Overweight and obesity have become a public health problem worldwide, particularly in children and adolescents(Reference Di Cesare, Sorić and Bovet1). Researchers and public health managers can assess the magnitude of this problem with various anthropometric indicators, such as BMI, waist circumference and subcutaneous fat measured as skinfold thickness (SFT). Up till now, BMI is still recommended as the most useful indicator to classify overweight and obesity in children and adolescents(Reference de Onis, Onyango and Borghi2). However, BMI is unable to distinguish between fat and lean mass(Reference Buss3). On the other hand, investigators widely use SFT measurements to assess body fat due to its low cost, simple and non-invasive procedure(Reference González Jiménez4). The two most frequently taken skinfold measurements are from triceps and sub-scapular sites(Reference González Jiménez4), but triceps SFT (TSFT) gives ‘the best results for obesity screening in adolescents aged 10–15 years’(Reference Sardinha, Going and Teixeira5). Bandini et al. even observed that the triceps skinfold predicted up to 68 %, whereas BMI predicted only 38 % of the body fat amount(Reference Bandini, Vu and Must6).
The literature has established the influence of adolescent physical activity (PA) on body fatness through to adulthood(Reference May, Bueno-de-Mesquita and Boshuizen7). Also, the relationship between changes in PA changes and BMI during adolescence is well documented(Reference Kimm, Glynn and Obarzanek8–Reference Cho and Kim10). Recently, a systematic review examining the longitudinal association between PA and body fat during adolescence concluded that PA had a protective effect on body fat with greater protection if practised at higher intensities(Reference Ramires, Dumith and Gonçalves11). As obesity is becoming a public health problem in many countries, weight gain prevention is essential. Changes in PA behaviour might lead to long-term weight control(Reference Field, Haines and Rosner12). An intervention study conducted on Brazilian students aged 10–15 years indicated that programmed PA improved or maintained body composition parameters and reduced overweight and obesity in the intervention group(Reference Farias, Paula and Carvalho13). Another systematic review of the efficacy of exercise intervention also suggested that exercise interventions in overweight and obese adolescents improve body composition, mainly by lowering body fat(Reference Stoner, Rowlands and Morrison14).
Concurrent with the rise in overweight and obesity was increasing sedentary behaviours and decreasing PA among adolescents in Ho Chi Minh City (HCMC)(Reference Trang, Hong and Van der Ploeg15,Reference Trang, Hong and van der Ploeg16) . However, longitudinal information from studies on the relationship between PA and changes in fatness among adolescents, especially Viet Nam youth, is limited. Further, we have yet to confirm the relationship between PA and excess adiposity. Recently, a systematic review conducted by Annette Rauner indicated that there should be the need for longitudinal studies ‘that would reveal the causality between PA and overweight’(Reference Rauner, Mess and Woll17). This paper uses the HCMC Youth Cohort(Reference Trang, Hong and Dibley18) data to examine PA’s effect on the child’s changing body fatness over 5 years.
Methods
Study population
We examined data from the HCMC Youth Cohort, a longitudinal study in urban areas of HCMC, Viet Nam. It aimed to assess the complex relationships in adolescents of changes in adiposity indicators, diet, PA and sedentary behaviours with home, neighbourhood and school microenvironments(Reference Trang, Hong and Dibley18). We selected a sub-sample of grade 6 or 7 students from the cross-sectional survey in 2004(Reference Tang, Nguyen and Dibley19) using systematic random sampling and then followed them annually. The data examined included anthropometry, dietary intake, PA, sedentary behaviours, family environment and parental characteristics. This selection consisted of 362 boys and 395 girls who have had SFT measurements in the 2004 baseline survey. Participation in the study required the consent of both adolescents and their parents. The Research Ethics Committee, Pham Ngoc Thach University of Medicine, HCMC, approved the cohort study (2838/GXN-TÐHYKPNT).
