesis, increased by 18·33 % in those consuming dried apple and only by 1·99 % in those consuming DP (Fig. 2). However, the mean difference between the groups was not statistically different after 12 months (Table 2). OPG, a decoy receptor for the RANKL, decreased in the dried apple group by 2·15 % and increased in the DP group by 4·87 %, although changes were not statistically significant (Fig. 2; Table 2). The RANKL:OPG ratio increased in the dried apple group and decreased in the DP group, but significant differences were not found. Sclerostin levels decreased in the DP group, but the levels were not significantly different from the dried apple group (Table 2).
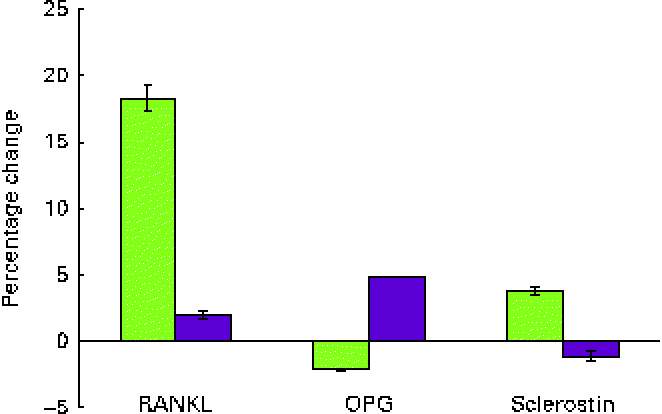
Fig. 2 Percentage changes from baseline in the serum levels of receptor activator of NF-κB ligand (RANKL), osteoprotegerin (OPG) and sclerostin after a 1-year consumption of dried apple (![]() ) or dried plum (
) or dried plum (![]() ). Values are means, with standard deviations represented by vertical bars. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
). Values are means, with standard deviations represented by vertical bars. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Table 2 Effect of dried apple and dried plum on bone biomarkers (Mean values and standard deviations)
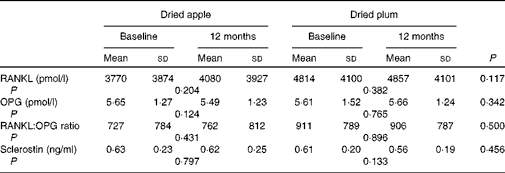
RANKL, receptor activator of NF-κB ligand; OPG, osteoprotegerin.
Discussion
Studies conducted in hormone-deficient rats have demonstrated that DP can prevent and even restore bone loss due to hormone deficiency, as well as improve bone microarchitecture( Reference Deyhim, Stoecker and Brusewitz 5 , Reference Bu, Lucas and Franklin 6 , Reference Franklin, Bu and Lerner 10 , Reference Arjmandi, Lucas and Juma 19 – Reference Johnson, Lucas and Hooshmand 21 ). As described in the present study and elsewhere( Reference Arjmandi, Khalil and Lucas 8 , Reference Hooshmand, Chai and Saadat 9 ), DP also demonstrates bone-protective effects in postmenopausal women. Studies of bone biomarkers have shown that DP may influence the activity of both osteoblasts and osteoclasts by modifying bone formation( Reference Deyhim, Stoecker and Brusewitz 5 , Reference Arjmandi, Khalil and Lucas 8 , Reference Franklin, Bu and Lerner 10 , Reference Johnson, Lucas and Hooshmand 21 ) as well as bone resorption( Reference Franklin, Bu and Lerner 10 , Reference Johnson, Lucas and Hooshmand 21 ), yet the exact mechanism of action remains to be clarified.
