Muscle of teleost fish, comprising 60 % or more of the fish body, is the main edible portion and constitutes the most valuable part of the derived products(Reference Periago, Ayala and López-Albors1). Muscle growth, the main determinant of fish growth, is the result of both the recruitment of new muscle fibres (hyperplasia) and hypertrophy of existing muscle fibres(Reference Greer-Walker2). In fish, muscle growth is known to be regulated by nutritional factors, especially amino acids(Reference Zhao, Li and Yin3–Reference Canada, Engrola and Mira6). As in animals(Reference Wang, Qiao and Yin7,Reference Hamard, Sève and Floc’h8) , threonine (Thr) is an indispensable amino acid and it directly participates in protein synthesis as a substrate(Reference Feng, Peng and Wu9), critically influences the protein utilisation efficiency of fish and ultimately affects fish growth and health(Reference Habte-Tsion, Ge and Liu10,Reference Dong, Jiang and Liu11) . The previous studies mainly focused on the effects of Thr on intestinal mucin synthesis and health(Reference Dong, Jiang and Liu11–Reference Faure, Moënnoz and Montigon14). However, information concerning the effect of Thr on muscle growth is limited in fish.
Muscle growth by hyperplasia and hypertrophy is controlled by several genetic factors such as growth hormone (GH), insulin-like growth factors (IGF), myogenic regulatory factors (MRF) and myostatin(Reference Johnston, Lee and MacQueen15). The GH/IGF axis is considered the most important endocrine system regulating skeletal growth in fish(Reference Mommsen16,Reference Fuentes, Valdés and Molina17) . Several studies in fish have demonstrated that GH enhances somatic growth via muscle hypertrophy or hyperplasia(Reference Hill, Kiessling and Devlin18–Reference Serrana, Devlin and Johnston20). The MRF, such as myoblast determination protein (MyoD), myogenic factor 5 (Myf5), myogenic regulatory factor 4 (Mrf4) and myogenin (MyoG), are critical for the determination and terminal differentiation of skeletal muscle(Reference Berkes and Tapscott21). MyoD and Myf5 regulate the activation and proliferation of satellite cells, whereas MyoG and Mrf4 act on cell differentiation(Reference Watabe22). Myostatin is a negative regulator of myogenesis, which inhibits myoblast cell proliferation and differentiation(Reference Rescan23). A number of studies have demonstrated that the GH differentially regulates the expression of MRF in fish muscle(Reference Fuentes, Valdés and Molina17). Despite increased understanding of the regulation of skeletal muscle growth by some of these factors, its regulation by nutrients remains poorly documented in fish. Studies on different aquatic species and pig already reported that dietary Thr significantly improved the growth rate(Reference Wang, Qiao and Yin7,Reference Habte-Tsion, Ge and Liu10,Reference Gao, Yang and Liu13,Reference Hong, Jiang and Kuang24) . However, actual role of Thr in regulating muscle growth in fish still needs to be clarified. Moreover, myogenic differentiation is a highly orchestrated sequential programme to generate mature skeletal muscle(Reference Jang and Baik25). Studies in C2C12 cells show myogenic differentiation is regulated by reactive oxygen species(Reference Hansen, Klass and Harris26–Reference Sagliocchi, Cicatiello and Di Cicco28). Previous studies have demonstrated that dietary Thr deficiency increased reactive oxygen species, malondialdehyde (MDA) and protein carbonyl (PC) contents and decreased antioxidant-related enzyme activities via the regulation of the NFE2-related factor 2 (Nrf2) signalling pathway in the gills of juvenile grass carp Ctenopharyngodon idellus (Reference Dong, Feng and Jiang29). Whether Thr modulates myogenic differentiation by regulating antioxidant capacity via the Nrf2 signalling pathway in muscle needs to be investigated.
Skeletal muscle protein deposition greatly contributes to overall growth in fish(Reference Greer-Walker2). The balance of protein synthesis and degradation is crucial to the protein deposition of skeletal muscle. A previous study has shown that nutrition can activate the IGF-1/phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signalling pathway and induce protein synthesis and accretion(Reference Fuentes, Björnsson and Valdés30). Target of rapamycin (TOR), a downstream component of the PI3K/AKT pathway, regulates protein synthesis via ribosomal S6 kinase (S6K) and the eukaryotic translation initiation factor 4E-binding protein (4E-BP) in fish(Reference Trevino, George and Hughes31,Reference Lansard, Panserat and Plagnes-Juan32) . Previous studies have demonstrated that Thr promotes protein synthesis of skeletal muscle in pigs and enterocyte via the regulation of TOR signalling pathway genes expression in fish(Reference Wang, Qiao and Yin7,Reference Feng, Peng and Wu9) . These results supported a nutritional stimulatory role on muscle growth. Nevertheless, studies exploring the role of Thr on fish muscle protein synthesis as well as the signalling pathways involved are very scarce.
Pelteobagrus vachelli♀ × Leiocassis longirostris♂ is a hybrid catfish that has been widely cultured in China in recent years. To the best of our knowledge, there is no available information on the nutrition of hybrid catfish. The objective of the present study was to investigate the effects of dietary Thr on growth performance and muscle growth, protein synthesis and antioxidant-related signalling pathways of hybrid catfish. Furthermore, the dietary Thr requirement for hybrid catfish was evaluated.
Materials and methods
Experimental design and diets
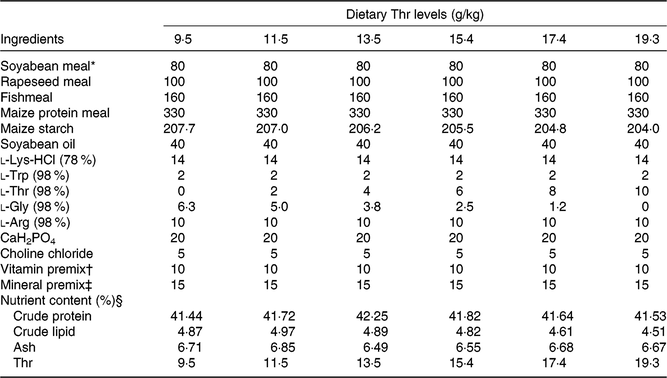
The experimental diet formulations are shown in Table 1. Soyabean meal, rapeseed meal, fishmeal and maize protein meal were used as dietary protein sources. Maize starch and soyabean oil were used as dietary carbohydrates and lipid sources, respectively. Six experimental diets were prepared with graded levels of Thr, ranging from 9·5 (control), 11·5, 13·5, 15·4, 17·4 to 19·3 g/kg. The diets were formulated to contain about 41·2 % crude protein and 5·0 % crude lipid. All dry ingredients were ground through a sixty-mesh screen. The diets were prepared by mixing the dry ingredients with oil using a mixer. Then, each diet was extruded in a twin-screw extruder (MY-165) with a 2-mm die. The processing conditions were as follows: 100 rpm screw speed, 127°C temperature and 30–45 atm pressure. Floating extruded pellets were air-dried and stored at 4°C in plastic bags until being used.
Table 1. Composition and nutrient content of diets
Thr, threonine.
* Soyabean meal (COFCO Oil Qinzhou Co. Ltd), rapeseed meal (Chengdu Huatai Grain and Oil Ltd), fishmeal (TASA steam dried fishmeal), maize gluten meal (Changchun Dacheng Industry Group), wheat meal (China National Cereals, Oils and Foodstuffs Corporation) and soyabean oil.
† Vitamin premix (IU or g/kg): retinyl acetate, 2 500 000 IU; cholecalciferol, 500 000 IU; α-tocopherol, 6700 IU; thiamine, 10; riboflavin, 6; pyridoxine hydrochloride, 12; nicotinic acid, 40; d-calcium pantothenate, 15; biotin, 0·25; folic acid, 0·4; inositol, 200; cyanocobalamin 0·02; menadione, 4. All ingredients were diluted with maize starch to 1 kg.
