The health benefits of consuming long-chain (LC) n-3 PUFA, particularly EPA and DHA, are frequently reported. These range from cardiovascular benefits1–Reference Siscovick, Raghunathan and King4 to aiding in the treatment of immune disorders, mild hypertension and rheumatoid arthritisReference Connor5, Reference Simopoulos6. LC n-3 PUFA are obtained predominately from fish, seafood, meat and eggsReference Meyer, Mann, Lewis, Milligan, Sinclair and Howe7 but in recent years food products such as bread, milk, margarine, eggs and luncheon meats have been enriched with LC n-3 PUFA in Australia. Considering the importance of LC n-3 PUFA in the human diet and that accurate assessment of their intakes could be useful in healthcare settings and nutritional research, there remains no single agreed method to estimate dietary intakes of these fatty acids. Possible dietary assessment methods are the 24 h recall, diet history, FFQ and food records (FR), which are discussed in more detail elsewhereReference Biro, Hulshof, Ovesen and Cruz8. Overall, the FFQ may be the best dietary assessment method, particularly for nutrients such as LC n-3 PUFA, where intake can vary considerably day to day, as it generally has a lower participant burden than other methods, is inexpensive and quick to administerReference Zulkifli and Yu9.
FFQ currently utilised to estimate LC n-3 PUFA intake have been in use for many years but may not be providing accurate estimates of intake. Some of these FFQ assess several nutrients or the whole diet and do not differentiate between different species of fish or include other types of seafoodReference Woods, Stoney, Ireland, Bailey, Raven, Thien, Walters and Abramson10–Reference Broadfield, McKeever, Fogarty and Britton12. Other sources of LC n-3 PUFA such as meat and eggs are often disregarded and fortified foods, being relatively new, are not included. Many fail to differentiate between different cuts of meat, for which LC n-3 PUFA content can vary significantlyReference Meyer, Tsivis, Howe, Tapsell and Calvert13. As LC n-3 PUFA are present in only a small range of foods, a more tailored FFQ is needed to estimate intakes. Up until recently, the databases available were the Composition of Foods AustraliaReference Cashel, English and Lewis14 where the data for fatty acids were expressed as g per 100 g of edible portion but only to 1 decimal place. Given that the LC n-3 PUFA can be present in foods at levels of 0·04 g or lower, these values would be rounded down to zero and hence these LC n-3 PUFA would be underreported. The limitation of these Composition of Foods Australia databases was rectified by Mann and colleaguesReference Mann, Sinclair, Percival, Lewis, Meyer and Howe15, who established a new fatty acid database with values to 2 decimal places, including 1044 foods derived from analytical data, to incorporate into Foodworks nutrient analysis software (Foodworks®, version 3.02; Xyris software, Brisbane, Australia).
It is important that any new FFQ is tested for reproducibility and validity. Reproducibility refers to how consistently the instrument produces the same values when it is retested under the same conditionsReference Zulkifli and Yu9. Validity is described as how accurately a method or instrument measures that which it is supposed to measure. A validity study determines to what degree a dietary method agrees with another dietary intake methodReference Cade, Thompson, Burley and Warm16. A weighed FR should be the first method chosen to validate a FFQ because, compared with other dietary methods, it has the least correlated errors with the FFQ as the portions are weighed and there is no reliance on memoryReference Cade, Thompson, Burley and Warm16, Reference Willet17. However, a limitation of the FR is that subjects begin to focus on what they are eating and hence may change their eating behaviour. A comparison of results of this FFQ to erythrocyte LC n-3 PUFA status has been published elsewhereReference Sullivan, Williams and Meyer18, but validation against another dietary intake method has not been previously reported and is necessary since erythrocyte levels may be affected by factors other than dietary intakes.
The aims of the present study were to: (1) assess the ability of a new FFQ to estimate mean dietary intakes of LC n-3 PUFA in healthy adults by comparing them with those intakes as assessed by a 3 d FR; (2) determine if there was any systematic bias in the new FFQ; (3) assess if the FFQ was reproducible; (4) determine how effectively the FFQ could rank individuals into quintiles according to intakes of LC n-3 PUFA.