Data collection
While the participants were in junior high schools, we collected the data at school. But when the study participants moved to senior high school, we used individual home follow-up to collect data that the same well-trained data collectors conducted. The anthropometric measurements included weight, height and SFT measurement. Standing height was measured with a portable direct-reading stadiometer to the nearest 0·5 cm using the standard stretch stature method(20). Body weight was measured with shoes and heavy clothes removed using a digital scale to the nearest 0·1 kg. BMI was calculated as weight in kg/m2. Trained doctors from the Nutrition Center took the SFT at triceps, sub-scapular, abdominal and mid-calf measurements twice to the nearest millimetre for each child using Harpenden Skinfold Calipers. When a discrepancy of more than 2 mm occurred in the measurements at one site, the data collectors took an additional measurement. Then we took the average of all measurements at each site to calculate the mean site-specific SFT. We used a standard measurement method(Reference Lohman, Roche and Martorell21) for all anthropometric measurements. However, in this paper, we focus the analysis on triceps and sub-scapular skinfolds and BMI. We chose to use TSFT as the primary measure of body fatness since the findings of previous studies indicated that triceps skinfold was the best indicator of percentage body fat in children(Reference Roche, Sievogel and Chumlea22) or most highly correlated with percentage body fat(Reference Ku, Shapiro and Crawford23). Sub-scapular SFT (SSFT) was an additional outcome because this measure may become a more meaningful indicator of body fatness for boys as they reach early school age(Reference Moore, Nguyen and Rothman24).
The adolescents completed all self-administered questionnaires at school or home. Information on sedentary behaviours was measured using the Adolescent Sedentary Activity Questionnaire(Reference Hardy, Booth and Okely25) validated in Vietnamese adolescents(Reference Tang, Nguyen and van der Ploeg26). It asked students to report the time spent outside of school hours for each day of the week in a range of sedentary activities, including time spent watching television. Our cohort study assessed PA objectively for 7 d with an Actigraph accelerometer (model GT1M) worn on the right hip. Daily moderate to vigorous PA (min/d) was calculated using age-adjusted cut points for accelerometer activity counts(Reference Trost, Pate and Sallis27). Among 362 boys and 395 girls selected from the 2004 baseline measurements, we only included participants who wore the accelerometer for ≥ 8 h/d on at least 4 d in the analysis. This criterion restricted the data to examine the association between PA levels and fat gain over 5 years to 235 boys and 247 girls. Participants included in the study were not different from those excluded in terms of the following characteristics: sex, BMI, age, energy intake, triceps and sub-scapular skinfolds measurements.
Family environment and parental characteristics, including parental BMI, were answered by parents in the family questionnaire form by the first year of the study. Children’s dietary intakes and behaviours were collected using a validated FFQ(Reference Hong, Dibley and Sibbritt28).
Data analysis
We express the results as means and standard deviations. We compared the baseline characteristics by sex and tested any differences using the Student’s t test (or Kruskal–Wallis if needed) and χ 2 tests. We used the International Obesity Task Force BMI cut-off values to define overweight and obesity (total adiposity)(Reference Cole, Bellizzi and Flegal29).
We computed energy and nutrient intakes using EIYOKUN v.1(Reference Hanh, Yoshimura and Takahashi30), a nutrient database developed from Vietnamese food consumption tables(31). We estimated PA from the average number of counts per hour, adjusting for age and sex differences for accelerometry(Reference Trost, Pate and Sallis27). In the absence of a recognised definition of PA levels that define active v. less active, we based the classification on the median physical counts as suggested in a similar study of Moore et al. (Reference Moore, Nguyen and Rothman24). We analysed the change in triceps and sub-scapular skinfolds and BMI over 5 years across PA levels.
We used linear regression analysis to calculate the slopes of each child’s TSFT, SSFT and BMI (from the first year to the last year). We classified them as increasing if the slopes were greater than zero or stable/decreasing if equal or less than zero. The ‘increasing slope’ was then defined as an indicator of fat gain.