‡ Mineral premix (g/kg): FeC6H5O7, 4·57; ZnSO4·7H2O, 9·43; MnSO4·H2O, 4·14; CuSO4·5H2O, 6·61; MgSO4·7H2O, 238·97; KI, 1·10 g; NaSeO3, 2·50 g; CoCl2·6H2O, 1·36. All ingredients were diluted with CaCO3 to 1 kg.
§ Crude protein, crude fat and ash were measured by using the Association of Official Analytical Chemists Methods. Thr concentrations were measured using HPLC (Agilent Technologies).
Fish management and feeding
The feeding trial was conducted at Experiment Station of Ya’an, Sichuan Agricultural University, China. Hybrid catfish were obtained from Rongsen Corporation. Fish were adapted to the experimental environment for 4 weeks. A total of 1200 fish with an average initial weight of 14·19 ± 0·13 g were randomly distributed into twenty-four concrete tanks (200 × 100 × 105 cm3), resulting in fifty fish in each tank. Fish were fed with their respective diets to satiation level two times (08.00 and 18.00 hours) per d for 8 weeks. Each of the diet was fed to four replicates of fish. The daily feed supplied was recorded, and the uneaten feed was collected 30 min after feeding, followed by drying, weighing and finally subtracted from the total amount of supplied diets to calculate feed intake. The water temperature and pH were maintained at 25°C ± 3·0°C and 7·0 ± 0·5, respectively. The rate of water flow was adjusted to dissolved O2 >5·0 mg/l. During the experimental period, fish were reared under natural light conditions. All experimental protocols were approved by Animal Care Advisory Committee of Sichuan Agricultural University.
Samples collection
At the termination of feeding trial, benzocaine solution (50 mg/l) was used to anaesthetise fish after fasting for 24 h. For each tank, total fish were taken for measuring the number and body weight, then fish were killed by a sharp blow to the head according to Lisbeth et al.(Reference Lisbeth, Tran Minh and Torbj Rn33). After slaughtering, the pituitary, liver and muscle samples from the left side of six fish each tank were quickly obtained and frozen in liquid N2 and then stored at −80°C for RNA extraction and Western blot analysis. Muscle samples from the right side of the same fish were obtained for biochemical analysis.
Biochemical analysis
Approximate compositions of diets and fish muscle were analysed according to the standard methods of the Association of Official Analytical Chemists. Crude protein (N × 6·25) was determined by the Kjeldahl method after an acid digestion was performed. Crude lipid was obtained by the diethyl ether-extraction method using the Soxhlet method. Muscle samples were homogenised in ice-cold physiological saline solution (10 volumes, w/v) and centrifuged at 6000 g and 4°C for 20 min. The supernatant was collected for enzyme activity analysis. Protein content was determined by the Bradford method(Reference Bradford34). Contents of MDA, PC and GSH were assayed as described by Yonar et al.(Reference Yonar, Yonar and Silici35). Catalase (CAT) activity was determined by measuring the decomposition of hydrogen peroxide(Reference Aebi36). Activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) were assayed as described by Zhang et al.(Reference Zhang, Zhu and Cai37). Glutathione-S-transferase (GST) activity was measured by monitoring the reduction of GSH concentration(Reference Lushchak, Lushchak and Mota38), and glutathione reductase (GR) activity was measured by the consumption of NADPH during the production of GSH according to Loar et al.(Reference Lora, Alonso and Segura39). Anti-superoxide anion and anti-hydroxy radical activities were measured as described by Cheng et al.(Reference Cheng, Lin and Yu40).
Real-time quantitative PCR
The procedures of RNA isolation, reverse transcription and quantitative real-time PCR were similar to the previous study(Reference Jiang, Wu and Zhou41). Total RNA was extracted from the muscle using an RNAiso Plus kit (TaKaRa) according to the manufacturer’s instruction and followed by DNAse I treatment. RNA purity and integrity were assessed by spectrophotometric (A260:280 nm ratio) analysis and agarose gel (1 %) electrophoresis. Subsequently, 2 μl of total RNA were used to synthesise cDNA using the PrimeScript® RT reagent kit with gDNA Eraser (TaKaRa). Specific primers for IGF-1, IGF-2, AKT, MyoD, MyoG, myostatin, GPx, GST and Kelch like ECH associated protein 1 (Keap1) were designed using published sequences of yellow catfish (Table 2). Specific primers for IGF-1 receptor, PI3K, TOR, 4E-BP, S6K1, proliferating cell nuclear antigen (PCNA), Myf5, Mrf4, myosin heavy chain (MyHC), CuZnSOD, CAT, γ-glutamylcysteine ligase catalytic subunit and Nrf2 were designed according to sequences of hybrid catfish cloned in our laboratory (Table 2). Real-time PCR analysis was performed in a CFX96 Real-Time PCR Detection System (Bio-Rad). Target gene mRNA concentration was normalised to that of reference genes (β-actin and 18S rRNA). Target and reference genes’ amplification efficiency was calculated according to specific gene standard curves generated from 10-fold serial dilutions. Results were calculated using the 2−ΔΔCT method after verifying that the primers amplified with an efficiency of approximately 100 % as described by Livak et al.(Reference Livak and Schmittgen42).
Table 2. Primer sequences and optimal annealing temperatures (OAT, °C) of genes selected for analysis by real-time PCR
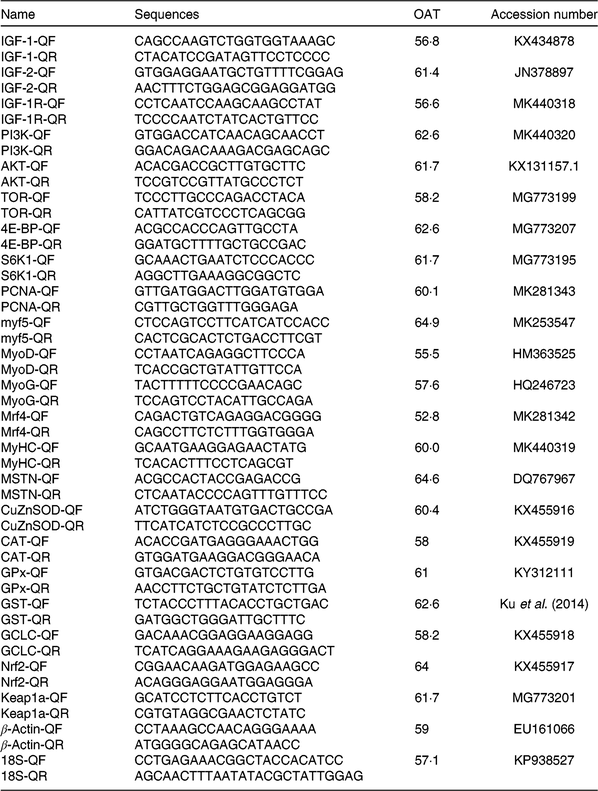
IGF-1, insulin-like growth factor 1; Q, quantitative PCR primer; F, forward; R, reverse; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; TOR, target of rapamycin; PCNA, proliferating cell nuclear antigen; Myf5, myogenic factor 5; MyoD, myoblast determination protein; Mrf4, myogenic regulatory factor 4; MyHC, myosin heavy chain; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GST, glutathione-S-transferase; GCLC, γ-glutamylcysteine ligase catalytic subunit; Nrf2, NFE2-related factor 2; Keap1a, Kelch like ECH associated protein 1a.
Protein extraction and Western blot analysis
Fish muscle tissues were homogenised with a glass Tenbroeck tissue grinder (Kimble Chase) on ice and lysed in RIPA with 1 mm phenylmethanesulfonyl fluoride (a protease inhibitor; Amresco) and 1 mm sodium β-glycerophosphate (Beyotime). A bicinchoninic acid protein assay kit (Pierce) was used to determine the protein concentration. In all, 20 μg of protein extractions were separated by 12 % SDS–polyacrylamide gel and then transferred to a polyvinyldifluoride membrane (Millipore, Inc.) using a wet Trans-Blot System (Bio-Rad). After blocking with TRIS-buffered saline Tween 20 (TBS/T) containing 5 % bovine serum albumin for 2 h at room temperature, the membranes were incubated with primary antibodies overnight at 4°C. Antibodies directed against AKT, phospho-AKT (Ser473), TOR and phospho-TOR (Ser2448) were purchased from Cell Signaling Technology Inc. The polyvinyldifluoride membranes were washed with TBS/T three times for 10 min each, incubated with second antibodies for 1 h at room temperature and then washed with TBS/T three times. Clarity Western-enhanced chemiluminescence substrate (Bio-Rad) was used to visualise signals. The Gel-Pro Analyser (Media Cybernetics) was used to quantify protein expression, and the ratio of target proteins expression was normalised to β-actin.