Experimental methods
Recruitment
Study volunteers were recruited for the validity study via poster advertisements and email to staff and students of the University of Wollongong, Australia. Friends and relatives of the study volunteers were also invited to participate in the study. There were no exclusion criteria with the sole requirement being that subjects had maintained a constant diet over the previous 3 months. Vegetarians were encouraged to volunteer to ensure a wide range of dietary habits among the subjects. Based on previous results, it was determined that a minimum of fifty subjects should be sufficient to detect an effect size of 1 with more than 99 % power at a significance level of 0·05, assuming that the correlation between methods is 0·6Reference Sullivan, Williams and Meyer18. The effect size is the difference between the means of the dietary intakes estimated by the FFQ and FR methods, divided by the standard deviation of either method. Subjects attended a single clinic appointment at the University of Wollongong. Digital scales and a wall-mounted stadiometer were used to measure weights and heights, respectively. Subjects then completed the FFQ and were given instructions on how to complete a 3 d weighed FR. Completed FR were, in most cases, returned up to 2 weeks later.
For the reproducibility study, subjects were recruited as described for the validity study and it included recruitment from the Australian Capital Territory, Australia.
All subjects were well informed about their role in the research project and gave their written consent. Approval to conduct the study was obtained from the University of Wollongong Human Research Ethics Committee.
FFQ development and analysis
A semi-quantitative FFQ was developed with a list of food items that are major contributors to LC n-3 PUFA intake, such as seafood, meat, eggs, LC n-3 PUFA-fortified products and fish oil capsulesReference Meyer, Mann, Lewis, Milligan, Sinclair and Howe7, Reference Ollis, Meyer and Howe19. As LC n-3 PUFA only occur naturally in fish, seafood, meat and eggs and more recently in fortified foods such as bread, milk and margarines, the questions were designed to capture all of these foods. The twenty-eight item semi-quantitative FFQ consists of a list of food items that are currently the only major contributors to LC n-3 PUFA intake. The FFQ assesses usual dietary habits related to LC n-3 PUFA intake over the previous 3 months. Frequency of consumption options for each food item included up to ten different choices ranging from never to intake per d, week and month. Questions assessed the specific type or brand of food consumed for items such as LC n-3 PUFA-fortified milk, bread, margarine and eggs. The quantity consumed was assessed with a multiple-choice format using natural units of quantity, for example, slices of bread or number of eggs. Open-ended questions allowed the subject to specify what types of meats and chicken were most commonly consumed and a diagram was used to assess the serving size generally eaten as a portion of a standard dinner plate. Participants were required to specify whether they normally consumed less than, the same as or more than the specified portion. The quantity of fish and seafood consumed was assessed using an open-ended question format due to the large variety of seafood products available and the varying portions consumed. In the case when the subject was unable to estimate how much fish or seafood was normally consumed, the portion size of meat and chicken normally eaten was used. Where the participants did not use a weight (g) to indicate how much food was normally consumed, an average portion size was used, based on typical weights of portions sold in Australian supermarkets.
The FFQ was self-administered by subjects and took approximately 15 min to complete. Subjects were encouraged to ask questions about how to answer specific questions in the FFQ before submission. The FFQ was checked for completeness and clarification of answers while the subject was present. To aid in the estimation of amounts of canned fish usually consumed, three different can sizes were used as prompts. For the reproducibility study, the FFQ was administered twice, 4 to 6 weeks apart.
Food record
The FR used as a comparison method has been used in a number of clinical trials at the University of WollongongReference Martin, Tapsell, Denmeade and Batterham20. Subjects were provided with kitchen scales (to 1 g accuracy), measuring cups, spoons, food recording sheets and written instructions on how to complete the FR. Each subject was shown an example FR and given verbal instructions on how to complete the FR and accurately measure foods. Subjects were told to estimate, to the best of their ability, the weight of foods that they consumed away from home. The importance of maintaining normal diets was heavily emphasised. The FR was completed by each subject on three different days – 2 week days and 1 d of the weekend (not necessarily consecutive days). Subjects were asked to indicate on their recording sheets if the food recorded represented normal dietary intake.
The Foodworks nutrient analysis software package (Foodworks®, version 3.02; Xyris software) was used to determine total daily energy intake for each subject from their FR. When the food recorded could not be matched exactly or a food package/wrapper or a recipe was not included, the best possible match was found in Foodworks and used as a substitute. For example, Turkish bread, a food item not included in Foodworks, was substituted with foccacia bread. Recipes provided by subjects were also entered into Foodworks. All subjects' FFQ and FR were coded and data were entered and analysed by the first author.