We conducted multilevel mixed-effects models to deal with repeated measurements, and the outcome was the slope of triceps with two categories. The following variables were potential confounders in the analysis: child’s sex, average hours of television watched per day and parents’ overweight/obesity status. We categorised the time spent on TV watching according to recommended guidelines (< 2 and ≥ 2 h/d)(32). We classified into two categories, that is, < median v. ≥ median other factors at baseline, such as child’s age, energy intakes and triceps/sub-scapular measurements. We defined overweight or obesity in mothers and fathers according to WHO recommendations for Asians(33).
We assessed the effects of the low levels of activity (defined by below median of the average number of counts) on the fat gain (defined by an increasing slope), using risk ratios and 95 % CI estimated with mixed-effects Poisson regression. We then stratified by baseline level of body fat to examine potential effect measure modification by this factor.
Firstly, we selected the covariates by univariate analysis to have crude models for each of the three outcome variables. We only retained potential confounders, significantly related to the outcomes with P < 0·05 in the crude models to examine in the next step. The final models included the variables which were significantly associated with the outcomes in multivariate analyses. But, the results show only risk ratios of activity effect (in the presence of other significant confounding factors). We examined three outcome variables (i.e. TSFT, SSFT and BMI) but carried out more detailed analyses for TSFT, our primary outcome variable. We conducted all analyses using Stata MP 14·2 (StataCorp 2015, StataCorp LLC).
Results
Overall, we included 235 boys (48·76 %) and 247 girls (51·24 %) with a mean age of 11·8 years old (ranged from 10·6 to 15·7) at the baseline in the data analysis. The percentage of mothers and fathers overweight/obese in this sample was 30·3 and 38·8 %, respectively. As shown in Table 1, the average number of G1TX counts per hour for the entire group was 6·4 (sd 2·6), and for each sex, it was 7·8 (sd 2·7) for boys and 5·0 (sd 1·7) for girls. The average number of boys’ counts per hour was significantly higher than girls (P < 0·001). Boys also had slightly higher energy intakes at the baseline than girls; however, the differences were not significant.
Table 1. Baseline characteristics of the study participants by sex
(Mean values and standard deviations; numbers and percentages; 95 % confidence intervals)
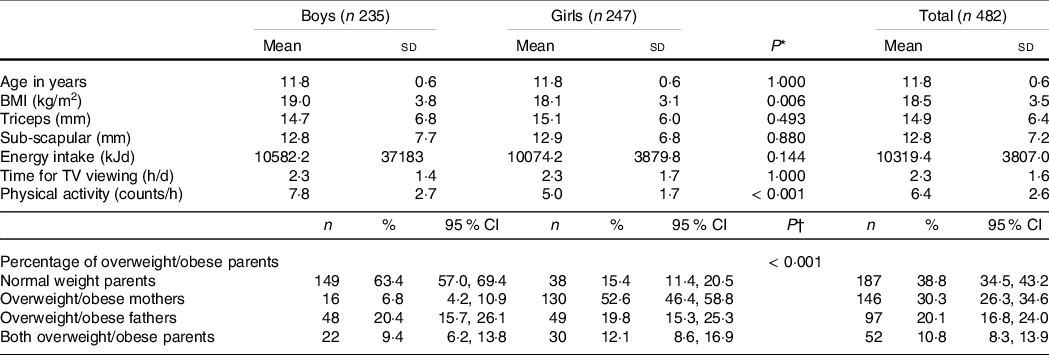
* P-values of t tests to compare data between two sexes.
† P-values of χ 2 test to compare data between two sexes.
Table 1 also found that girls had slightly larger SFT than the boys (though the differences were not significant), but boys’ BMI was significantly higher than girls (P = 0·006).
Table 2 presents the median changes in TSFT for boys and girls over 5-year period. On average, boys gained 0·01 mm while girls gained 0·58 mm in their TSFT during 5-year period. In detail, active boys lost 0·12 mm in their TSFT during the study period, while inactive boys gained 0·28 mm. Active girls gained 0·51 mm in their TSFT from the baseline to the last year compared with 0·61 mm gain for inactive girls. Overall, active students lost 0·03 mm, while inactive students gained 0·55 mm. The change in TSFT varied widely for active and inactive students, from losing 0·54 mm to gaining 1·11 mm.