Statistical analysis
Results were presented as mean values with their standard errors. All data being tested for normality of distribution using the Shapiro–Wilk test and homogeneity of variance using Levene’s test then were subjected to a one-way ANOVA. Differences between the treatment means were determined using Duncan’s multiple-range test at a P < 0·05 level of significance. Pearson correlation coefficient analysis was conducted using the Bivariate Correlation program. Statistical analyses were done using SPSS 13.0 (SPSS Inc.). Dietary Thr requirement of hybrid catfish was estimated by the quadratic regression method.
Results
Growth performance and muscle composition
All experimental diets were well accepted by the fish. The dietary Thr did not have a significant effect on the survival rate (>97 %) of hybrid catfish. Table 3 shows growth and feed utilisation parameters of hybrid catfish fed diets with graded levels of Thr. Compared with the control group, final body weight, feed intake, feed efficiency and protein efficiency ratio were increased with Thr level increasing up to 15·4, 13·5, 15·4 and 15·4 g/kg diet, respectively (P < 0·05). Fish fed diets containing 13·5 and 15·4 g/Thr per kg diet had higher PWG and specific growth rate than those fish fed the control diet (P < 0·05). Based on the quadratic regression analysis of specific growth rate, the dietary Thr requirement of hybrid catfish was estimated to be 13·77 g/kg of the diet, corresponding to 33·40 g/kg of dietary protein (Fig. 1). Fish fed diet containing 13·5 g/Thr per kg had higher muscle protein content than those fish fed the control diet (Table 4, P < 0·05). The Thr treatment had no effects on muscle moisture, lipid and ash contents (Table 4, P > 0·05).
Table 3. Initial body weight (IBW, g/fish), final body weight (FBW, g/fish), percentage weight gain (PWG, %), specific growth rate (SGR, %/d), feed intake (FI, g/fish), feed efficiency (FE) and protein efficiency ratio (PER) of hybrid catfish fed diets with graded levels of Thr (g/kg) for 56 d
(Mean values with their standard errors of three replicates, while quadratic regression was run with the triplicate data points)
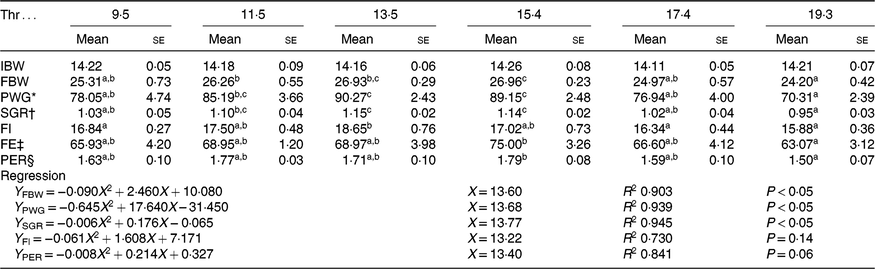
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* PWG = weight gain (g)/initial weight (g) × 100.
† SGR = (ln FBW − ln IBW)/d × 100.
‡ FE = weight gain (g)/feed intake (g) × 100.
§ PER = weight gain (g)/protein intake (g).
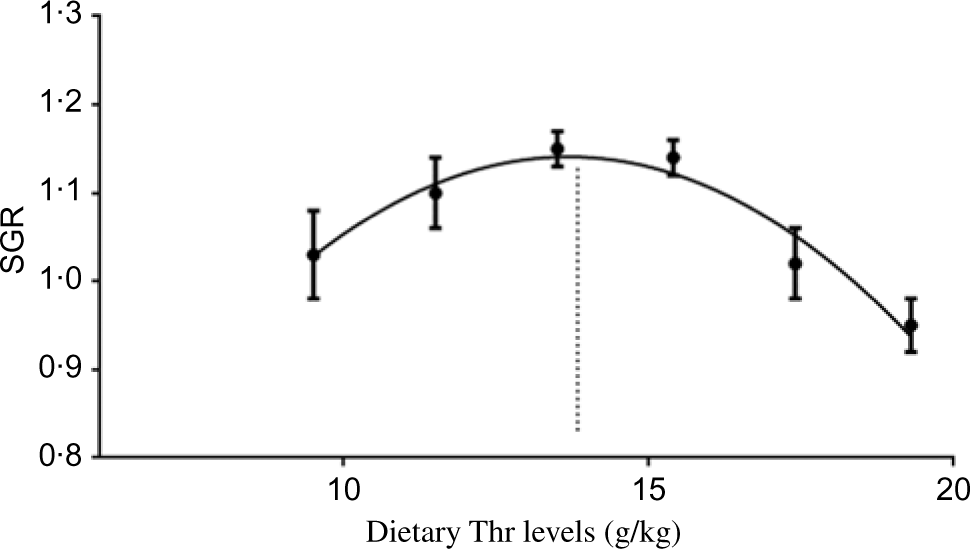
Fig. 1. Quadratic regression analysis of specific growth rate (SGR) for hybrid catfish fed diets containing graded levels of Thr for 56 d. Y = − 0·0064X 2 + 0·1763X − 0·0647; R 2 0·9446; X = 13·77.
Table 4. Muscle composition of hybrid catfish fed diets with graded levels of Thr (g/kg) for 56 d
(Mean values with their standard errors)
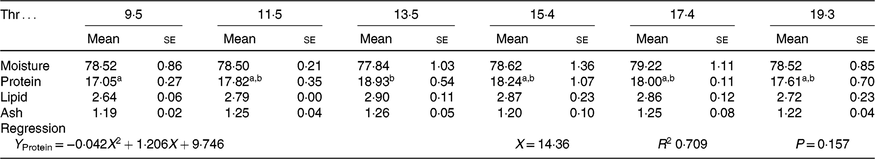
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
Muscle growth-related gene mRNA expression
The relative gene mRNA expressions of GH, IGF-1, IGF-2 and IGF-1 receptor are displayed in Fig. 2. Fish fed diets containing 11·5, 13·5 and 15·4 g/Thr per kg had higher GH mRNA level in pituitary than those fish fed the other diet groups (P < 0·05). Compared with the control group, liver IGF-1 mRNA level was higher in fish fed 13·5 and 15·4 g/Thr per kg diets (P < 0·05). However, dietary Thr did not significantly affect IGF-2 and IGF-1 receptor mRNA levels in fish liver (P > 0.05). As shown in Fig. 3, fish fed diet containing 17·4 g/Thr per kg had higher mRNA levels of PCNA and Myf5 in muscle than those fish fed the control diet (P < 0·05). In comparison with the control group, muscle mRNA levels of MyoD and MyHC were higher in fish fed 11·5 and 13·5 g/Thr per kg diets (P < 0·05). Fish fed 13·5 g/Thr per kg diet had higher MyoG mRNA level in muscle than those fish fed other diet groups (P < 0·05). Dietary Thr level (>9·5 g/Thr per kg diet) significantly up-regulated Mrf4 mRNA expression. Muscle myostatin mRNA levels were higher in fish fed the control diet (P < 0·05), and no significant differences were found among other groups (P > 0·05).