A subset of subjects (n 18) were asked to do a repeat 3 d FR (recording the other weekend day and 2 out of the other 3 weekdays that they had not previously recorded) within 6–8 weeks of completing the first FR to determine how reliably one 3 d FR measures LC n-3 PUFA.
Long-chain n-3 PUFA intakes
The Royal Melbourne Institute of Tecnhology (RMIT) fatty acid database within the Foodworks nutrient analysis software package, which is based on the Composition of Foods Australia Nutrient Database21, was used to determine the average daily intake of LC n-3 PUFA for both the FFQ and FR. Where the database did not have any LC n-3 PUFA contents for specific brands of canned tuna or salmon, the contents were taken from published literatureReference Sinclair, Oon, Lim, Li and Mann22 or data were sought from manufacturers. The fatty acid compositions of eel and some fish varieties were not available in Australian databases and were obtained from New Zealand fatty acid composition tablesReference Quigley, Burlingame, Milligan and Gibson23 and added to the database. LC n-3 PUFA contents were expressed per 100 g edible portion.
As many subjects did not know the exact brand of the canned tuna they normally consumed and the LC n-3 PUFA contents of most brands of tinned tuna were unknown, an average value was derived to use in such cases to analyse both the FFQ and FR. The LC n-3 PUFA content of some fortified foods and supplements was also not listed in the databases and therefore this was determined from nutrition information panels located on food labels. If this information was not specific, for example, individual values were not given for EPA, docosapentaenoic acid (DPA) and DHA, the manufacturer/distributor was contacted. When this information could not be obtained, values were assigned based on similar products. Total EPA, DPA and DHA intakes were determined by multiplying food intake (g/d) with EPA, DPA and DHA contents of foods (mg/g edible portion). Total LC n-3 PUFA intakes for both methods were calculated by adding the EPA, DPA and DHA intakes.
Comparison of FFQ and food records
Mean (sd) daily total LC n-3 PUFA, EPA, DPA and DHA intakes were determined using both the FFQ and FR. Because the FFQ and FR intake data were not normally distributed, Wilcoxon signed rank tests were used to compare differences between the two methods. The calculation of non-parametric Spearman's correlation coefficients determined the existence of significant linear relationships between the FFQ and FR intakes. Bland–Altman analyses were used to: (i) determine if there was any systematic bias between the FFQ and FR; (ii) assess the agreement between the FFQ and FR methodsReference Bland and Altman24.
Subjects were also ranked in ascending order of their daily total intake of LC n-3 PUFA estimated from the FFQ and FR and separated into quintiles. The percentage agreement was determined between the quintile assignments using the FFQ and FR.
Statistical analysis
Statistical analysis was performed using JMP statistical analysis program (version 5.1; SAS Institute, Cary, NC, USA). Statistical significance was set at α = 0·05 for all analyses.
Results
Fifty-three subjects (twenty males and thirty-three females) were recruited for the validity study, including seven self-identified vegetarians. Two vegetarians were vegans, one ate extremely small amounts of fish and two individuals were lacto-ovovegetarians. The remaining two vegetarians reported eating significant amounts of fish in the FFQ and/or FR. Subject characteristics are presented in Table 1.
Table 1 Characteristics of the validation study group*
(Mean values, standard deviations and ranges for thirty-three females and twenty males)

* For details of subjects and procedures, see Experimental methods.
All fifty-three subjects completed the FFQ. Two subjects out of the total fifty-three did not submit a FR, five subjects were excluded because their daily energy intakes were unrealistically lowReference Goldberg, Black, Jebb, Cole, Murgatroyd, Coward and Prentice25 and one subject was excluded on the basis that they had changed their diet significantly between the FFQ and the FR. In total, forty-five subjects (seventeen males and twenty-eight females) were included in the final analysis. Three subjects reported that they were taking fish oil capsules in the FFQ but did not record using them when they completed their FR. After determining that the subjects were no longer taking the supplements, their FFQ intakes used for this analysis were calculated without the fish oil capsules.