We postulated that modifications of the RANKL/RANK/OPG pathway may explain the mechanism of action of DP in bone health. In the present study, serum levels of OPG decreased by 2·15 % in the dried apple group and increased by 4·87 % in the DP group. Total RANKL levels increased by 18·33 % in those consuming dried apple and by 1·99 % in those consuming DP. However, the levels were not significantly different from baseline. In an earlier animal study that assessed the effect of DP on the RANKL/OPG pathway, mRNA levels of RANKL and OPG were measured in orchidectomised male rats supplemented with DP( Reference Franklin, Bu and Lerner 10 ). The study showed that medium and high doses of DP significantly reduced the gene expression levels of both RANKL and OPG and led to a non-significant reduction in the RANKL:OPG ratio. This suggests that DP has an impact on bone turnover by down-regulating the expression levels of RANKL and OPG. Similar reductions in the RANKL:OPG ratio were demonstrated in the present study, although changes did not reach a significant level. This could be in part due to the measuring of serum levels of RANKL and OPG in the present study, whereas Franklin et al. ( Reference Franklin, Bu and Lerner 10 ) measured gene expression in bone. Unfortunately, serum levels are difficult to interpret due to the limitations of available ELISA kits( Reference Kostenuik 14 , Reference Jabbar, Drury and Fordham 15 ) and conflicting results among studies because of the complexity of the relationship between RANKL, OPG and bone metabolism( Reference Findlay and Atkins 22 ). Many different cell types express RANKL and OPG, and how circulating levels reflect activity in the bone microenvironment is uncertain( Reference Rogers and Eastell 23 ). Findlay et al. ( Reference Findlay, Chehade and Tsangari 24 ) found that gene expression of RANKL in bone, as measured by RT-PCR, correlated with the measurements of BMD, yet serum levels were inversely related. This was only true for the male population as the relationship was not seen in the female participants. Overall, mRNA levels of RANKL may better reflect RANKL activity in bone; unfortunately, due to the invasive nature of measuring bone mRNA, we chose to measure serum levels in the present study.
Furthermore, serum RANKL exists in a free form and in a bound form with OPG( Reference Findlay and Atkins 22 ). We measured total RANKL levels because only a small portion of circulating RANKL is unbound and the levels can be so minute that available ELISA kits are unable to detect them( Reference Findlay and Atkins 22 , Reference Drake, Srinivasan and Mödder 25 ). Total RANKL levels are in higher quantities in the circulation and easier to measure. Unfortunately, interpretation of free v. total RANKL is poorly understood( Reference Findlay and Atkins 22 ). In the present study, there was large variability of total RANKL levels among postmenopausal women, inhibiting our ability to find statistical significance. These results are similar to those reported by Reyes-Garcia et al. ( Reference Reyes-Garcia, Muñoz-Torres and Garcia 26 ) and Findlay et al. ( Reference Findlay, Chehade and Tsangari 24 ) who found high levels of variability in total RANKL levels among postmenopausal women treated with alendronate and in osteoarthritic males.
We measured the effect of DP on osteocyte activity by measuring the levels of sclerostin. To our knowledge, the present study was the first to evaluate this relationship. Sclerostin inhibits bone formation by interfering with the Wnt-signalling pathway and preventing osteoblast activity( Reference Guo, Peng and Liang 27 ). After menopause, sclerostin levels increase, suggesting that postmenopausal bone loss is in part due to a lessening in osteoblastogenesis( Reference Mirza, Padhi and Raisz 28 ). Alternatively, reductions in sclerostin levels by alterations in the SOST gene can increase bone formation and lead to sclerosteosis and van Buchem disease, two conditions characterised by high BMD( Reference Costa and Bilezikian 29 , Reference Silverman 30 ). Therefore, osteoporosis therapies that can regulate sclerostin expression have the potential to improve BMD. In the present study, DP reduced sclerostin levels by 1·1 %, while dried apple increased the levels by 3·8 %. Further research with a larger sample size may help to explain whether the positive effects of DP on BMD are in part due to the decreased levels of sclerostin.
The present study had several limitations. There was a small sample size of participants in each group, and we only measured the levels of OPG, RANKL and sclerostin at two time points during the study (baseline and 12 months). Assessment of serum biomarkers at 3-month intervals throughout the experimental period would have provided a more detailed and precise view of what changes were taking place over time. In addition, as we mentioned previously, bone biopsy gives a more accurate view of changes in the expression levels of RANKL and OPG. Future research may benefit from trying to measure bone levels of these biomarkers as opposed to serum levels alone.
In conclusion, although the present study showed a possible role for the OPG, RANKL and sclerostin pathways in increasing BMD in the DP group, these changes in serum biomarkers did not reach a significant level. Further mechanistic studies are needed to evaluate more closely the role of DP treatment on these pathways.
Acknowledgements
The present study was partly supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service (grant no. 2005-35200-17053). The funder had no role in the design, analysis or writing of this article.
S. H. contributed to the formulation of the research question, design of the study, analysis of the data and writing of the manuscript. J. R. Y. B. contributed to the design and conduct of the study, analysis of the data and writing of the manuscript. B. H. A. assisted in the formulation of the research question, design of the study and writing of the manuscript.
The authors have no conflicts of interest to report.