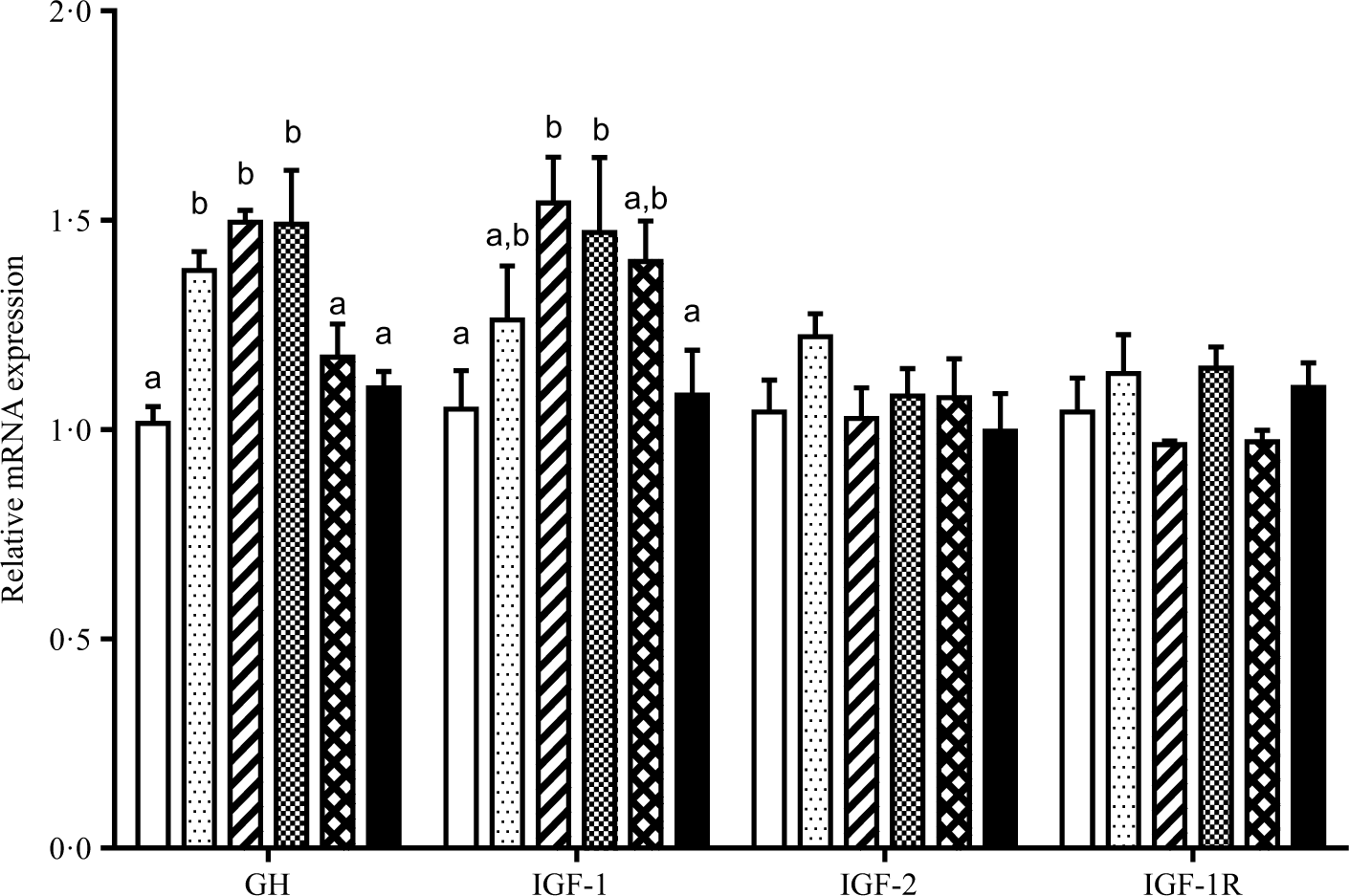
Fig. 2. Effects of dietary Thr on growth hormone (GH) in pituitary, insulin-like growth factor 1 (IGF-1) and IGF-2 in liver, and IGF-1 receptor (IGF-1R) in muscle gene expressions of hybrid catfish. Values are means with their standard errors, of three replicates, with six fish in each replicate. a,b Mean values with unlike letters were significantly different (P < 0·05). (![]() ), 9·5; (
), 9·5; (![]() ), 11·5; (
), 11·5; (![]() ), 13·5; (
), 13·5; (![]() ), 15·4; (
), 15·4; (![]() ), 17·4; (
), 17·4; (![]() ), 19·3 g Thr/kg.
), 19·3 g Thr/kg.
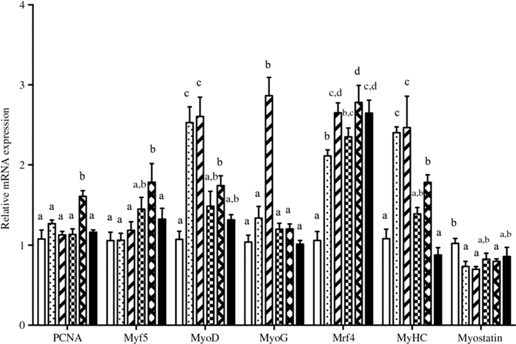
Fig. 3. Effects of dietary Thr on proliferating cell nuclear antigen (PCNA), myogenic factor 5 (Myf5), myoblast determination protein (MyoD), myogenin (MyoG), myogenic regulatory factor 4 (Mrf4), myosin heavy chain (MyHC) and myostatin gene expressions in the muscle of hybrid catfish. Values are means with their standard errors, of three replicates, with six fish in each replicate. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05). ![]() ), 9·5; (
), 9·5; (![]() ), 11·5; (
), 11·5; (![]() ), 13·5; (
), 13·5; (![]() ), 15·4; (
), 15·4; (![]() ), 17·4; (
), 17·4; (![]() ), 19·3 g Thr/kg.
), 19·3 g Thr/kg.
Protein synthesis-related gene mRNA and protein expression in muscle
As shown in Fig. 4, fish fed 13·5 and 15·4 g/Thr per kg diets had higher PI3K mRNA level than those fish fed the control diet (P < 0·05). Dietary Thr level (>9·5 g/Thr per kg diet) significantly up-regulated AKT mRNA expression. The TOR mRNA level was higher in fish fed 11·5 and 13·5 g/Thr per kg diets than those fish fed other diet groups (P < 0·05). Inversely, the 4E-BP mRNA level was lower in fish fed Thr at 13·5 g/kg diet (P < 0·05). The S6K1 mRNA level was gradually increased with increasing dietary Thr levels up to 17·4 g/kg diet and decreased thereafter (P < 0·05). As shown in Fig. 5, compared with the control group, fish fed diets containing 13·5 and 19·3 g/Thr per kg increased the level of phospho-AKT:total AKT (P < 0·05). The level of phospho-TOR:total TOR was the highest for fish fed 13·5 g/Thr per kg diet, then followed by 19·3 g/Thr per kg diet, and the lowest for fish fed the control diet (P < 0·05).

Fig. 4. Effects of dietary Thr on phosphoinositide 3-kinase (PI3K), protein kinase B (AKT), target of rapamycin (TOR), 4E-binding protein (4E-BP) and S6 kinase 1 (S6K1) gene expressions in muscle of hybrid catfish. Values are means with their standard errors, of three replicates, with six fish in each replicate. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05). (![]() ), 9·5; (
), 9·5; (![]() ), 11·5; (
), 11·5; (![]() ), 13·5; (
), 13·5; (![]() ), 15·4; (
), 15·4; (![]() ), 17·4; (
), 17·4; (![]() ), 19·3 g Thr/kg.
), 19·3 g Thr/kg.