FFQ and food record analysis
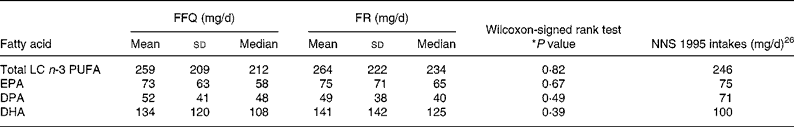
Total LC n-3 PUFA intakes ranged from between 0–1110 mg/d and 0–1060 mg/d for the FFQ and FR, respectively. The mean, standard deviation and median LC n-3 PUFA intakes (mg/d) are shown in Table 2. Mean differences between the two methods (FR – FFQ) were found to be 5, 1, − 3 and 7 mg/d for total LC n-3 PUFA, EPA, DPA and DHA respectively. There was no significant difference found between any of the estimates made by the FFQ and FR. Furthermore, the mean FFQ intakes were slightly higher than or consistent with the 1995 Australian National Nutrition Survey intakes (Table 2). The FFQ DPA intakes were lower than those reported in the 1995 Australian National Nutrition SurveyReference Howe, Meyer, Record and Baghurst26 and this is most likely because the present study group included 10 % vegans. Spearman's correlation coefficients between intakes estimated using the FFQ and FR were determined. Strong significant correlations of 0·75 were found between the two methods for total LC n-3 PUFA as shown in Fig. 1. Correlation coefficients for EPA, DPA and DHA were 0·64, 0·62 and 0·72, respectively.
Table 2 Intake of total long-chain (LC) n-3 PUFA, EPA, docosapentaenoic acid (DPA) and DHA estimated from the FFQ and measured from 3 d food record (FR) in mg/d and comparison with the Australian 1995 National Nutrition Survey (NNS) intakes (n 10 851)†
(Mean values, standard deviations and medians for twenty-eight females and seventeen males)
* Significant at P < 0·05.
† For details of subjects and procedures, see Experimental methods.
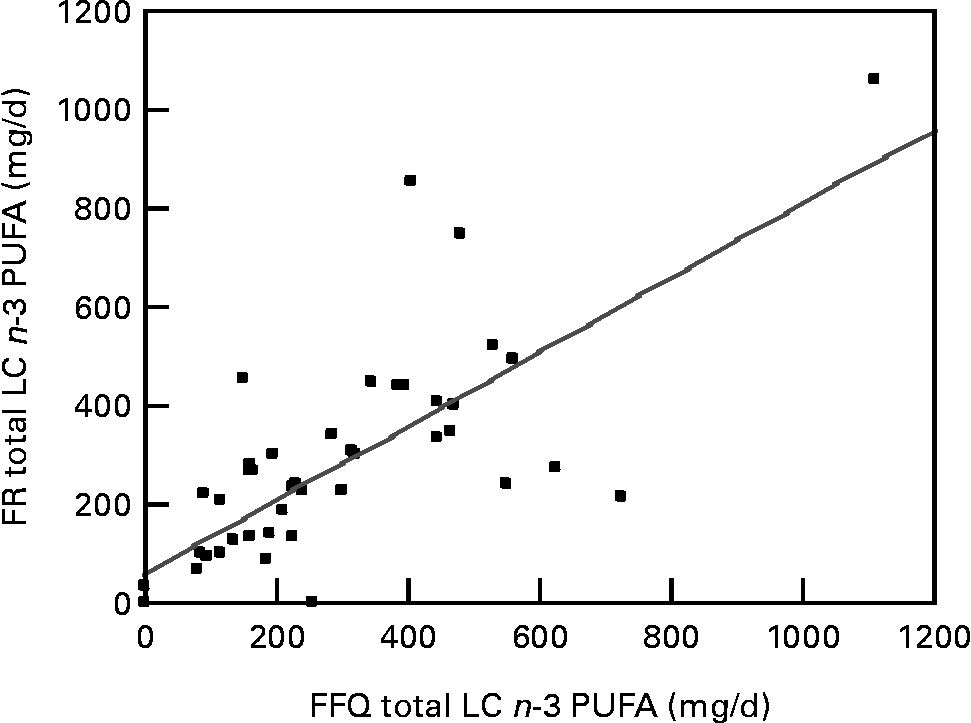
Fig. 1 Bivariate plot of long-chain (LC) n-3 PUFA intakes (mg/d) measured from the food record (FR) v. LC n-3 PUFA intakes (mg/d) estimated from the FFQ. Spearman correlation coefficient r 0·75 (P < 0·001). For details of subjects and procedures, see Experimental methods.