Table 2. Change in triceps skinfold thickness (in mm) from the first year to the last year of the study participants by sex
(Median values and interquartile range)

* Active: G1TX counts per hour greater than median.
† Inactive: G1TX counts per hour below median.
Table 3 shows the crude risk ratios and 95 % CI for an increasing slope of TSFT, SSFT and BMI for inactive compared with active students: 1·43 (95 % CI 1·22, 1·67); 1·09 (95 % CI 1·00, 1·18); 1·06 (95 % CI 1·02, 1·10), respectively. The effect of PA was stronger and more significant for the slope of TSFT than for those of SSFT and BMI, and this effect was higher in boys than in girls.
Table 3. Crude RR (unadjusted) for the effect of activity level on slopes of anthropometry indices by sex
(Risk Ratios and 95% Confidence Intervals)
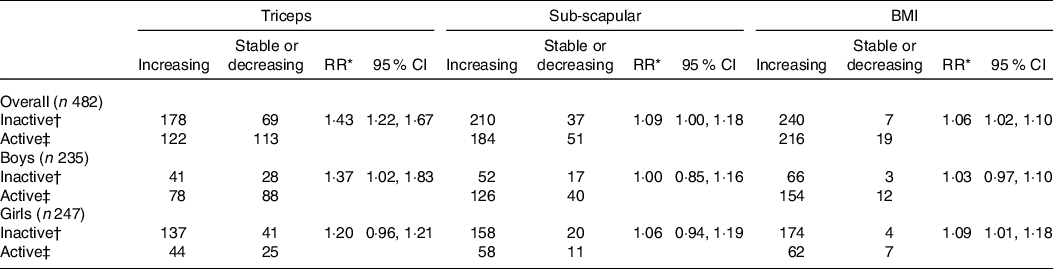
* Crude RR from the multilevel mixed-effects models with the slope of anthropometry (increasing v. stable/decreasing) as dependent variable and activity level (inactive v. active) as the independent variable.
† Inactive: G1TX counts per hour below the median.
‡ Active: G1TX counts per hour greater than the median.
Table 4 shows the Poisson regression results for TSFT slope only in the whole sample and those thinner or heavier than the median at baseline (i.e. baseline triceps ≤ median v. > median). This table examined the effect of PA adjusted for each potential confounder, such as baseline TSFT, parents’ BMI, time spent watching TV, baseline age, baseline energy intake, sex and combined confounding factors. For all subjects, the effect of activity on the slope of TSFT was slightly confounded by parents’ BMI, and sex singly. In the final model, the confounding factors included baseline TSFT, baseline age and baseline energy intake with the adjusted risk ratio = 1·39 (95 % CI 1·19, 1·63). Among those who were leaner at baseline, the combination of baseline age and sex slightly confounded the effect of PA level on fat gain. Leaner subjects had a 1·23-fold increased risk (95 % CI 1·06, 1·42) associated with a low activity level. Among those heavier at baseline, there seemed to be negative confounding by baseline age and sex. The final model results for children with larger TSFT at baseline include baseline age and baseline energy intake, which show an approximately 1·62-fold increased risk of an increasing TSFT slope (95 % CI 1·14, 2·30).
Table 4. Results of adjusted effect (derived from stratified analysis and multivariate analysis) of activity level on slopes of triceps by categories of triceps at baseline
(Risk ratios and 95 % confidence intervals)
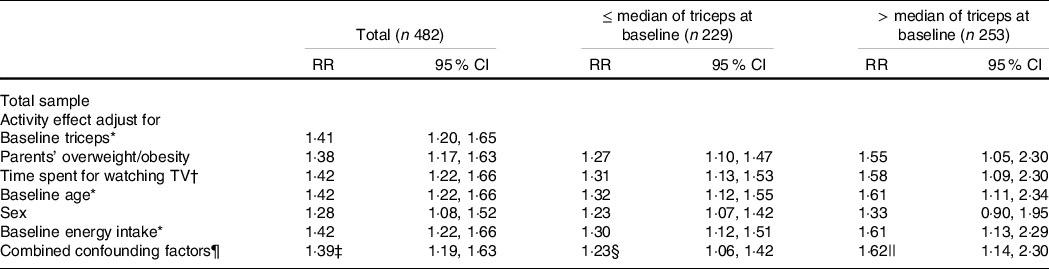
* Categorisation is based on the median value of this variable.