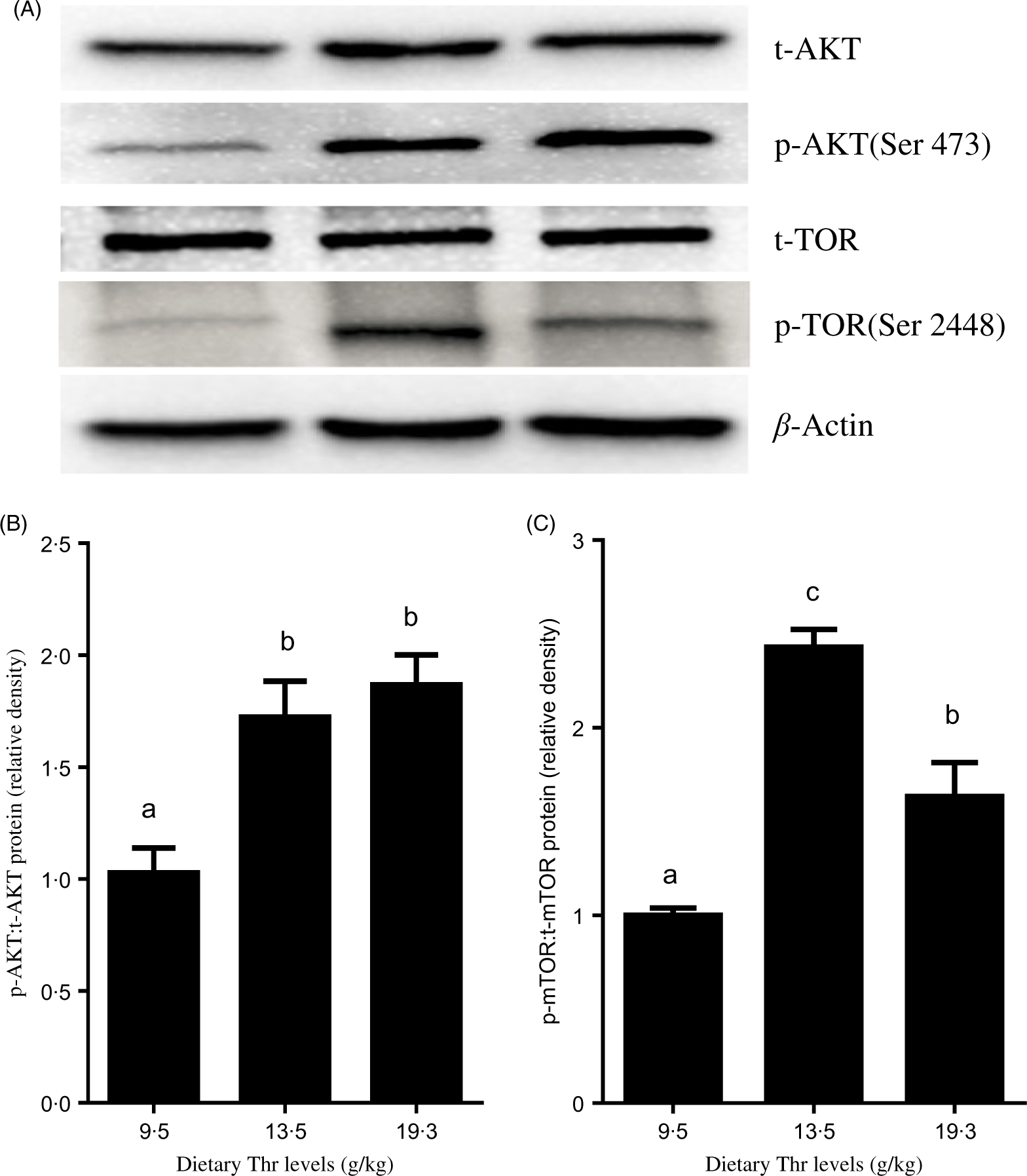
Fig. 5. Effects of dietary Thr on the protein kinase B (AKT)/target of rapamycin (TOR) signalling pathway in the muscle of hybrid catfish. The total AKT (t-AKT) and phospho-AKT (p-AKT) (A and B) and total TOR (t-TOR) and phospho-TOR (p-TOR) (A and C) protein levels were determined by Western blot analysis. Equal loading was monitored with anti-β-actin antibody. Values are means with their standard errors, of three replicates, with three individuals in each replicate. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
Antioxidant-related parameters in muscle
As shown in Table 5. The contents of MDA and PC were decreased with the increasing dietary Thr level up to 13·5 g/kg diet and then increased with further increasing of dietary Thr levels (P < 0·05). Fish fed 13·5 g/Thr per kg diet had higher CAT activity than those fish fed 19·3 g/Thr per kg diet (P < 0·05). Compared with the control group, GST activity was increased in fish fed 11·5, 13·5 and 15·4 g/Thr per kg diets (P < 0·05). The activity of GR was a maximum for fish fed 15·4 g/Thr per kg diet and minimal for fish fed 19·3 g/Thr per kg diet (P < 0·05). The GSH content was increased with the increasing dietary Thr level up to 15·4 g/Thr per kg diet (P < 0·05) and plateaued thereafter (P > 0·05). No significant difference in SOD, GPx, anti-superoxide anion and anti-hydroxy radical activities among treatments was detected (P > 0·05).
Table 5. Malondialdehyde (MDA, nmol/mg protein), protein carbonyl (PC, nmol/mg protein) and GSH (mmol/g tissue) contents and superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), glutathione peroxidase (GPx), glutathione reductase (GR), anti-superoxide anion (ASA) and anti-hydroxyl radical (AHR) activities (U/mg protein) in the muscle of hybrid catfish fed with graded levels of Thr (g/kg) for 56 d
(Mean values with their standard errors of three replicates with six fish in each replicate)
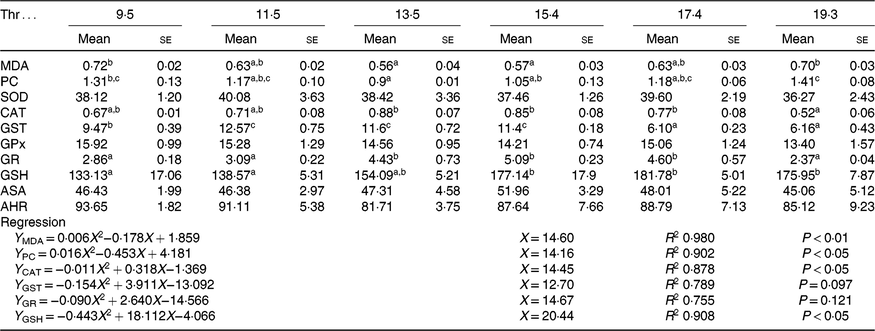
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
As shown in Figs. 6 and 7. Fish fed diet containing 19·3 g/Thr per kg had lower level of CuZnSOD mRNA than those fish fed diets with other Thr levels (P < 0·05). Significantly lower level of CAT mRNA was found in fish fed the control and 19·3 g/kg Thr diets. The GST, GPx and γ-glutamylcysteine ligase catalytic mRNA levels gradually increased with increasing Thr levels up to 15·4, 15·4 and 13·5 g/kg diet, respectively, and decreased thereafter (P < 0·05). The Nrf2 mRNA level was the maximum for fish fed the diet of 13·5 g/Thr per kg and was the minimum for fish fed the diet of 19·3 g/Thr per kg (P < 0·05). Interestingly, the Keap1 mRNA level showed the opposite of trend with respect to Nrf2 mRNA level (P < 0·05).
