As in other animals and humans, increasing the supply of digestible energy promotes weight gain in fish. Fish use a large proportion of dietary proteins to cover their energy requirements. As protein sources are costly ingredients, research has been undertaken in order to increase the dietary input of non-protein digestible energy. Dietary carbohydrates are less efficiently utilised as an energy source by high trophic-level fish species such as salmonids than by low trophic-level fish species. In contrast, dietary lipids are highly digestible and constitute a major source of energy in salmonid diets. For a given dietary protein level, an increase in fat level in the diets of rainbow trout generally enhances growth and protein utilisation( Reference Lee and Putnam 1 , Reference Takeuchi, Watanabe and Ogino 2 ). The improvement in protein utilisation is due to increasing contribution of non-protein energy sources to overall energy expenditure, which leads to a reduction in N excretion and improves the quality of effluent discharge from fish farms( Reference Kaushik and Medale 3 ). Information on the effects of changes in dietary protein:energy ratio on the growth processes of fish during early stages is lacking, despite the fact that there is increasing evidence from studies in human and various animal models, including fish, that the nutritional regulation of growth is life-cycle stage-specific, and that early stages are particularly sensitive to the deficiency or excess of some nutrients. Fish hatch immature, with a yolk sac, morphology, anatomy (e.g. unopened mouth) and physiology that are very different from those of the adult. The period of development and growth that follows the first exogenous feeding is typified by an increase in the activity of enzymes involved in digestive function and muscle glycolytic metabolism( Reference Mazurais, Darias and Zambonino-Infante 4 , Reference Alami-Durante and Bacou 5 ), and a remodelling of the relative volume of the main tissues and organs that make up the body, with positive allometry of axial locomotor muscles( Reference Alami-Durante 6 ).
In rainbow trout (Oncorhynchus mykiss), the growth of white skeletal muscle, which constitutes the bulk of the axial locomotor muscle, occurs after hatching by both the recruitment of new muscle fibres (hyperplasia) and the enlargement of the previously formed fibres (hypertrophy)( Reference Weatherley, Gill and Rogers 7 , Reference Stickland 8 ), as in other aquaculture fish reaching a large adult body size( Reference Rowlerson, Veggetti and Johnston 9 ). The relative contributions of hyperplasia and hypertrophy to the growth of white muscle in trout change when the length of the trout increases( Reference Weatherley, Gill and Rogers 7 , Reference Stickland 8 ), as indicated by length-related changes in white muscle cellularity (i.e. in the total number of white muscle fibres and their size distribution): hyperplasia contributes more to white muscle growth in small rather than large trout. In addition to this developmental regulation, the cellularity of fish white muscle has been established as being triggered by temperature from the embryonic to the adult period of life( Reference Stickland, White and Mescall 10 – Reference Macqueen, Robb and Olsen 13 ) and affected by starvation and feed restriction in fingerlings, juveniles and mature fish( Reference Salze, Alami-Durante and Barbut 14 – Reference Kiessling, Storebakken and Asgard 17 ). The understanding of the regulation of muscle cellularity by specific nutrients in fish fed to apparent satiety is fragmentary: only a few nutritional conditions have been tested, and they have been tested in a limited number of species at different growth stages. In first-feeding larvae, the cellularity of white axial skeletal muscle has been successively demonstrated as being regulated by dietary protein sources in common carp and pikeperch( Reference Alami-Durante, Fauconneau and Rouel 18 , Reference Ostaszewska, Dabrowski and Wegner 19 ), by dietary DHA:EPA ratio in Atlantic cod( Reference Galloway, Kjorsvik and Kryvi 20 ), by dietary lecithin source in gilthead sea bream( Reference Alves Martins, Estevez and Stickland 21 ) and by dietary cholecalciferol content in European sea bass( Reference Alami-Durante, Cluzeaud and Bazin 22 ). In fingerlings, white muscle cellularity has been shown to be sensitive to the form of delivery of lysine and glycine in common carp( Reference Kamaszewski, Prasek and Ostaszewska 23 ). During the subsequent period of life (i.e. juvenile life), the cellularity of white muscle has been shown to vary according to dietary lipid source in rainbow trout( Reference Fauconneau, Andre and Chmaitilly 24 ), protein level in Atlantic salmon and blackspot sea bream( Reference Johnston, Manthri and Alderson 25 – Reference Silva, Valente and Galante 27 ), and protein sources and amino acid profile in rainbow trout( Reference Alami-Durante, Medale and Cluzeaud 28 , Reference Alami-Durante, Wrutniack-Cabello and Kaushik 29 ). To our knowledge, no information on the effects of dietary lipid levels on muscle cellularity (which is a phenotypic consequence of muscle hyperplasia and hypertrophy) in fish is currently available.
The first aim of the present study was to characterise the ontogenetic changes in the expression of genes that regulate early myogenesis in trout and to characterise the corresponding changes in muscle cellularity. The genes selected were myogenic regulatory factors (MRF; myogenic differentiation 1 (MyoD1), myogenic factor 5 (Myf5) and myogenin (Myog)), a marker of cell proliferation (proliferating cell nuclear antigen (PCNA)) and involved in muscle structure and function (fast myosin heavy chain (fast-MHC) and slow myosin heavy chain (slow-MHC)). The second aim of the study was to determine how an early decrease in dietary protein: energy ratio by fat addition regulates muscle growth and its mechanisms (hyperplasia and hypertrophy) in rainbow trout alevins by means of histological and molecular analyses. The third aim of the study was to determine whether an early decrease in dietary protein: energy ratio by fat addition (i.e. early feeding with a high-fat (HF) diet instead of a low-fat (LF) diet) had a long-term effect on the subsequent muscle growth processes of juveniles fed a commercial diet.