Eighteen subjects (six males and twelve females) submitted a repeat FR. Mean intakes of LC n-3 PUFA, EPA, DPA and DHA for the sub-group (n 18) who completed the repeat FR were 253 (sd 190), 74 (sd 61), 41 (sd 24) and 137 (sd 125) mg/d, respectively. Comparison of these subjects' repeat FR with their first FR showed no significant differences in intakes. Re-analysis of the data with the inclusion of the repeat FR showed no significant improvement with correlation coefficients of 0·84, 0·81, 0·71 and 0·77 for total LC n-3 PUFA, EPA, DPA and DHA, respectively (P < 0·05).
The Bland–Altman plots showed that there was no systematic variation between the two methods for total LC n-3 PUFA (Fig. 2 (a)), EPA (data not shown) and DHA (data not shown), i.e. one method did not consistently measure intakes higher or lower than the other. Some systematic bias was apparently present for DPA (Fig. 2 (b)) and the FFQ appears to be affected by random error with approximately 29 % of subjects having different intakes between the FFQ and FR. As mean intakes increased, differences between FFQ and FR intakes increased both negatively and positively.
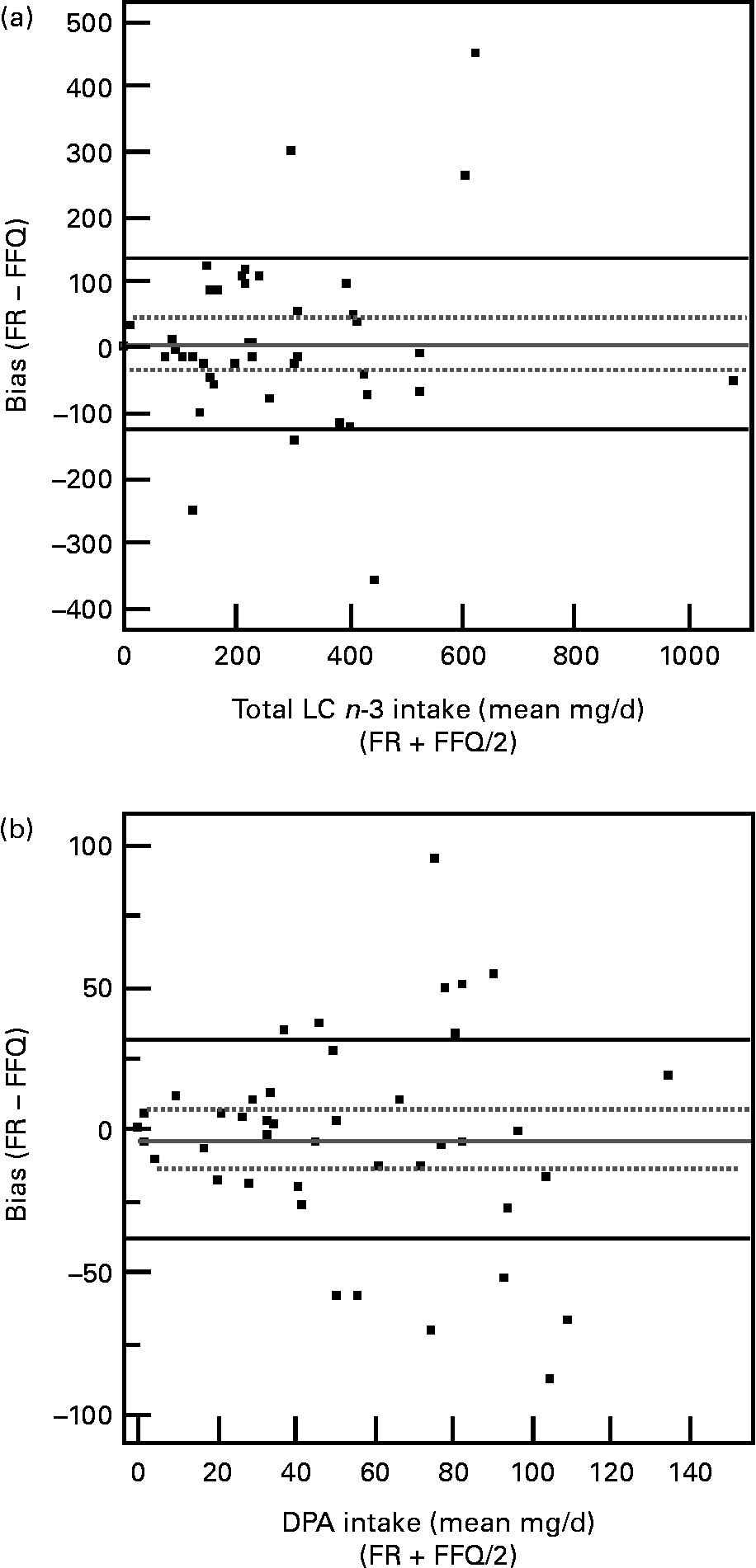
Fig. 2 Agreement between the FFQ and food record (FR) (mg/d) in estimates of (a) total long-chain (LC) n-3 PUFA intakes and (b) docosapentaenoic acid (DPA) intakes as assessed by the Bland-Altman techniqueReference Bland and Altman24. – Mean difference (sd 2). For details of subjects and procedures, see Experimental methods.