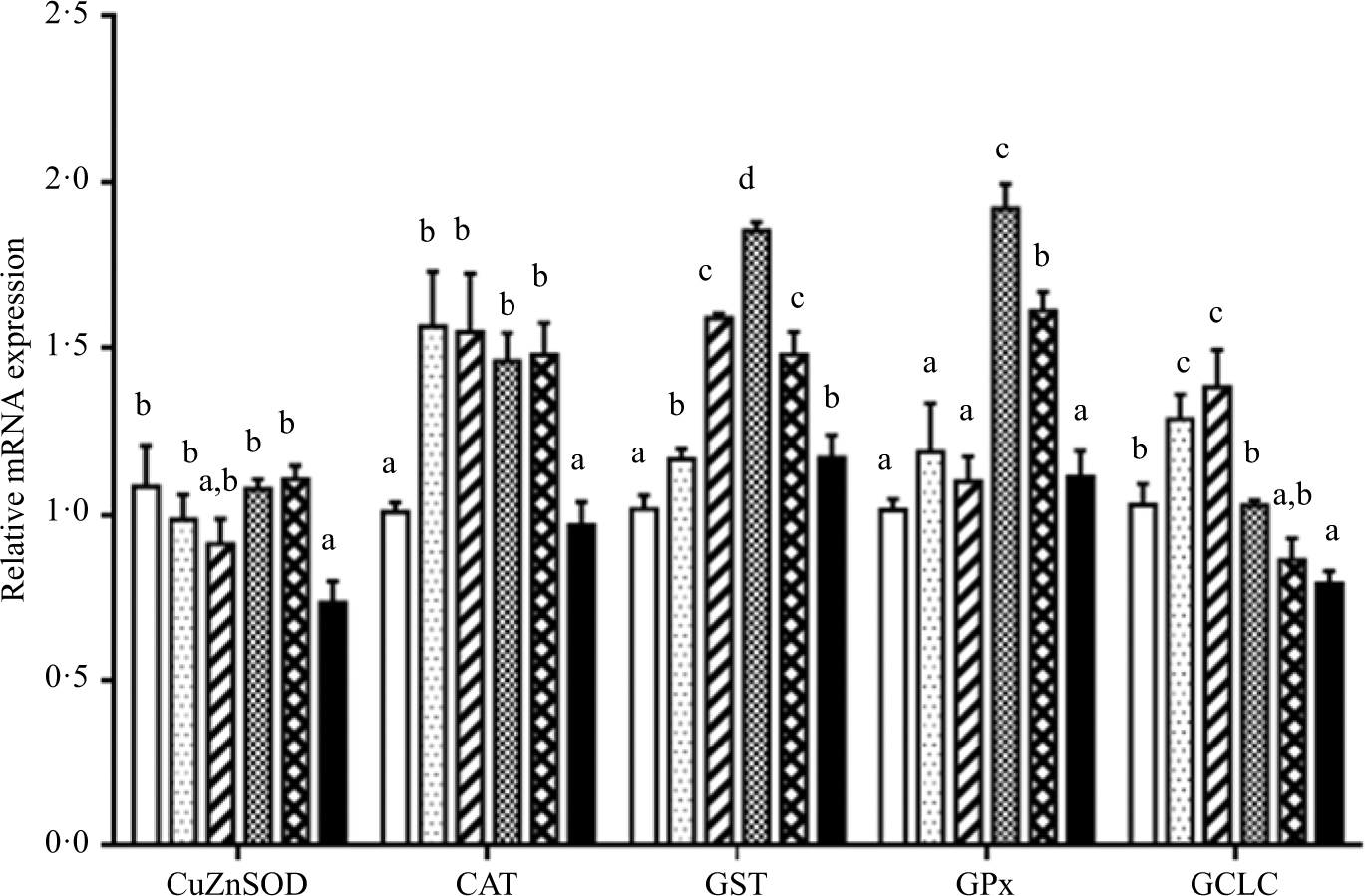
Fig. 6. Effects of dietary Thr on copper,zinc-superoxide dismutase (CuZnSOD), catalase (CAT), glutathione-S-transferase (GST), glutathione peroxidase (GPx) and γ-glutamylcysteine ligase catalytic subunit (GCLC) gene expressions in the muscle of hybrid catfish. Values are means with their standard errors, of three replicates, with six fish in each replicate. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05). (![]() ), 9·5; (
), 9·5; (![]() ), 11·5; (
), 11·5; (![]() ), 13·5; (
), 13·5; (![]() ), 15·4; (
), 15·4; (![]() ), 17·4; (
), 17·4; (![]() ), 19·3 g Thr/kg.
), 19·3 g Thr/kg.
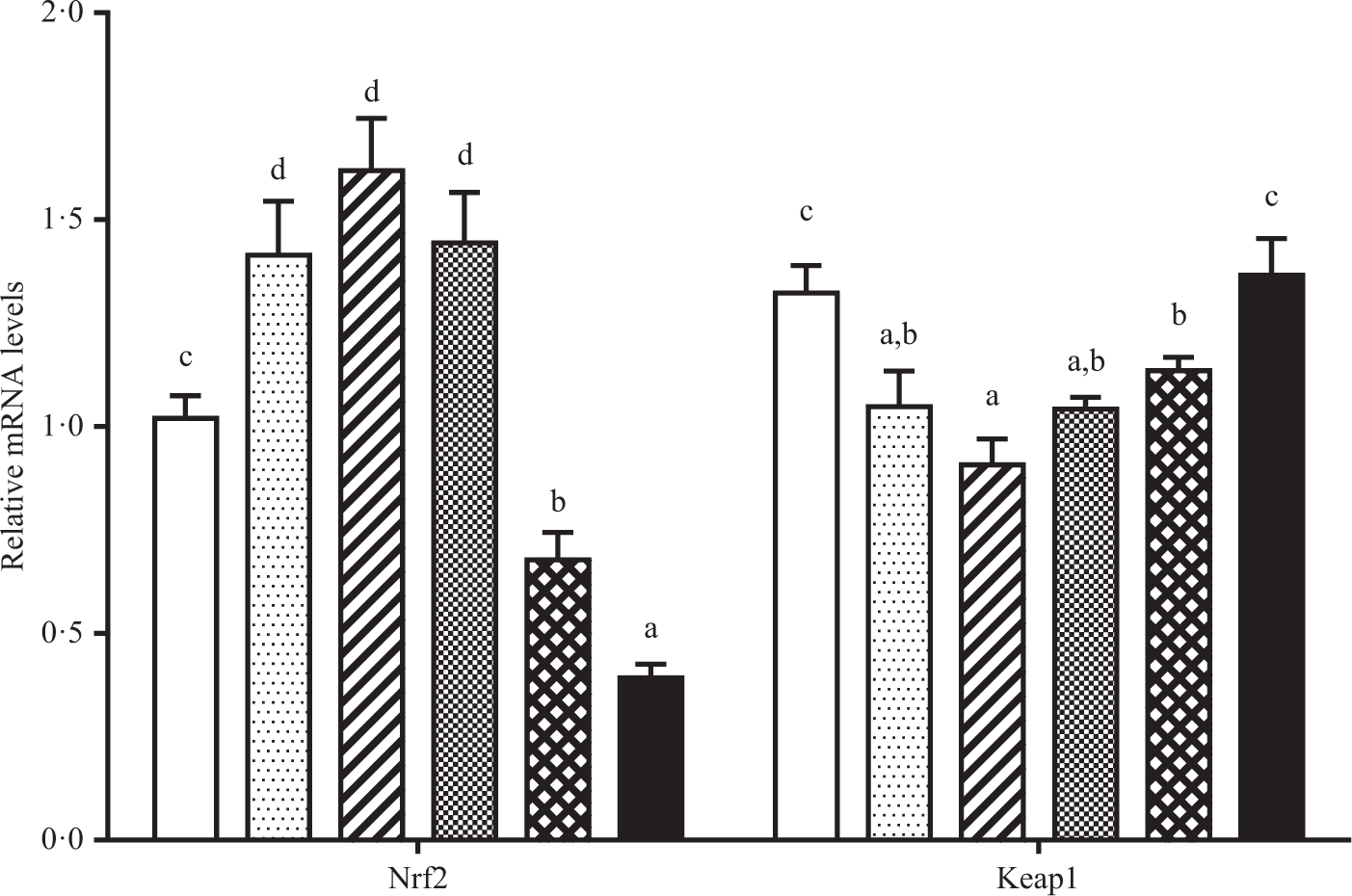
Fig. 7. Relative mRNA expressions of NFE2-related factor 2 (Nrf2) and Kelch like ECH associated protein 1 (Keap1) in the muscle of hybrid catfish fed diets containing graded levels of Thr for 56 d. Values are means with their standard errors, of three replicates, with six fish in each replicate. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05). (![]() ), 9·5; (
), 9·5; (![]() ), 11·5; (
), 11·5; (![]() ), 13·5; (
), 13·5; (![]() ), 15·4; (
), 15·4; (![]() ), 17·4; (
), 17·4; (![]() ), 19·3 g Thr/kg.