Experimental methods
Growth experiment and sampling procedures
The eggs of diploid rainbow trout (O. mykiss) were obtained in February from a commercial fish farm (Viviers de France) and incubated/reared in an INRA experimental fish farm (Lees-Athas, France) in spring water at a temperature range of 7·5 to 8·4°C. Embryos were sampled at the eyed stage (270 degree days post-fertilisation (°d)). Alevins were sampled at hatching (333°d), first exogenous feeding (528°d), yolk-sac resorption (602°d = 10 d of feeding (df)), and after 30 df (758°d), 60 df (992°d), 75 df (1109°d) and 90 df (1226°d) with the experimental diets. To characterise early myogenesis and its regulation by a HF diet from first feeding onwards accurately, eggs and alevins of rainbow trout were subjected to three successive gradings. The first grading was a size grading that was performed at the embryonic eyed stage. This grading was performed because egg size significantly affects both yolk utilisation and early body growth in rainbow trout( Reference Escaffre and Bergot 30 , Reference Escaffre and Bergot 31 ). Therefore, the eggs used in the present study were sorted with grids and those with diameters < 4·8 or >5·4 mm were discarded, in order to retain an egg population with a mean diameter of 5·1 mm. The two subsequent gradings were developmental grading, in order to work on the best possible ‘developmentally coordinated’ population of alevins. The first developmental grading was applied at hatching. As hatching lasted 6 d in the study conditions (7·5–8·4°C), the alevins that hatched from the 2nd to the 4th day of hatching were retained, and those that hatched earlier and later were discarded. The ‘hatching’ stage that was sampled corresponded to the peak of hatching (333°d). The alevins of rainbow trout hatch with important yolk reserve, and they develop on these endogenous reserves until they ‘emerge’ from the river substrate in the natural environment/tank bottom in aquaculture conditions to search for exogenous food. The alevins emerge before the complete resorption of their yolk reserve and their development thus relies on ‘mixed’ feeding (endogenous reserves+exogenous food) until complete yolk-sac resorption. The second developmental grading applied was at emergence, in order to homogenise the ‘first exogenous feeding stage’ of alevins. As emergence in rainbow trout lasts numerous days at the conditions of 7·5 to 8·4°C, the alevins that emerged ‘precociously’ (during the first 3 d of emergence) were discarded; those that emerged between the 4th and the 6th day were sorted daily and fed immediately to satiety with the experimental diets, and those emerging later were discarded. This schedule was applied in order to avoid the negative effect of delayed feeding on early body growth in trout( Reference Escaffre and Bergot 31 ). It was also verified that the alevins sampled at the ‘yolk-sac resorption’ stage (602°d) were totally free of macroscopically visible remaining yolk reserves.
The following two successive feeding trials were conducted: the first trial was conducted with two experimental diets from first feeding onwards to assess the effects of an early decrease in dietary protein:energy ratio by the addition of lipids on the muscle growth mechanisms of alevins, and the second trial was conducted in juveniles fed with a commercial diet to assess whether the changes in early nutritional conditions tested had a long-term effect on the muscle growth of trout (Fig. 1).
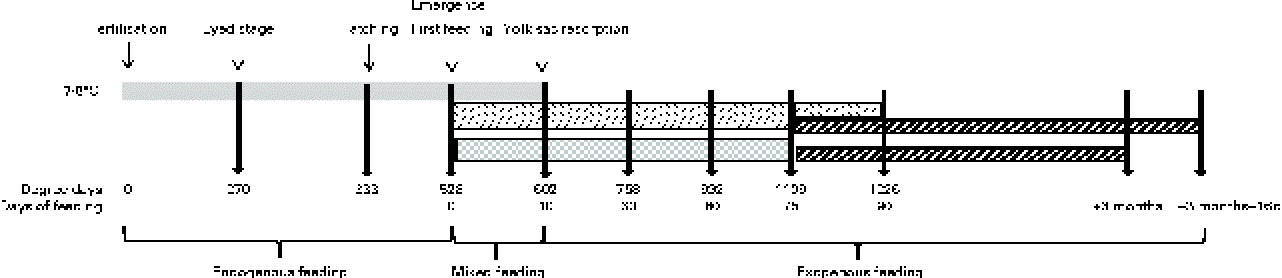
Fig. 1 Schematic diagram of the feeding trials with changes in nutritional conditions and sampling stages/ages (arrows) with corresponding degree days from fertilisation and days of exogenous feeding. ![]() , Yolk reserves;
, Yolk reserves; ![]() , low-fat diet;
, low-fat diet; ![]() , high-fat diet;
, high-fat diet; ![]() , commercial diet.
, commercial diet.
For the first trial, two experimental diets were formulated to contain the same level of protein and different dietary protein: energy ratios (Table 1). The common basis was made of fishmeal and extruded pea as protein sources, sodium alginate, and vitamin mix and mineral mix in order to meet the nutritional requirements of trout fry. In the HF diet, non-protein energy was mainly supplied by fish oil, and in the LF diet, fish oil was replaced by crude wheat starch. The ingredients were mixed and the mixture was processed as food particles of different diameters to match the increasing mouth size of the alevins. The analytical composition of the two diets was assessed using the following procedures: DM was assessed after drying at 105°C for 24 h; protein content (N × 6·25) was determined by the Kjeldahl method; fat level was assessed by weighing before and after petroleum diethyl ether extraction (Soxtherm, Gerhardt); starch content was determined according to the method described by Thivend et al. ( Reference Thivend, Mercier, Guilbot, Whilster and Bemillier 32 ); gross energy content was determined after combustion in an adiabatic bomb calorimeter. Protein content was found to be high and similar in the two diets (60·3 v. 61·6 % DM). Energy content was found to be higher in the HF diet (23·2 kJ/g dry weight) than in the LF diet (20·3 kJ/g dry weight) due to the higher lipid content of the HF diet (20 %) than that of the LF diet (6·8 %), as shown in Table 1.
Table 1 Ingredients and analytical composition of the experimental diets
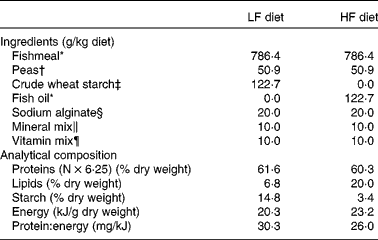
LF, low fat; HF, high fat.
* Sopropèche, France.
† Sotexpro, France.
‡ Roquette, France.
§ Ets Louis François, France.
∥ CM 762, INRA, France.
¶ CV 763, INRA, France.
The HF and LF diets were distributed from emergence (first exogenous feeding = day 0 of the feeding trial) onwards to triplicate groups of 3200 alevins that were hand-fed six times per d to excess or visual satiety. At 75 df, the alevins were sorted. Three replicates of 1400 alevins from the HF diet-fed group were fed a commercial diet (17 % crude lipids, 54 % crude protein and 22·5 kJ/g DM, Ecostart 18; BioMar). The alevins from the LF diet-fed group were divided into six replicates of 1400 fish: three replicates were fed the commercial diet, while the other three replicates were maintained on the LF diet until they reached the weight of HF diet-fed fish at 75 df, 15 d later. The groups transferred to the commercial diet at 75 df were fed to satiety for 3 months with this diet. Thereafter, rearing of the initially LF diet-fed juveniles was continued until they reached the same weight (+16 d) as that of the initially HF diet-fed fish after 3 months of feeding (mf) with the commercial diet. Juveniles were sampled after 3 mf and 3 mf+16 df with the commercial diet. These sampling schedules (Fig. 1) were constructed in order to compare the alevins and juveniles not only at a similar age but also at a similar body size.