Quintile assignments
The agreement of quintile assignment for intake estimates is shown in Table 3. This analysis correctly assigned 49 % of subjects into the same quintiles. A total of 87 % of subjects were correctly assigned either to the same or adjacent quintiles.
Table 3 Agreement of quintile assignment between the FFQ and food record based on total long-chain n-3 PUFA intakes (twenty-eight females and seventeen males)*‡
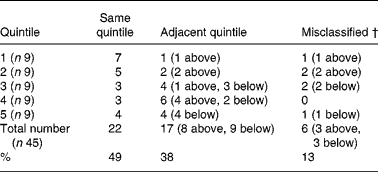
* Number of subjects who have agreement of quintile assignment out of the total number of subjects assigned to that quintile.
† Number of subjects not classified into same or adjacent quintile.
‡ For details of subjects and procedures, see Experimental methods.
Reproducibility
Ten male and twenty-three female subjects, with a mean age of 37 years, were recruited for the reproducibility study. All thirty-three subjects submitted the first FFQ but three failed to return the repeat FFQ. One subject was excluded due to an unusually high intake of fish in the repeat FFQ. Therefore, data for twenty-nine subjects (nine males and twenty females) were used for the analysis. Intakes of total LC n-3 PUFA ranged from 114–1273 mg/d for the first FFQ and 35–1635 mg/d for the repeat FFQ.
The mean (sd) intakes of total LC n-3 PUFA, EPA, DPA and DHA estimated from the two FFQ completed 4–6 weeks apart did not differ and Spearman correlation coefficients are high (Table 4).
Table 4 Intakes of total long-chain (LC) n-3 PUFA, EPA, docosapentaenoic acid (DPA) and DHA estimated from the first FFQ (FFQ 1) and repeat FFQ (FFQ 2)‡
(Mean values and standard deviations for twenty females and nine males)
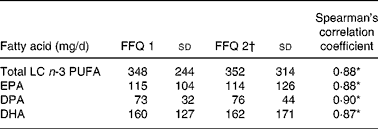
* Significant at P < 0·05.
† Repeated 4–6 weeks after FFQ 1.
‡ For details of subjects and procedures, see Experimental methods.
Discussion
The present study has found that the new FFQ to estimate dietary intakes of LC n-3 PUFA is comparable to a 3 d weighed FR. The FFQ does not show any significant systematic bias, it is reproducible and results from the quintile assignments show that it is a good tool for ranking individuals from lowest to highest intakes. The lack of significant differences found between the intakes estimated from the FFQ and intakes measured from the FR, coupled with strong Spearman correlation coefficients, indicates that the FFQ and FR are comparable methods for determining LC n-3 PUFA intake. The LC n-3 PUFA intakes estimated from the FFQ in this validity study (Table 2) were slightly higher than or consistent with those determined by others in Australian populationsReference Meyer, Mann, Lewis, Milligan, Sinclair and Howe7, Reference Ollis, Meyer and Howe19, Reference Howe, Meyer, Record and Baghurst26, further reinforcing the validity of the FFQ. The intakes estimated using this FFQ have also been shown to be significantly related to a biomarker measure of n-3 intake, namely, erythrocyte LC n-3 PUFAReference Sullivan, Williams and Meyer18. This tailored FFQ to assess LC n-3 PUFA intake is an improvement over previously published tools.