), 19·3 g Thr/kg.
Discussion
As an essential amino acid, dietary Thr level had a clear effect on growth and feed utilisation of hybrid catfish. The dietary Thr requirement of hybrid catfish (14·19–25·77 g) was estimated to be 13·77 g/kg of the diet, corresponding to 33·40 g/kg of dietary protein (Fig. 1). This value (g/kg of dietary protein) was close to that (36·10 g/kg of dietary protein) reported in grass carp(Reference Gao, Yang and Liu13), and Japanese flounder Paralichthys olivaceus (32·20 g/kg of dietary protein)(Reference Alam, Teshima and Koshio43), lower than that reported in Jian carp Cyprinus carpio (51·30 g/kg of dietary protein)(Reference Feng, Peng and Wu9), and blunt snout bream Megalobrama amblycephala (46·20 g/kg of dietary protein)(Reference Habte-Tsion, Liu and Ren44). These discrepancies might be ascribed to the differences in genetics of species, selection of dietary protein source, growth environment or growth stages. Correlation analysis showed that PWG was positively related to feed intake (r +0·835, P = 0·039, Table 6) and feed efficiency (r +0·865, P = 0·026, Table 6), suggesting that the improved growth by Thr was partly due to increased feed intake and feed utilisation, which was also observed in Jian carp(Reference Feng, Peng and Wu9). Fish weight gain is primarily attributed to the accretion of protein and fat(Reference Bureau, Azevedo and Tapia-Salazar45). Muscle protein synthesis in teleostean fish contributes to 50 % of fish growth(Reference Mommsen16). The present study showed that Thr significantly enhanced hybrid catfish muscle protein content, indicating that Thr has beneficial effects on protein synthesis and muscle growth, which were in accordance with the results for young pigs, rats and broiler chickens(Reference Wang, Qiao and Yin7,Reference Sidransky and Verney46,Reference Ciftci and Ceylan47) .
Table 6. Correlation analysis of parameters in the muscle of hybrid catfish

PWG, percentage weight gain; FI, feed intake, FE, feed efficiency; IGF-1, insulin-like growth factor 1; GH, growth hormone; MyoD, myoblast determination protein; MyoG, myogenin; MyHC, myosin heavy chain; TOR, target of rapamycin; PCNA, proliferating cell nuclear antigen; Myf5, myogenic factor 5; Mrf4, myogenic regulatory factor 4; MDA, malondialdehyde; PC, protein carbonyl; CAT, catalase; GPx, glutathione peroxidase; GST, glutathione-S-transferase; GCLC, γ-glutamylcysteine ligase catalytic subunit; Nrf2, NFE2-related factor 2; Keap1, Kelch like ECH associated protein 1.
The lower PWG observed in fish fed 9·5 g/Thr per kg diet or 19·3 g/Thr per kg diet compared with that of fish fed 13·5 g/Thr per kg diet indicated that both excess and insufficiency of Thr in diets could induce a decrease in growth performance of hybrid catfish, being in agreement with the results reported in Jian carp(Reference Feng, Peng and Wu9), grass carp(Reference Gao, Yang and Liu13), Japanese flounder(Reference Alam, Teshima and Koshio43) and blunt snout bream(Reference Habte-Tsion, Liu and Ren44). Fish growth is regulated by the GH/IGF axis(Reference Picha, J Turano and Tipsmark48). Several of the components of the GH and IGF system described in mammals have been isolated, characterised and assessed in fish(Reference Mckay, Trautner and Smith49–Reference Zou, Kamei and Modi53). In the present study, relative expressions of GH and IGF-1 gene were up-regulated by Thr. Similarly, studies in growing pigs and rats have demonstrated that a low-Thr diet significantly reduced plasma IGF-1 levels(Reference Katsumata, Kawakami and Kaji54,Reference Takenaka, Oki and Takahashi55) . The previous study also observed dose dependency of IGF-1 mRNA expression to Thr concentrations in cultured pig hepatocytes(Reference Brameld, Gilmour and Buttery56). Correlation analysis showed that PWG was positively correlated with mRNA levels of GH (r +0·902, P = 0·014) and IGF-I (r +0·765, P = 0·076), which partially explained the variations in PWG values among different experimental treatments. This further demonstrated that dietary Thr levels can affect the expressions of pituitary GH and hepatic IGF-1 to alter fish growth in hybrid catfish.
In fish, the majority of growth is invested in accretion of muscle tissue, since muscle may account for more than half of the fish’s body mass. As mammals, the GH/IGF axis is the most important endocrine system regulating muscle growth in fish(Reference Fuentes, Valdés and Molina17). GH exerts its action directly via binding to the GH receptor in its target tissues such as muscle or indirectly via local GH-induced IGF-1 production. The present results showed that dietary Thr had GH-stimulating effects and increased IGF-1 mRNA level. Correlation analysis showed that GH was positively correlated with mRNA level of IGF-1 (r +0·841, P = 0·036), which suggested that the up-regulated IGF-1 mRNA expression by Thr was partly due to increased GH expression. Fish muscle growth is complicated and precisely controlled processes, including proliferation and differentiation of myoblasts(Reference Johnston57). IGF-1 stimulates both proliferation and differentiation of myoblasts(Reference Coolican, Samuel and Ewton58) to promote muscle growth in fish(Reference Rius-Francino, Acerete and Jiménez-Amilburu59,Reference Montserrat, Capilla and Navarro60) . PCNA is a DNA polymerase δ associated peptide that is synthesised early in the G1 and S-phase of the cell cycle, which expression is correlated with DNA synthesis(Reference Bravo, Frank and Blundell61,Reference Baserga62) . Expression of PCNA in association with MyoD in myogenic cells is a marker of myogenic progenitor cell activation(Reference Yablonka-Reuveni and Rivera63). During myogenesis, the MyoD and Myf5 are required for the initial specification of the myogenic lineage, while MyoG and Mrf4 are activated during myoblast differentiation and cell fusion(Reference Cornelison, Olwin and Rudnicki64). MyHC plays important roles in fish muscle growth via hyperplasia and hypertrophy of muscle fibres(Reference Biga and Goetz65). The present results for the first time show that dietary Thr up-regulated PCNA, Myf5, MyoD, Mrf4, MyoG and MyHC mRNA expressions. Correlation analysis indicated that the IGF-1 was positively correlated with MyoD (r 0·604, P = 0·204), MyoG (r 0·658, P = 0·155) and MyHC (r 0·656, P = 0·157) mRNA levels, indicating that dietary Thr increasing the muscle growth in fish might be partly related to up-regulated IGF-1 transcription. Myostatin acts as a negative regulator, which inhibits satellite cell proliferation during muscle development and growth(Reference Seiliez, Sabin and Gabillard66). In the present study, the myostatin mRNA level followed an opposite pattern to MyoD mRNA level. Correlation analysis indicated that the myostatin was negatively correlated with GH (r −0·767, P = 0·075) and IGF-I (r −0·726, P = 0·102) mRNA expressions, suggesting that the dietary Thr decreasing myostatin might partly be associated with the increased GH and IGF-I mRNA levels in fish muscle. Similar results were found in rainbow trout and gilthead sea bream Sparus aurata (Reference Vélez, Perelló and Azizi67,Reference Gahr, Vallejo and Weber68) . All the above results indicate that there may be a relationship between the improvement of the MRF and the enhanced IGF-1 mRNA level by Thr. However, more studies are required to elucidate a more detailed mode in which Thr regulated muscle growth-related gene expression in fish.