For the analysis of the expression levels of muscle growth-related genes, samples were pools of whole (yolk sac-removed) embryos at the eyed stage and alevins at hatching; pools of ‘trunks’ (posterior body part without the viscera, from the anterior insertion of the dorsal fin to the vent; Fig. 3(A)) from first feeding to 90 df; and individual pieces of dorsal white muscle of juveniles. These samples were immediately frozen in liquid N2 and stored at − 80°C until molecular analysis. For analysis of muscle cellularity, ten fish were sampled per batch. These histological samples (whole alevins at first feeding and at 30, 75 and 90 df; transverse sections of 1 cm width cut at the vent level of previously weighed and measured juveniles), were fixed and dehydrated according to the method described by Alami-Durante et al. ( Reference Alami-Durante, Olive and Rouel 12 ) until further histological analysis. For the qualitative histochemical determination of the location of lipid deposits, whole alevins were individually sampled after 75 df, frozen in liquid N2 and stored at − 80°C. Other alevins were frozen in liquid N2 at 75 df (fifteen pools of three (HF) or four to five (LF) alevins) and 90 df (eight pools of two to three (LF) whole alevins) for quantification of lipid content in the whole body, and at 75 df for quantification of the lipid content of the trunk compared with the remaining part of the anterior body (three pools of five alevins/body part per diet). The experiment was conducted according to the Guidelines of the National Legislation on Animal Care of the French Ministry of Research and complied with European Community Directive 86/609/EEC.
Quantification and histochemical location of lipid deposits
Lipid deposits in the whole body, trunk and anterior part of the frozen alevins were quantified according to the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 33 ). For histochemical location of lipid deposits, other frozen alevins were cut transversely at − 25°C in slices of 10 μm thick at two anatomical levels, namely the level of the anterior insertion of the dorsal fin (anterior body level) and the level of the vent (tail level), as shown in Fig. 3(B). Lipid deposits were stained with dark Sudan Black( Reference Gabe 34 ) on just a few alevins, to obtain qualitative information on the location of lipid deposits in muscle.
Quantitative histological analysis
Alevins kept in butanol were weighed and measured. The tails were then cut, dehydrated, embedded in paraffin and cut transversely into sections (10 μm thick) that were stained with haematoxylin and orange G as described in Alami-Durante et al. ( Reference Alami-Durante, Olive and Rouel 12 ). The sections of juveniles were similarly paraffin embedded, cut and stained. Muscle cellularity was examined on one section per fish, located at the vent level. The total cross-sectional areas of white and red muscles and the individual cross-sectional areas of white muscle fibres were quantified with Optimas software (Media Cybernetics). To quantify stratified and mosaic hyperplasia, and maximum hypertrophy and to measure the numbers of muscle fibres greater than 500 fibres in all fish, white muscle cellularity was studied in three whole sections of myotomes at first feeding, two whole sections of myotomes at 30 df, one whole section of myotomes at 75 and 90 df, and a transverse section of a myotome in juveniles. The individual equivalent diameter of white muscle fibres (diameter of a circle of equal area to that of the muscle fibre, hereafter referred to as ‘fibre diameter’) was determined. The total number of white muscle fibres was estimated from the total cross-sectional area of white muscle and the mean cross-sectional area of white muscle fibres. Cellularity was analysed in seven alevins at first feeding, nine alevins at 30 df (HF and LF diets), 75 df (HF and LF diets) and 90 df (LF diet), and six to ten juveniles per condition.
RNA extraction and quantitative RT-PCR
Total RNA was extracted using TRIzol® reagent (Invitrogen), according to the manufacturer's instructions, and stored in nuclease-free water (Dutscher) at − 20°C (n 5–11 pools/condition). Samples were subjected to electrophoresis on 1 % agarose gels to confirm the integrity of 28S and 18S rRNA bands, and RNA purity was assessed by the absorbance ratio of 260:280 nm, with the ratio ranging between 1·7 and 2·1 being acceptable. RNA integrity was verified using an Agilent Technologies method (Agilent 2100 Bioanalyzer and RNA 6000 Nano LabChip kit). Published primers were used to quantify the expression of skeletal fast-MHC ( Reference Alami-Durante, Medale and Cluzeaud 28 ), slow-MHC ( Reference Alami-Durante, Medale and Cluzeaud 28 ), MyoD1 ( Reference Alami-Durante, Medale and Cluzeaud 28 ), PCNA ( Reference Alami-Durante, Medale and Cluzeaud 28 ), Myog ( Reference Chauvigné, Gabillard and Weil 35 ) and 18S ( Reference Kamangar, Gabillard and Bobe 36 ) mRNA. Primers were designed for Myf5 (forward gcttaccttctcgccctcca; reverse tcaaagcggtatgcggttga) using GeneBank sequence AY751283.1 and primer 3 software (University of Massachusetts). All primers were synthesised by Eurogentec and amplicons confirmed by sequencing (Millegen). Complementary DNA (cDNA) was generated from 2 μg of total RNA using 200 U SuperSript™ III Reverse Transcriptase (Invitrogen) and 500 ng random primers (Promega) in a total volume of 20 μl. RNA samples were heated at 25°C for 10 min, at 65°C for 3 min, and then at 55°C for 1 h to generate cDNA, followed by heat inactivation at 70°C for 15 min. Real-time PCR was performed in an iCycler iQ™ (Bio-Rad) on duplicates of each RT product using the iQ™ SYBR® Green Supermix (Bio-Rad). All PCR assays were set up using 200 nmol/l of each primer and 10 μl cDNA (diluted at 1:30 except for 18S and fast-MHC (at 1:120)) in a reaction volume of 25 μl. Overall, thirty-five steps of PCR were performed, each consisting of heating at 95°C for 20 s to denature cDNA, and at the annealing temperature for 30 s for polymerisation. Following the final cycle of PCR, melting curves were systematically monitored (increasing the set-point temperature from 55 to 94°C by 0·5°C/10 s) to confirm the production of a single product and for dissociation analysis of the PCR products. Controls were included to test the absence of contamination by genomic DNA or assay reagents. Standard curves, consisting of five serial dilutions in triplicate of a pool of cDNA, were obtained for each cDNA template by plotting C T values against the log10 of the different dilutions. Real-time PCR efficiency was calculated from standard curves according to the method described by Pfaffl( Reference Pfaffl 37 ). Abundance of 18S RNA was similar for all conditions and was used for the normalisation of quantitative PCR data. The 18 S-adjusted data were analysed using the ΔΔC T method( Reference Pfaffl 37 ), with the first-feeding stage as the control for alevins and the initially LF diet-fed juveniles after 3 mf with the commercial diet as the control for juveniles.