† Categorisation is based on < 2 and ≥ 2 h/d.
‡ The final model includes only baseline triceps, baseline age and baseline energy intake.
§ The final model includes only baseline age and sex.
|| The final model includes only baseline energy intake and baseline age.
¶ Combined confounding factors include the following variables: parents’ BMI, time spent for watching TV, baseline age, baseline energy intake, sex – with baseline triceps (for the total group) or without baseline triceps (for each group stratified by the median of triceps at baseline).
** Crude RR was calculated using mixed-effects models.
Discussion
Our results showed significantly lower changes in BMI, SSFT and especially TSFT among physically active compared with inactive adolescents at baseline from a cohort of junior high school students followed over 5 years. This effect differed between those who were leaner or heavier at baseline. The risk of increasing body fatness associated with low activity levels was lower in thin students than heavier ones. Inactivity had a more adverse effect on junior high school students who already had a more considerable degree of body fatness.
Some systematic reviews indicate that adolescent PA has both short- and long-term impacts on health(Reference Hallal, Victora and Azevedo34). During childhood and adolescence, high PA levels are protective against child and adolescent obesity(Reference Jiménez-Pavón, Kelly and Reilly35,Reference Ramires, Dumith and Gonçalves36) . The first of two systematic reviews used BMI as the only adiposity measure. The other reviews employed different fatness measurement methods because BMI alone incorporates fat and lean mass, which are likely to be influenced by PA in opposite directions(Reference Wells, Coward and Cole37). In our study, we did not use BMI alone. The primary focus of the analysis was the TSFT and SSFT measurements. The results revealed that the effect of PA was stronger for the slope of TSFT than for other outcomes and was modified by sex with a larger effect in boys than in girls. TSFT is widely used to diagnose obesity in children and adolescents(Reference Addo and Himes38–Reference Ramirez-Velez, Lopez-Cifuentes and Correa-Bautista41) because of its higher correlation with total body fat measures than BMI(Reference Roche, Sievogel and Chumlea22,Reference Seltzer, Goldman and Mayer42) and its low cost and easy examiner training. A longitudinal study in Montreal, Canada, examined the relationship between PA and body fat in adolescents aged 12–13 years at baseline. The authors found statistically significant associations between the fluctuation of PA and triceps skinfold for both boys and girls(Reference Belanger, O’Loughlin and Karp43). The results from a large prospective cohort of black and white girls from the USA showed a significant relationship between PA and changes in the sum of SF thickness(Reference Kimm, Glynn and Obarzanek8). Another study in Cameroon found PA to be inversely related to TSFT(Reference Navti, Atanga and Niba44). Earlier studies showed that TSFT was the best clinical measure of adiposity and a predictive measure of body fat in young children(Reference Roche, Sievogel and Chumlea22,Reference Seltzer, Goldman and Mayer42) . This study’s findings confirm the impact of PA on the fat mass index. A previous study(Reference Vasickova, Groffik and Fromel45) explained that ‘higher moderate to vigorous PA had an inverse effect’ on BMI.
An essential question needed an appropriate assessment is sex differences in PA to help develop appropriate health and education policies. Vietnamese adolescent girls are less physically active than boys, and this finding has been consistently reported internationally in many studies(Reference Belanger, O’Loughlin and Karp43,Reference Vasickova, Groffik and Fromel45) . It may be the different involvement in PA between two sexes, such as lower enrolment in organised sports clubs(Reference Vilhjalmsson and Kristjansdottir46), fewer motives for participation and perceived barriers to doing exercise that leads to different PA levels(Reference McMurray, Harrell and Creighton47,Reference Mundt, Baxter-Jones and Whiting48) .