In regard to reproducibility, the FFQ performed better than other more general FFQ, which reported correlation coefficients ranging anywhere from 0·31 to 0·87 for various nutrients and foodsReference Woods, Stoney, Ireland, Bailey, Raven, Thien, Walters and Abramson10, Reference Erkkola, Karppinen, Javanainen, Rasanen, Knip and Virtanen27, Reference Pisani, Faggiano, Krogh, Palli, Vineis and Berrino28. The FFQ also performed better than other validated FFQ for PUFA. A semi-quantitative FFQ to measure dietary fatty acid intakes (over the previous month) was validated with a 7 d FR in a sample of thirty-one subjects from the UKReference Broadfield, McKeever, Fogarty and Britton12. Pearson correlation coefficients between the two methods for EPA and DHA were significant at 0·59 and 0·55. The UK FFQ did not discriminate between cuts of meat and only had very limited choices for fish, which may account for the weaker correlations compared with the present study.
A semi-quantitative FFQ with 181 food items, to estimate total dietary intake over the previous month, was validated with two 5 d FR in a group of pregnant Finnish womenReference Erkkola, Karppinen, Javanainen, Rasanen, Knip and Virtanen27. A significant unadjusted Pearson's correlation coefficient of 0·30 was obtained for the relationship between total n-3 fatty acids (including α-Linolenic acid) measured by the FFQ and FR. Although frequency of consumption options was open-ended, portion sizes were based on commonly used portions from other Finnish dietary studies. Compared with the FR, the FFQ tended to overestimate intakes of total n-3 PUFA and most of the other nutrients by between 19 to 65 %, possibly due to the portion sizes used. The authors do not state whether specific fish and meat varieties or only general categories were included in the FFQ. The FFQ validated in the present study may have performed better than the afore-mentioned FFQ because, for some food items, subjects could indicate gram weights usually consumed on each occasion and the portion-size diagram aided the subjects in indicating the most common portion of meat and chicken consumed. Several questions were open-ended, allowing subjects to report varieties of fish and meat most commonly eaten.
The high correlation coefficients obtained from the FFQ validated in the present study of 0·75, 0·64, 0·62 and 0·72 (for total LC n-3 PUFA, EPA, DPA and DHA, respectively) are comparable with other nutrient-specific FFQ. Intakes of soya isoflavones, genistein and daidzein from a forty-item semi-quantitative soya FFQ were compared with an extensive 122-item FFQReference Frankenfield, Patterson, Kalhorn, Skor, Howald and Lampe29. Pearson product moment correlations between the two methods for genistein and daidzein were highly significant at 0·83 and 0·82, respectively. Montomoli and colleaguesReference Montomoli, Gonnelli, Giacchi, Mattei, Cuda, Rossi and Gennari30 obtained a correlation of 0·90 when they compared Ca intakes determined from a Ca-specific FFQ and a 14 d weighed FR. The strong correlation obtained could be attributed to a consistent intake of Ca by the study population, but it is more likely to be because of its nutrient specificity. These results and those obtained from the present study suggest that nutrient-specific FFQ are able to better estimate intakes of nutrients as opposed to FFQ that assess total dietary intake. While only consisting of twenty-eight items, the FFQ in the present study was detailed enough to capture intakes of all major sources of LC n-3 PUFA and took only 10–15 min to complete.
The Bland–Altman plots created for the total and individual LC n-3 PUFA showed that mean differences between measurements from the FFQ and FR were close to zero and that no systematic variation existed for total LC n-3 PUFA, EPA and DHA, i.e. neither method consistently over- or underestimated LC n-3 PUFA intakes compared with the FR. Although there was some suggestion of greater variability at higher intake levels, the number of data points is too few to demonstrate any consistent trend. An increased number of data points may have clarified whether or not higher intakes suggest greater variability and this is a limitation of the study. However, as the FFQ estimated intake over the previous 3 months and was compared with a 3 d FR, large variations between the two methods are not inconceivable. Increasing the number of recording days in the FR may have improved the consistency between methods as it is difficult to capture habitual consumption of some nutrients, particularly when only 3 d food intake is recorded; however, this would have imposed significant burdens on study subjects and could have reduced compliance and the reliability of records. However, the repeat FR did not alter our results in any way, suggesting that the 3 d FR was long enough to capture accurate dietary intakes of LC n-3 PUFA. Other studies using Bland-Altman analysis have used 7 d FR and still found large variation between intakes of various nutrientsReference MacIntyre, Venter and Vorster31. Dietary intakes of free-living individuals vary from day to dayReference Willet17. Variability, therefore, is inherent in measuring nutrient intakes and, thus, there will never be perfect agreement between methods.