Statistical analysis
Data that were ln transformed (weight, total length, total cross-sectional area of white and red muscles and white muscle fibre diameters), arcsine transformed (percentage of white muscle fibres in a diameter class) or square root transformed (total number of white muscle fibres) were analysed by ANOVA and Student–Newman–Keuls tests with R 2.15.3 software (University of Auckland). ANOVA were performed on pools of embryos, alevins or trunks (relative gene expression levels and lipid deposits) or on individuals (muscle fibre diameters). Results for cellularity were compared both at three equivalent durations of the feeding period (30 and 75 df with the HF and LF diets, and 3 mf with the commercial diet) and, as white muscle cellularity is size dependent in trout and other teleosts( Reference Weatherley, Gill and Rogers 7 , Reference Weatherley, Gill and Lobo 38 ), at a similar body size (HF diet-fed alevins after 75 df v. LF diet-fed alevins after 90 df; initially HF diet-fed juveniles after 3 mf with the commercial diet v. initially LF diet-fed juveniles after 3 mf+16 df with the commercial diet). A P value of 0·05 was considered as statistically significant. Data are presented as means with their standard errors.
Results
Somatic growth of alevins and juveniles
During the first 10 df, no significant differences were observed in the increase of the body weight of rainbow trout alevins (Fig. 2) . Later, the alevins fed the HF diet showed significantly increased body weight compared with those fed the LF diet; from 60 df onwards, LF diet-fed alevins reached the weight of HF diet-fed alevins at an interval of 15 d (P< 0·0001).
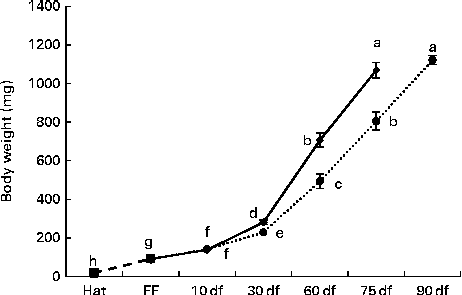
Fig. 2 Body growth of rainbow trout alevins fed the high-fat (![]() ) and low-fat (
) and low-fat (![]() ) diets from first feeding onwards. Values are means, with their standard errors represented by vertical bars. a,b,c,d,e,f,g,hMean values with unlike letters were significantly different (P< 0·0001). Hat, hatching; FF, first exogenous feeding; df, days of feeding.
) diets from first feeding onwards. Values are means, with their standard errors represented by vertical bars. a,b,c,d,e,f,g,hMean values with unlike letters were significantly different (P< 0·0001). Hat, hatching; FF, first exogenous feeding; df, days of feeding. ![]() , Endogenous feeding.
, Endogenous feeding.
The body weights and total lengths of the initially HF diet-fed juveniles after 3 mf with the commercial diet (P= 0·0394 and P= 0·020, respectively) were significantly higher than those of the initially LF diet-fed juveniles after 3 mf with the commercial diet, but were, as planned in the protocol, similar to those of the initially LF diet-fed juveniles after 3 mf+16 df with the commercial diet (Table 3).
Lipid content of alevins and location of lipid deposits
As shown in Fig. 3(A), the bodies of HF diet-fed alevins contained 84 % more lipids than those of LF diet-fed alevins after 75 df (P< 0·001). The body lipid content of HF diet-fed alevins at 75 df was also about twice as high as that of LF diet-fed alevins of similar body weight, because the total lipid content of LF diet-fed alevins did not increase between 75 and 90 df (P= 0·575).

Fig. 3 (A) Quantification of lipid deposits (% fresh weight) in the whole body, trunk and anterior part of alevins. Data presented in the table are mean values with their standard errors. (B) Anatomical positions of the two sections and four areas selected for visualising the tissular location of lipid deposits by Sudan Black histochemistry. The arrows indicate lipid deposits in white and red muscles. df, Days of feeding; LF, low fat; HF, high fat. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
The comparison of the lipid content of the anterior part of alevins with that of the trunk part first showed that in both LF diet-fed (P= 0·017) and HF diet-fed (P= 0·0019) alevins, more lipids were deposited in the anterior part than in the trunk (LF diet +46 %; HF diet +58 %). It also showed that the distribution of the HF diet, not the LF diet, significantly increased the quantity of lipids deposited in both the anterior part (P= 0·001) and the trunk (P= 0·025) of alevins. The histochemical staining of lipids confirmed that more lipids were deposited in HF diet-fed than in LF diet-fed alevins, and that these lipids were mainly deposited in the anterior part, around the viscera (data not shown). As shown in Fig. 3(B), lipids were also deposited in red muscle and in specific areas of white muscle. In the white muscle of HF diet-fed alevins, lipids were mainly deposited in the apical area (A1), the deep area close to the spinal cord (A2) and the abdominal area, close to the pyloric caeca (A4).
Growth dynamics of white and red axial skeletal muscles
As shown in Table 2, feeding the alevins with the HF diet from first feeding onwards had no effect on the total cross-sectional area of white muscle at 30 df (758°d); however, it significantly increased the total cross-sectional area of white muscle at 75 df (1109°d), without reaching the total cross-sectional area of white muscle observed in LF diet-fed alevins of similar body size, i.e. at 90 df (1226°d; P< 0·0001). The total cross-sectional area of red muscle was not significantly different in HF diet- and LF diet-fed alevins at 30 and 75 df; however, it was lower in HF diet-fed alevins at 75 df than in LF diet-fed alevins of similar body size, i.e. at 90 df (P< 0·0001). The total number of white muscle fibres increased with days of feeding (P< 0·0001); however, it was not significantly affected by dietary lipid content. The mean diameter of white muscle fibres increased between first feeding (528°d) and 75 df (P< 0·0001) but remained constant thereafter. The median diameter of white muscle fibres increased from first feeding to 75 df but decreased thereafter (P< 0·0001). The maximum diameter of white muscle fibres increased from first feeding to 90 df (P< 0·0001).
Table 2 Changes in the white muscle cellularity of rainbow trout alevins during the first 90 d of feeding (df) with the high-fat (HF) and low-fat (LF) diets (Mean values with their standard errors)

a,b,c,d,eMean values within a row with unlike superscript letters were significantly different (P< 0·05).