It is well established that overweight or obesity in childhood or adolescence is strongly associated with overweight or obesity in adulthood. In this study, the risk was slightly higher for students with more body fat at baseline. One explanation could be that students with low body fat at baseline had higher metabolic rates at rest in our study, leading to a lower risk of weight gain despite low activity levels. Furthermore, the thinner students at baseline were assumed to have lower levels of energy intake and might also have low levels of energy expenditure; thus, they were less likely to become obese despite having experienced low levels of activity. We examined the energy intake of children in different groups of baseline body fat. We found that children in the lower group of baseline triceps also consumed lower kilocalories per day (9988·0 kJ v. 10668·4 kJ).
The effect of activity level on the slope of triceps skinfolds slightly changed after controlling for every factor such as sex, baseline age, parental BMI status, baseline energy intake and time spent watching TV. In the presence of sex, the risk ratio of the lower level of PA on an increasing slope of triceps became smaller than that from the model examining the crude activity effect. The sex disparity in activity level (girls were less likely to be active than boys) made the PA influence on fat gain different across sexes; however, we do not have data to explain the differences between the sexes. With the parents’ overweight and obesity variable, the confounding effect of activity level changed to a smaller effect. Different PA levels among parents who were overweight/obese or not overweight/obese may affect the relationship between adolescent activity level and fat gain. The combined effect, when including all possible potential confounders, was different from the crude effect, indicating that the development of children and adolescents overweight and obesity ‘involves a complex set of factors from multiple contexts that interact with each other to place a child at risk of overweight’(Reference Davison and Birch49). We should not consider these factors as individual risk or protective factors but instead combined them to determine the risk of developing overweight or fat gain.
This study’s key strength includes its longitudinal design that examined changes in PA and fat gain during adolescence. Another strength of the study was the use of accelerometers to measure PA time objectively. Thus, the accelerometers provide more precise information, which may account for the stronger relation between activity and body fatness.
However, this study also showed some limitations. Firstly, the self-reported/questionnaire-administered measurement of parents’ obesity status, time for watching TV and energy intake may have led to underestimating the risk of increased slopes of triceps sub-scapular skinfolds and BMI. Secondly, the number of subjects we analysed in the secondary data analysis may not be enough to examine the true effect of activity level stratified by body fatness at baseline or allow many covariates in the multivariable regression model. Thirdly, this study’s data did not explain the adjusted risk ratio changes compared with the crude risk ratio with the sex variable in the model, which requires examination in future studies. Additionally, categorising both exposure and outcome into binary variables may limit our ability to identify potential dose–response trends. However, this classification method and the evidence of this effect on fat gain seem easier to understand and use in prevention campaigns. Furthermore, we did not consider the dietary intake in the last year, which may affect fat gain over 5 years among adolescents. Despite these limitations, this is the fırst prospective cohort study to provide data on fat gain measured by different anthropometric indicators, including TSFT over 5 years in Viet Nam’s major urban area and potential confounders among adolescents.
The results from the present study confirm that changes in activity levels of junior high school students during adolescence significantly affected changes in BMI and adiposity. Thus, strategies to prevent adolescent obesity in HCMC should consider the critical role of PA to control this problem in adolescents effectively. There is also a need to implement other longitudinal analysis models that assess changes in PA levels throughout adolescence to establish a relation between PA and body fat rather than analysing PA only at baseline.
Acknowledgements
We gratefully acknowledge the support of the staff from the Pham Ngoc Thach University of Medicine and the Nutrition Centre, Ho Chi Minh City, in data collection and entry. We appreciate and thank the help from Associate Professor Patrick Kelly (University of Sydney) and Associate Professor Steve Bowe (Deakin University) in advices in data analyses.
The Nestlé Foundation, Switzerland, funded grants to support the 2004 survey and the 5-year cohort study.
H. K. T. contributed to study designing, data analysing, preparing the first manuscript and interpreting the results as well as literature reviewing. M. J. D. contributed to interpreting the results, the analytical strategy and revised the manuscript for important intellectual content. Both authors read and approved the final manuscript.
The authors have no conflict of interest to declare for this paper.