For some subjects, there were considerable differences between FFQ and FR intakes. The FFQ was administered prior to the FR and even though there was a large emphasis on recording on days that represented habitual intake, some subjects may have increased consumption of LC n-3 PUFA-rich foods when completing the FR. A few subjects indicated low frequencies of consumption for meat, chicken and fish in their FFQ but then reported eating significant amounts of them in their FR. It is highly probable that day-to-day variations of LC n-3 PUFA intake caused these differences. As micronutrients and nutrients such as LC n-3 PUFA are likely to be concentrated in certain foods, depending on the food choices for any particular day, intakes could be very low or very highReference Willet17. For nutrients that are in relatively few food sources and may have high within-person variability, such as vitamin C and possibly LC n-3 PUFA, a nutrient-specific FFQ may provide more accurate estimates of usual intake compared with a short FRReference Willet17.
There is a lack of data on the variability of LC n-3 PUFA intakes in human populations and also Australian data on seasonal consumption of meat and seafood. It has been suggested that seasonal variations in intakes of nutrients may existReference Willet17. The present study took place in autumn–winter and it was a concern that intakes could have been different if it had taken place over spring–summer. However, data from the 1995 National Nutrition SurveyReference McLennan and Podger32 suggest that intakes of the major dietary sources of LC n-3 PUFA vary by less than 20 % throughout the year. The FR took into account some of the weekday/weekend variation of food intakes; however, the weekdays during which food was recorded were not equally represented. Data from the European Investigation into Cancer and Nutrition study show that, for both men and women, there was high variability of fish intakesReference Welch, Lund and Amiano33. However, as there are no Australian data for food intakes on individual days of the week, it is unknown if the 3 recording days chosen contributed to the large variations in intakes observed in some individuals. However, in the present study, eighteen subjects completed a second 3 d FR, which did not differ from their previous 3 d FR. Re-analysis of the data including the repeat FR showed no significant improvement, suggesting that intakes of LC n-3 PUFA did not vary considerably.
The FFQ used in the present study performed well and was able to classify the majority of subjects into the same or adjacent quintile on the basis of total LC n-3 PUFA intakes. The level of agreement was highest at the lowest levels of intake, suggesting the FFQ would be particularly useful in screening for low intakes of LC n-3 PUFA. Quintile assignment in the present study was better than or consistent with other studies that measured n-3 PUFA intakes. The FFQ used by Erkkola and colleaguesReference Erkkola, Karppinen, Javanainen, Rasanen, Knip and Virtanen27 classified 62 % of individuals into the same or adjacent quintiles based on total n-3 PUFA intakes. A more recent study by McNaughton et al. Reference McNaughton, Hughes and Marks34 reported a 42 % agreement for LC n-3 PUFA intakes. The ability of this FFQ to classify 49 % of individuals correctly and 87 % of individuals into identical or adjacent quintiles is an improvement over previously published studies.
There are two limitations of the present study: one is the small sample size; the other is the sample selection being more toward an educated group. However, the results from our study group are comparable with the 1995 National Nutrition Survey, which is a survey conducted on over 10 000 Australians (Table 2).
Data from the present study have shown that the new FFQ was well able to estimate LC n-3 PUFA intakes and these intakes were consistent with those from other published studies. The variability of LC n-3 PUFA intakes observed in this study was not a major surprise, considering that LC n-3 PUFA are found in only a small range of foods, and is consistent with the variability inherent in measuring nutrient intakes. The FFQ was able to effectively rank the majority of individuals according to total LC n-3 PUFA intakes. The FFQ is therefore an adequate dietary assessment tool for the estimation of total LC n-3 PUFA intakes in healthy Australian adults. It is an appealing alternative to other dietary assessment methods due to its validity, low subject burden and low cost. The future development of an electronic version of this FFQ will make it more user-friendly and readily accessible for a larger proportion of the Australian population.
Acknowledgements
The authors would like to acknowledge the University of Wollongong for funding the study; Sr Sheena McGhee, Liz Grigonis-Deane and staff at the Exercise Science and Rehabilitation Centre for assistance in, and use of, the clinic facilities; A/Professor Ken Russell for statistical advice and the study volunteers for their involvement in the study.