The shape of the distribution of white muscle fibre diameters changed with time: it was unimodal at first feeding, bimodal at 30 and 75 df, before returning to unimodality at 90 df (Fig. 4(A)). The percentage of white muscle fibres constituting the first mode (10–15 μm) of the distribution of white muscle fibre diameters decreased between first feeding and 75 df and increased between 75 and 90 df, up to the value observed at 30 df (P< 0·0001). White muscle fibres with diameters >30 μm appeared after first feeding. The percentages of white muscle fibres constituting the diameter classes 30–35 μm and 35–40 μm increased between 30 and 75 df and decreased between 75 and 90 df (P< 0·0001 for both classes). Fibres with diameters >40 μm appeared at 75 df and their percentages increased between 75 and 90 df (40–45 μm: P< 0·0001; 45–50 μm: P< 0·0001; 50–55 μm: P< 0·0002). The decrease in dietary protein:energy ratio resulting from the addition of lipids slightly affected the distribution of white muscle fibres at 30 df (Fig. 4(B)) without significantly modifying the mean, median and maximum diameters of white muscle fibres (Table 2), or the percentages of white muscle fibres constituting the different diameter classes (Fig. 4(B)). Significant effects of dietary lipid levels on muscle cellularity were recorded in older fish (Table 2). The distribution of white muscle fibre diameters in HF diet-fed alevins at 75 df was intermediate between that of LF diet-fed alevins at 75 and 90 df (Fig. 4(C)), with significant differences between the batches of alevins in terms of the percentage of white muscle fibres in the diameter class constituting the mode (10–15 μm) of the first population of muscle fibres (P= 0·014), in terms of the percentage of white muscle fibres in the diameter class constituting the mode (30–35 μm) of the second population of muscle fibres (P= 0·0003) and in the percentage of large white muscle fibres with diameters ranging between 45 and 50 μm (P= 0·0066). The maximum diameter of white muscle fibres (Table 2) and the percentage of white muscle fibres in the largest class of diameters (Fig. 4(C)) were both significantly higher in LF diet-fed alevins at 90 df than in HF diet- and LF diet-fed alevins at 75 df.

Fig. 4 Changes in the distribution of white muscle fibre diameters with age (A), according to dietary lipid levels at 30, 75 and 90 d of initial feeding with the high-fat (HF) and low-fat (LF) diets (B, C), and after 3 months (m) and 3 m+16 d of additional feeding with the commercial diet (CD) (D). Values are means, with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different in a diameter class (P< 0·05). (A) ![]() , First exogenous feeding;
, First exogenous feeding; ![]() , LF at 30 d;
, LF at 30 d; ![]() , LF at 75 d;
, LF at 75 d; ![]() , LF at 90 d. (B)
, LF at 90 d. (B) ![]() , HF at 30 d;
, HF at 30 d; ![]() , LF at 30 d. (C)
, LF at 30 d. (C) ![]() , HF at 75 d;
, HF at 75 d; ![]() , LF at 75 d;
, LF at 75 d; ![]() , LF at 90 d. (D)
, LF at 90 d. (D) ![]() , HF at 75 d+CD for 6 m;
, HF at 75 d+CD for 6 m; ![]() , LF at 75 d+CD for 6 m;
, LF at 75 d+CD for 6 m; ![]() , LF at 75 d+CD for 6 m and 16 d.
, LF at 75 d+CD for 6 m and 16 d.
Early feeding conditions had consequences on the subsequent white muscle growth processes in juveniles. After 3 mf with the commercial diet, the total cross-sectional area of white muscle of the fish transferred to the commercial feed at 75 df continued to be smaller in initially LF diet-fed juveniles than in initially HF diet-fed juveniles (Table 3). The distributions of white muscle fibre diameters in initially HF diet- and LF diet-fed juveniles were very similar (Fig. 4(D)), as were the mean, median and maximum diameters of white muscle fibres (Table 3). Differences in muscle growth mechanisms were evidenced when juveniles were compared at similar body size (initially HF diet-fed juveniles after 3 mf with the commercial diet v. initially LF diet-fed juveniles after 3 mf+16 df with the commercial diet): the mean diameter of white muscle fibres, the median diameter of white muscle fibres and the number of white muscle fibres with diameters ranging between 70 and 80 μm were significantly higher (P= 0·047, 0·030 and 0·032, respectively) in initially LF diet-fed juveniles than in initially HF diet-fed juveniles (Table 3; Fig. 4(D)).
Table 3 Changes in the white muscle cellularity of rainbow trout juveniles at the end of the commercial diet (CD) feeding period (Mean values with their standard errors)

LF, low fat; HF, high fat.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Expression of muscle growth-related genes
Developmental regulation
As shown in Fig. 5 for the LF diet-fed batch, the relative expression levels of all the genes selected for the study were developmentally regulated. The expression of Myf5 mRNA was stable between the eyed stage (270°d) and first feeding (528°d) and decreased between first feeding and 10 df (yolk-sac resorption: 602°d; P= 0·0027), but did not vary significantly thereafter. The expression of MyoD1 mRNA did not vary significantly between the eyed stage and hatching (333°d) and increased between hatching and 10 df (P= 0·0252). The expression of PCNA mRNA was also not significantly different at the eyed stage and hatching, then decreased between the hatching and first-feeding stages (P< 0·0001) and did not change significantly thereafter. The expression of Myog mRNA decreased gradually between the eyed stage and first feeding (P< 0·0001), but did not change significantly thereafter. The expression of fast-MHC mRNA increased gradually between the eyed stage and 10 df, and increased between 30 and 75 df (P< 0·0001). The expression of slow-MHC mRNA increased significantly between the eyed stage and 10 df, and between 10 and 75 df (P< 0·0001).

Fig. 5 Ontogenetic changes in the relative expression levels of muscle growth-related genes in rainbow trout alevins fed the low-fat diet: (A) myogenic factor 5 (Myf5); (B) myogenic differentiation 1 (MyoD1); (C) proliferating cell nuclear antigen (PCNA); (D) myogenin (Myog); (E) fast myosin heavy chain (fast-MHC) and (F) slow-MHC. Values are means, with their standard errors represented by vertical bars. a,b,c,d,eMean values with unlike letters were significantly different (P< 0·05). ES, eyed stage; Hat, hatching; FF, first exogenous feeding; df, days of feeding.
Effects of early nutrition
As shown in Table 4, there was a significant effect observed in the very short term (10 df) and the short term (30 df) of the early decrease in dietary protein:energy ratio by the addition of lipids on the relative expression of fast-MHC mRNA, with higher expression levels being shown at 10 and 30 df in LF diet-fed alevins compared with HF diet-fed alevins (P= 0·0383 and 0·0018, respectively). However, no significant effects of dietary lipid levels on the relative expression levels of slow-MHC, Myf5, MyoD1, Myog and PCNA mRNA at 10 and 30 df were observed. There were also no significant effects of dietary lipid levels on the expression of fast-MHC, slow-MHC, Myf5, MyoD1 and Myog mRNA after 75 df (Table 4), but comparison of alevins at similar body weights (i.e. LF diet-fed alevins at 90 df v. HF diet-fed alevins at 75 df) indicated that the relative expression of PCNA mRNA was significantly higher in the former than in the latter (P= 0·0014).