In addition to MRF, the PI3K/AKT pathway plays crucial roles in fish muscle protein synthesis and myoblast differentiation and hypertrophy(Reference Fuentes, Björnsson and Valdés30,Reference Montserrat, Sánchez-Gurmaches and Serrana69) . AKT phosphorylation is an important marker of the activation of the PI3K/AKT pathway and muscle growth(Reference Rommel, Bodine and Clarke70). Despite their significance however, their regulation by nutrients remains poorly understood in fish. The present study demonstrated for the first time dietary Thr supplementation resulted in up-regulation of PI3K and AKT mRNA expressions. The phospho-AKT:total AKT ratio was increased by dietary Thr, indicating that dietary Thr increased muscle protein synthesis via the PI3K/AKT signalling pathway. TOR, a downstream component of the PI3K/AKT pathway, promotes cellular growth by stimulating protein synthesis via 4E-BP and S6K in fish(Reference Trevino, George and Hughes31). The present data showed that dietary Thr supplementation increased muscle TOR and S6K1 mRNA levels and the phosphorylation of TOR. This result was in good agreement with reports on rainbow trout Oncorhynchus mykiss and gilthead sea bream myocytes, which reported that Thr could stimulate TOR phosphorylation and gene expressions of its downstream effectors(Reference Vélez, Lutfi and Jiménez-Amilburu71,Reference Seiliez, Gabillard and Skiba-Cassy72) . The correlation analysis also indicated that muscle protein content was positively correlated with PI3K (r 0·764, P = 0·077), AKT (r 0·681, P = 0·137), TOR (r 0·633, P = 0·177) and S6K1 (r 0·633, P = 0·178) mRNA levels. A negative correlation was observed between muscle protein content and 4E-BP (r −0·892, P = 0·017) mRNA level. These results suggested that dietary Thr increasing muscle protein content might be partly related to elevate muscle protein synthesis via the PI3K/AKT/TOR signalling pathway. Meanwhile, the present results also showed IGF-I was positively correlated with mRNA expressions of PI3K (r 0·882, P = 0·020), AKT (r 0·770, P = 0·073) and S6K1 (r 0·782, P = 0·066), which suggested that dietary Thr promotes muscle protein synthesis by activating the PI3K/AKT/TOR signalling pathway via IGF-I. Similar results were observed in muscle of rainbow trout and fine flounder(Reference Fuentes, Björnsson and Valdés30,Reference Montserrat, Sánchez-Gurmaches and Serrana69) . Moreover, muscle protein mass is regulated primarily through alterations in protein synthesis(Reference Millward, Garlick and Nnanyelugo73). TOR is essential for satellite cell function and skeletal muscle regeneration through controlling the expression of myogenic genes(Reference Zhang, Liang and Shan74). Rion et al. reported mTOR controls embryonic and adult myogenesis via mTORC1(Reference Rion, Castets and Lin75). As a result, the rate of whole-body growth can be delineated from an assessment of muscle protein synthesis. The present study showed that a positive correlation was observed between the AKT, TOR and S6K1 and muscle growth-related gene mRNA levels (Table 6). These findings suggest that, as in mammals, the Thr could activate the PI3K/AKT/TOR signalling pathways via IGF-I and contribute to muscle protein synthesis and growth in fish.
In fish, PC and MDA contents are widely used as markers for protein oxidation and lipid peroxidation, respectively(Reference Jiang, Wu and Zhou76). Increasing evidence indicates that the efficiency of myogenic differentiation is reduced by oxidative damage(Reference Barbieri and Sestili27,Reference Ardite, Barbera and Roca77,Reference Kozakowska, Pietraszek-Gremplewicz and Jozkowicz78) . In the present study, both PC and MDA contents in fish muscle were decreased by Thr. The correlation analysis indicated that muscle PC and MDA contents were negatively correlated with MyoD (r PC = −0·687, P = 0·131; r MDA = −0·656, P = 0·157), MyoG (r PC = −0·807, P = 0·052; r MDA = −0·753, P = 0·081) and MyHC (r PC = −0·747, P = 0·088; r MDA = −0·675, P = 0·141) mRNA levels. These results suggested that Thr may improve muscle growth via suppressing oxidative damage in fish. However, no information is available to date about the effect of Thr on oxidative damage in fish muscle. Recent studies from our laboratory showed that lipid peroxidation and protein oxidation in intestine and gill could be effectively prevented by Thr in grass carp(Reference Hong, Jiang and Kuang24,Reference Dong, Feng and Jiang29) . Fish antioxidant systems are composed of antioxidant enzymes (SOD, CAT, GPx, GST and GR) and non-enzymatic compounds (GSH)(Reference Martínez-Álvarez, Morales and Sanz79,Reference Srikanth, Pereira and Duarte80) . The present study showed that CAT, GST and GR activities in muscle were increased by dietary Thr. Similar results are found in the intestine of grass carp(Reference Hong, Jiang and Kuang24). Antioxidant enzyme activities were closely related to their mRNA levels in fish(Reference Jiang, Wu and Zhou76). The present study indicated that dietary Thr increased mRNA levels of CAT and GST in muscle, which showed the same trend to their enzyme activities. The cellular pool of GSH is maintained by the activity status of the enzyme γ-glutamylcysteine ligase catalytic subunit(Reference Hirzel, Lindinger and Maseneni81). The present study showed dietary Thr also up-regulated the muscle γ-glutamylcysteine ligase catalytic mRNA levels. Nrf2 is a key regulator to transcriptions of antioxidant enzymes, which could be prevented by blinding to Keap1 from translocation into the nucleus in fish(Reference Ma82,Reference Giuliani and Regoli83) . When activated, Nrf2 is bound to antioxidant transcription elements in the promoter regions of phase 2 detoxification enzyme genes and certain antioxidant genes(Reference Ma82,Reference Jiang, Liu and Jiang84) . Keap1 is identified as a Nrf2-binding protein that prevents Nrf2 translocation to the nucleus and promotes the ubiquitination-proteasomal degradation of Nrf2(Reference Kaspar, Niture and Jaiswal85). In the present study, dietary Thr supplementation elevated Nrf2 mRNA expression and blocked the increase in Keap1 mRNA expression in muscle of hybrid catfish. Similar results were found in gills of grass carp fed with diets containing graded dietary Thr levels(Reference Dong, Feng and Jiang29). Correlation analysis indicated that CAT (r 0·786, P = 0·064) and GST (r 0·948, P = 0·004) activities were positively correlated with Nrf2 mRNA level, whereas muscle Keap1 mRNA level was negatively correlated with CAT (r −0·887, P = 0·018), GST (r −0·678, P = 0·139) and GR (r −0·722, P = 0·105) activities. These results suggested that increased antioxidant enzyme activities by Thr might partly attribute to the Nrf2/Keap1 signalling in fish muscle. Thus, dietary Thr could improve muscle growth (myogenic differentiation) via suppressing oxidative damage through regulating the Nrf2/Keap1 signalling pathway to elevate antioxidative capacity.
In summary, the present work showed that dietary Thr improved the growth of hybrid catfish. For the first time, we found that dietary Thr up-regulated muscle growth-related gene (GH, IGF-1, PCNA, Myf5, MyoD, MyoG, Mrf4 and MyHC) expression, improved muscle protein content via the AKT/TOR signalling pathway. Furthermore, Thr supplementation improved muscle antioxidant capacity via regulating the Nrf2/Keap1 signalling pathway, reducing oxidative damage and promoted muscle growth. Based on the quadratic regression analysis of specific growth rate, the dietary Thr requirement of hybrid catfish (14·19–25·77 g) was estimated to be 13·77 g/kg of the diet, corresponding to 33·40 g/kg of dietary protein.
Acknowledgements
The authors would like to express their sincere thanks to the personnel of these teams for their kind assistance.
The present study was financially supported by National Natural Science Foundation of China (31702362 and 31601952), the Applied Basic Research Programs of Science and Technology Commission Foundation of Sichuan Province, China (2015JY0067) and Sichuan Province Science and Technology Support Program, China (2014FZ0026).
Y. Z. and Q. J. conducted the trial, performed the RT-PCR experiments and wrote the manuscript. J. J. and X.-Q. Z. contributed to the design of the study. L. F. and W.-D. J. assisted in the manuscript preparation. S.-X. X. and J. Z. assisted with all data analysis. Y. L. and P. W. assisted with the trail.
There are no conflicts of interest.
