Table 4 Effects of early feeding with the high-fat (HF) and low-fat (LF) diets on the relative mRNA expression of muscle growth-related genes in rainbow trout alevins and juveniles (Mean values with their standard errors)

df, Days of feeding after the first meal; Myf5, myogenic factor 5; MyoD1, myogenic differentiation 1; Myog, myogenin; PCNA, proliferating cell nuclear antigen; fast/slow-MHC, fast/slow-myosin heavy chain; CD, commercial diet; m, months.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Long-term effects of early nutrition after transfer to a common commercial diet at 75 d of feeding
A significant effect of an early decrease in dietary protein:energy ratio by the addition of lipids was recorded after 3 mf with the commercial diet, with a significantly higher relative expression level of PCNA mRNA (P= 0·0122) in juveniles initially fed the LF diet (Table 4). The comparison of juveniles at similar body weights (i.e. juveniles fed the HF diet for 75 d+the commercial diet for 3 months v. juveniles fed the LF diet for 75 d+the commercial diet for 3 months+16 d) revealed a significantly higher relative expression levels of MyoD1 (P= 0·0184) and Myog (P= 0·0019) mRNA in the latter than in the former (Table 4).
Discussion
Ontogenetic changes in muscle growth dynamics and expression of muscle growth-related genes
We characterised the ontogenetic changes occurring in the distribution of white skeletal muscle fibres during the first 90 df of rainbow trout alevins and demonstrated the maintenance of a population of white muscle fibres of small diameter ( < 20 μm), which indicated the persistent recruitment of new white muscle fibres. The changes in the percentage of these small fibres with time showed that the recruitment of new fibres decreased between 30 and 75 df, but increased between 75 and 90 df, as confirmed by the changes in the total number of white muscle fibres. This showed that the contribution of hyperplasia to muscle growth changed during the growth of rainbow trout alevins, and confirmed the results of earlier studies( Reference Weatherley, Gill and Rogers 7 , Reference Stickland 8 ). Myogenesis is regulated by a family of transcription factors called MRF that are expressed in a temporally distinct pattern during determination, activation and proliferation of muscle precursor cells (Myf5 and MyoD) and in cells entering the terminal differentiation programme (Myog)( Reference Sabourin and Rudnicki 39 ). Proliferative muscle precursor cells also express PCNA, which functions as a cofactor of DNA polymerase-δ and is necessary for cell-cycle progression and cell proliferation( Reference Bravo, Frank and Blundell 40 ). The present results demonstrated that MyoD1 and Myf5 are not similarly regulated during early muscle development in rainbow trout. Our findings of a high relative expression level of Myf5 mRNA during embryonic endogenous feeding, which decreased between first feeding (528°d) and 10 df (yolk-sac resorption: 602°d), and a low relative embryonic expression of MyoD1 mRNA, which increased between hatching and yolk-sac resorption, suggest a sequential significance of these two MRF during early myogenesis in trout. This is consistent with the changes in the expression levels of Myf5 and MyoD mRNA that occur during mammalian myogenic lineage progression( Reference Sabourin and Rudnicki 39 ). The ontogenetic changes that we observed herein in rainbow trout between hatching (333°d) and yolk-sac resorption (602°d; 10 df) at the relative expression level of MyoD1 mRNA (increasing significantly) confirmed previous results obtained for the same species at 8°C( Reference Wilkes, Xie and Stickland 41 ) and extended them by indicating that the relative expression of MyoD1 mRNA did not vary significantly during the subsequent 65 df (i.e. up to 1109°d). In contrast, others( Reference Mennigen, Skiba-Cassy and Panserat 42 ) have reported a decrease in the relative expression of MyoD1 mRNA in trout alevins between 550°d (endogenous feeding) and 638°d (mixed feeding). This difference is probably linked to the fact that alevins were fed later (at only 638°d) in the study of Mennigen et al. ( Reference Mennigen, Skiba-Cassy and Panserat 42 ), while in the present study, the first meal was distributed at 528°d. The alevins in their study( Reference Mennigen, Skiba-Cassy and Panserat 42 ) had probably suffered from this delay in first feeding, which might have impaired the myogenic process and thus lowered the relative expression of MyoD1 mRNA. A delay in first feeding had indeed already been demonstrated to have a negative effect on the body growth of rainbow trout alevins( Reference Escaffre and Bergot 31 ), and the onset of exogenous feeding, by providing access to an unlimited source of nutrients and energy, is known to trigger myogenesis, and in particular hyperplasia, in different fish species( Reference Rowlerson, Veggetti and Johnston 9 , Reference Alami-Durante, Rouel and Kentouri 11 , Reference Ostaszewska, Dabrowski and Wegner 19 , Reference Stoiber, Haslett and Wenk 43 ). Briefly, delayed first feeding (2–3 d) is also known to decrease myofibre area and induce myonuclear apoptosis in chickens( Reference Mozdziak, Evans and McCoy 44 ) and to decrease skeletal muscle growth, satellite cell proliferation and Myog mRNA expression in turkey poults( Reference Halevy, Nadel and Barak 45 ). The ontogenetic pattern of Myog mRNA expression that we observed between 270°d (eyed stage) and 602°d (yolk-sac resorption) was different from that reported by Johansen & Overturf( Reference Johansen and Overturf 46 ) between 210 and 555°d for the same species. This might be linked to the differences in experimental temperatures (7·5–8·4°C in the present study v. 15°C in Johansen & Overturf's study( Reference Johansen and Overturf 46 )), as some ontogenetic changes in the expression level of Myog mRNA are known to be temperature dependent in rainbow trout( Reference Wilkes, Xie and Stickland 41 ). As in the latter study, we found that the expression level of fast-MHC mRNA increased in the alevins of rainbow trout between the hatching and yolk-sac resorption stages. In agreement with the increased expression levels of genes involved in muscle sarcomeric content (fast-MHC and slow-MHC) after exogenous feeding, we demonstrated that the maximum hypertrophy of white muscle fibres increased during the first 90 df.
Effects of early feeding with the high-fat and low-fat diets on alevin body growth and lipid deposits
The present results showed that rainbow trout alevins responded in the same way as rainbow trout juveniles and various other fish juveniles when the dietary protein:energy ratio was changed by the addition of lipids: body growth rate was stimulated and lipid deposits were increased( Reference Shearer 47 , 48 ). Moreover, the results reported herein showed that the better growth of alevins fed the HF diet was not fully explained by increased muscle growth. Indeed, the higher weight of HF diet-fed alevins at 30 df was not associated with greater total cross-sectional areas of white and red skeletal muscles at the vent level, suggesting greater weight of the anterior part of alevins, with heavier visceral tissues and/or perivisceral adipose tissue. The subsequent comparison of alevins at similar body weights (HF diet after 75 df v. LF diet after 90 df) indicated greater total cross-sectional areas of white and red skeletal muscles at the vent level in LF diet-fed alevins, which again suggests a heavier anterior part in HF diet-fed alevins. This heavier anterior part in HF diet-fed alevins was supported by a 70 % higher lipid content than in LF diet-fed alevins. This is consistent with the findings obtained for larger rainbow trout showing that when the dietary protein:energy ratio is changed by the addition of lipids, there is an increase in lipid deposition around the viscera( Reference Takeuchi, Watanabe and Ogino 2 , Reference Diaz, Corraze and Arzel 49 ). The present findings are original in that they add to the understanding of the early stages and demonstrate that lipid storage in alevins is, at high dietary protein levels, governed by dietary energy independently of the body weight of alevins. Indeed, the present results demonstrated that the early distribution of HF diets to rainbow trout alevins led to a fatty phenotype with less muscle at similar body weights than that formed with a leaner diet.
Effects of early feeding with the high-fat and low-fat diets on the expression of muscle growth-related genes and muscle growth dynamics in alevins
We demonstrated that the decrease in dietary protein:energy ratio by fat addition from first feeding onwards led to a very rapid decrease (highly significant after only 10 d of distribution) in the relative expression level of fast-MHC mRNA. This significant decrease persisted after 20 further days of HF diet feeding. In contrast, the distribution of the HF diet from first feeding onwards appeared from the present results to affect neither the relative expression levels of Myf5 and MyoD1 mRNA, which are involved in the determination and activation of muscle precursor cells, nor the relative expression level of Myog mRNA, which is involved in terminal differentiation and fusion. Few other findings are available on the nutritional regulation of the expression levels of MRF and MHC during early life in fish. What is known about fast-MHC is that its mRNA expression is modulated by starvation, with an age-related relationship (increased at 1–3 d but decreased at 9 d), in Atlantic cod larvae( Reference MacNamara, Caldarone and Buckley 50 ), and decreased by a low dietary cholecalciferol level (0·28 mg/kg) and promoted by an intermediate dietary cholecalciferol level (0·69 mg/kg) in 22- and 44-d-old European sea bass larvae( Reference Alami-Durante, Cluzeaud and Bazin 22 ). However, it is not modified by nucleotide enrichment of live feed in Atlantic cod larvae( Reference Lanes, Bolla and Fernandes 51 ); however, it is sensitive to dietary DHA content with an age-related relationship (higher at 26 d with 1 % DHA, but higher at 35 d with 5 % DHA) in European sea bass larvae( Reference Betancor, Izquierdo and Terova 52 ). The expression levels of MyoD and Myog mRNA have been shown to be independent of diet type (Artemia nauplii v. commercial diet) in pacu larvae( Reference Leitao, Dal Pai-Silva and Losi Alves de Almeida 53 ), and MyoD mRNA has also not shown to be modified by the nucleotide enrichment of live feed in Atlantic cod larvae( Reference Lanes, Bolla and Fernandes 51 ). In one study( Reference Alami-Durante, Cluzeaud and Bazin 22 ), the importance of dietary cholecalciferol level for the expression of MRF in European sea bass larvae has been revealed, and it has been shown that according to larval age, not all MRF were similarly regulated by this nutrient. The understanding of the nutritional regulation of the early expression levels of MRF and MHC is thus only partial; however, it showed that the effects of nutrients are a function of larval age, and thus of larval stage of development, and consequently on the advancement of the myogenic process. Any nutrient that might have an impact on the early expression of MRF might have an impact on the number of myotubes formed and consequently, as fibre hypertrophy is limited by diffusion distances, govern future fish growth.
Despite the significant effects of the early decrease in dietary protein:energy ratio by fat addition on the expression of fast-MHC at 10 and 30 df, there were no significant differences in the distribution of white muscle fibre diameters of HF diet- and LF diet-fed rainbow trout alevins after 30 df, indicating a delay between the changes in fast-MHC mRNA expression and myosin protein synthesis, and/or post-transcriptional regulation. The cellularity of rainbow trout white muscle thus appeared to be independent of large differences in first-feeding dietary lipid content after 30 df. This might be due to a positive post-hatching effect of the high lipid reserves of rainbow trout embryos. The protein sources( Reference Alami-Durante, Fauconneau and Rouel 18 , Reference Ostaszewska, Dabrowski and Wegner 19 ), DHA:EPA ratio( Reference Galloway, Kjorsvik and Kryvi 20 ), lecithin sources( Reference Alves Martins, Estevez and Stickland 21 ) and cholecalciferol level( Reference Alami-Durante, Cluzeaud and Bazin 22 ) utilised in first-feeding diets have been differently shown to provide a significant impact on white muscle cellularity of larvae having reduced reserves at hatching. These differences in cellular responses to the changes in dietary composition might be linked to specific nutrient effects and/or to species-specific differences (quantity and quality of the reserves present at hatching). Further studies are needed to clarify the early nutritional regulation of muscle growth dynamics in different species of aquaculture interest.
The hypothesis of a delay necessary to observe a phenotypic consequence of the decrease in the relative expression level of fast-MHC mRNA in alevins fed the HF diet is supported by the changes in white muscle cellularity after a longer time of feeding (75–90 df) with this diet. The comparison of the fish at a similar age (75 df) showed that HF diet-fed alevins had a greater total cross-sectional area of white muscle, achieved by greater maximum hypertrophy of white muscle fibres. Given that the size of HF diet- and LF diet-fed alevins was different at 75 df, their differences in white muscle cellularity at this age could be attributed both to the changes that occur normally in trout white muscle cellularity when the body length increased( Reference Weatherley, Gill and Rogers 7 , Reference Stickland 8 ) and to potential changes linked to the changes in dietary protein:energy ratio by the addition of lipids. The comparison of alevins of similar body size (HF diet-fed alevins at 75 df v. LF diet-fed alevins at 90 df) provided an explanation regarding which muscle growth processes the alevins fed the two diets utilised to reach a similar body size. To reach a similar body size, LF diet-fed alevins made more muscle at the vent level than HF diet-fed alevins, and they made more muscle both by a significantly higher recruitment of new white muscle fibres, as indicated by a total number of white muscle fibres being 58 % higher, and by higher hypertrophy of large fibres, as indicated by the greater number of large fibres and the maximum diameter of large fibres. Thus, there was in this experiment no relationship between high white muscle hyperplasia and rapid somatic growth. Such a relationship has been proved to exist during the early life of some other fish species fed differently, such as common carp larvae and pikeperch larvae fed compound diets that contained different protein sources( Reference Alami-Durante, Fauconneau and Rouel 18 , Reference Ostaszewska, Dabrowski and Wegner 19 ), Atlantic cod larvae fed enriched rotifers that differed in their DHA:EPA ratio( Reference Galloway, Kjorsvik and Kryvi 20 ) and gilthead sea bream larvae fed compound diets that contained different lecithin sources( Reference Alves Martins, Estevez and Stickland 21 ). The fact that there was no relationship between high white muscle hyperplasia and rapid growth when trout alevins were fed high lipid levels is probably due to the fact that the greater increase in the body weight of HF diet-fed alevins was not fully explained by their increase in the total cross-sectional area of white muscle at the vent level. The present results, with fewer white muscle fibres and lower maximum hypertrophy of white muscle fibres in HF diet-fed alevins when compared with LF diet-fed alevins at similar body weights (75 df for the former v. 90 df for the latter), suggest preferential deposition of supplementary energy in tissues other than caudal skeletal muscle. A recent study in weaning rats has led to similar conclusions: a maternal postnatal HF diet (i.e. a HF diet provided from birth onwards) promoted an obese phenotype, characterised by high body fat, fat accumulation in the retroperitoneal area, and altered skeletal muscle morphology with reduced myofibre density( Reference Pantaleao, Teodoro and Torres-Leal 54 ). We did not find a link between the relative expression level of fast-MHC mRNA, white muscle cellularity and body growth in rainbow trout alevins fed the HF and LF diets. The higher expression level of fast-MHC mRNA in LF diet-fed alevins at 30 df did not correlate with greater muscle growth, a different muscle growth process or greater body growth. Similarly, there was no link observed between fast-MHC mRNA expression, muscle cellularity and body growth in juvenile rainbow trout fed different protein sources with different amino acid profiles( Reference Alami-Durante, Medale and Cluzeaud 28 , Reference Alami-Durante, Wrutniack-Cabello and Kaushik 29 ) and no correlation observed between fast-MHC mRNA expression and body growth in the juveniles of Senegalese sole fed different dietary lipid levels( Reference Campos, Valente and Borges 55 ). In contrast, a link between fast-MHC mRNA expression, muscle cellularity and body growth has been demonstrated in European sea bass larvae fed different levels of cholecalciferol( Reference Alami-Durante, Cluzeaud and Bazin 22 ). These examples suggest that the correlation between fast-MHC mRNA expression, muscle cellularity and body growth might be species-specific, stage-specific and nutrient-specific. Early postnatal (mammals) and post-hatching (birds, reptiles, amphibians and fish) stages are, for all vertebrates, stages during which cell multiplication and differentiation continue to occur with, in addition to organ specificity, group specificity and species specificity within each group. The early stages of fish that hatch with yolk reserves and an unopened mouth appeared from the present study to be very sensitive to an early decrease in dietary protein:energy ratio by the addition of lipids, which was shown here to modify the balance between myogenesis and adipogenesis in trout alevins. These findings, which are innovative for fish, expand the growing understanding of the negative effects of early-life obesity. The present results in trout are consistent with those obtained when young mice are fed a HF diet (heavier animals with reduced muscle mass( Reference Woo, Isganaitis and Cerletti 56 )).
Long-term effects of initial feeding with a high-fat or a low-fat diet
Interestingly, we also demonstrated that an early decrease in dietary protein:energy ratio by the addition of lipids had a long-term effect on the body growth of juvenile rainbow trout that were fed the HF diet during the first 75 df maintaining their higher body weights after three additional months of feeding with a commercial diet. Our findings confirmed that first feeding dietary composition had an impact on juvenile growth, as already demonstrated by Koedijk et al. ( Reference Koedijk, Folkvord and Foss 57 ) with Atlantic cod larvae fed various live prey, and extended understanding by highlighting the importance of a change in a specific nutritional component for first-feeding rainbow trout. There was a persistent effect of initial feeding with the HF diet on the muscle growth of rainbow trout juveniles, as indicated by a greater total cross-sectional area of white muscle after 3 mf with the commercial diet in initially HF diet-fed juveniles than in initially LF diet-fed juveniles. The higher body weight and the greater total cross-sectional area of white muscle of initially HF diet-fed juveniles did not correlate with the greater expression level of fast-MHC mRNA. Initial feeding of Atlantic cod larvae with different live prey also had no effect on the expression levels of MHC in juveniles fed a commercial diet to satiety for 3 months( Reference Koedijk, Le François and Blier 58 ). However, we provided evidence that an early decrease in dietary protein:energy ratio by the addition of lipids induced long-term differences in the expression levels of genes involved in the early steps of myogenesis with, when compared at similar body weight after the commercial feeding period, higher relative expression levels of MyoD1 and Myog mRNA in initially LF diet-fed juveniles (i.e. in fish that promoted muscle growth when compared at similar body weights at the end of the experimental feeding period). This is consistent with the findings showing that overfeeding mice during the postnatal life with a HF diet reduces myogenic stem cell function( Reference Woo, Isganaitis and Cerletti 56 ). Taking into account that there was a delay between changes at the mRNA and cellular levels in alevins, a longer commercial feeding period might be similarly necessary in juveniles to observe the cellular consequences of higher expression levels of MyoD1 and Myog mRNA in initially LF diet-fed juveniles. Another proof of a persisting effect of initial feeding on muscle growth came from a comparison of juveniles at similar body weights (initially HF diet-fed juveniles after 3 mf with the commercial feed v. initially LF diet-fed juveniles after 6 mf+16 df with the commercial feed). This comparison showed that initially HF diet- and LF diet-fed juveniles did not achieve the same total cross-sectional area of white muscle by the same process: initially LF diet-fed juveniles retained the ability to maximise fibre enlargement that they had after 90 df for at least three further months. To our knowledge, this constitutes the first evidence of a long-lasting effect of early nutrition on the expression of MyoD1 and Myog mRNA and on the cellularity of white axial skeletal muscle in a fish species. This finding demonstrates that the activity of muscle precursor cells present in the white muscle of trout juveniles (i.e. satellite cells) might be programmed by early nutrition.
Acknowledgements
The authors thank P. Aguirre and P. Peyrotte for preparation and analysis of the HF and LF diets, as well as J. P. Fourriot and F. Vallee for their assistance with fish rearing and sampling.
The authors' contributions are as follows: H. A.-D. conceived the reserach on muscle development and growth. H. A.-D. and F. M. designed the grading, sampling and feeding protocols. M. C., C. D. and V. G.-D. performed the molecular, histological and chemical–histochemical analyses of alevins; P. M. was responsible for fish farm work; H. A.-D. analysed the data on body growth and the data on muscle development and growth. F. M. analysed the quantitative data on lipid deposits; H. A.-D. interpreted the results and wrote the paper.
There are no conflicts of interest.











