Dietary energy is derived from three broad macronutrient classes: protein, lipid and carbohydrates (CHO) (such as starch). Metabolism of these macronutrient classes is known to occur with different levels of efficiency in terrestrial animals(Reference Azevedo, van Milgen and Leeson1,Reference Hua, Birkett and De Lange2) and has been examined in several fish species(Reference Bureau, Hua, France and Kebreab3,Reference Dumas, France and Bureau4–Reference Pérez-Jiménez, Hidalgo and Morales8) . In past studies, varying the dietary macronutrient composition while maintaining the dietary digestible energy content resulted in significant changes in growth performance, feed utilisation and body composition(Reference Saravanan, Schrama and Figueiredo-Silva5,Reference Schrama, Saravanan and Geurden6,Reference Glencross, Blyth and Irvin9) . These studies across three different fish species showed that increasing dietary CHO composition reduced feed intake, growth and the efficiency of dietary energy utilisation, with a preference for protein-derived energy. This clearly indicated that dietary macronutrients were not utilised equally, despite the prevailing theory that only total dietary energy was important, but did not reveal how these effects might be occurring. Many studies have sought to understand the metabolic utilisation of glucose and other CHO in carnivorous fish(Reference Polakof, Panserat and Soengas10,Reference Moon11) . Despite possessing shared metabolic pathways, fundamental differences in the hepatic and extra-hepatic regulation of dietary starch metabolism are beginning to be discovered in fish(Reference Viegas, Trenkner and Rito12–Reference Panserat, Rideau and Polakof15).
Barramundi (Lates calcarifer) are an obligate carnivorous fish species at trophic level 3·8(Reference Froese and Pauly16) and are the basis of a significant aquaculture industry in Southeast Asia and Australia(Reference Jerry17). A series of factorial bioenergetic nutritional models have been developed to provide estimations of feed demand and idealised feed compositions to support growth performance(Reference Bermudes, Glencross and Austen18–Reference Glencross and Bermudes21). Although the aim in finfish aquaculture is to reduce dependence on protein (for costs and sustainability), there has been a resulting shift in dietary energy proportion towards formulations with higher levels of lipids and CHO, with a range of implications for farmed fish(Reference Kamalam, Medale and Panserat13). As expected for a carnivorous fish, growth performance and feed conversion in barramundi were more efficient when a high proportion of dietary energy was supplied as protein, followed by lipid energy(Reference Glencross, Blyth and Irvin9). However, a high proportion of dietary starch was also shown to restrict protein utilisation, suggesting a limited capacity for barramundi to utilise starch-derived energy over protein or lipid energy(Reference Glencross, Blyth and Bourne22). The types of physiological adaptations or underlying molecular mechanisms responsible for such differential macronutrient utilisation and subsequent growth performance are yet to be defined.
Molecular tools such as quantitative gene expression analysis have been successfully applied to understanding the effects of dietary nutrients on intermediary metabolism(Reference Skiba-Cassy, Panserat and Larquier23–Reference Panserat and Kaushik26). This includes key genes regulating glycolysis or gluconeogenesis (glucokinase (gck), pyruvate kinase (pk), glucose-6-phosphatase (g6pca) and fructose-1,6-bisphosphatase (fbp1)), lipogenesis or lipid oxidation (ATP citrate lyase (acyl), stearoyl CoA desaturase (scd), fatty acid synthase (fasn), carnitine palmitoyl transferase 1a (cpt1a), glucose-6-phosphate dehydrogenase (g6pd) and sterol responsive element binding protein (srebf1)). In addition, the activation of signalling cascades (serine/threonine specific protein kinase B (Akt), mammalian target of rapamycin (mTOR), ribosomal S6 kinase (S6), ribosomal protein S6 kinase beta-1 (S6K1), forkhead homebox protein O1 (FoxO1) and FoxO1-3) has been defined as primary regulators of growth and metabolism in fish(Reference Dai, Panserat and Mennigen25,Reference Lansard, Panserat and Seiliez27) . When sampled at peak times after feeding, these studies provide a snapshot of the molecular regulation underlying dietary metabolic changes(Reference Wade, Skiba-Cassy and Dias24). As a complementary technique, the use of stable isotopes is further able to define metabolite flux and re-routing of major macronutrient metabolic pathways within tissues of fish fed different diets. Deuterated water (2H2O) has been particularly effective for assessing contributions of gluconeogenesis to circulating glucose(Reference Viegas, Mendes and Leston28), direct or indirect pathways of hepatic glycogen synthesis(Reference Viegas, Rito and Jarak29,Reference Viegas, Rito and Jarak30) or lipogenesis of hepatic TAG(Reference Viegas, Jarak and Rito31) in European sea bass (Dicentrarchus labrax).
This study sought to evaluate the underlying mechanisms that regulate nutrient utilisation that potentially drive fish performance. Two experiments were used to investigate gene expression, signalling pathways and metabolic labelling changes that define metabolic nutrient preference and metabolic flux re-routing. The first used liver samples from barramundi fed either a high-protein or high-starch diet in a 12-week growth performance study(Reference Glencross, Blyth and Irvin9). The second used liver samples from a complementary metabolic labelling experiment that fed fish the same two diets in the presence of 2H2O to track the fate of dietary starch using 2H NMR(Reference Viegas, Trenkner and Rito12). These experiments defined several potential mechanisms that underlie the utilisation of dietary starch in barramundi. It also highlighted the diversity of mechanisms that regulate growth, metabolism and nutrient utilisation in fish as dietary protein is replaced by CHO.
Methods
All experiments were performed in accordance with the Australian code of practice for the care and use of animals for scientific purposes and were approved by the CSIRO Animal Ethics Committee (approval numbers: A8-2010 and A8-2016).
Fish Expt 1 – macronutrient source
Samples from the protein and starch treatments from a previous experiment were used in this study(Reference Glencross, Blyth and Irvin9), where fish were fed diets formulated to the same digestible energy specifications but were biased to increase the relative contributions from protein or starch (Table 1). Twenty juvenile barramundi (81·2 (se 1·48) g) were allocated to each of the six 300-litre tanks, maintained at 27·8 (se 0·45)°C, dissolved O2 5·6 (se 0·18) mg/l, at flow rates of approximately 3 litres/min and under a 12 h light–12 h dark photoperiod. Three replicate tanks were hand fed one of the two experimental diets for a period of 12 weeks. Diets were fed twice daily (09.00–09.30 and 16.30–17.00 hours) to slight excess based on the loss of observed feeding behaviour. All feed fed and all uneaten feed were accounted for and correction factors applied to obtain an accurate estimate of feed intake. At the end of the 12-week trial, four random fish from each of the three tank replicates for each treatment were sedated in anaesthetic Aqui-S® (0·02 ml/l) 2 h after their final meal, the time by which key gene regulatory pathways of intermediary metabolism in barramundi liver tend to peak(Reference Wade, Skiba-Cassy and Dias24). Blood was collected by caudal vein puncture using a syringe pre-treated with a solution containing 0·2m EDTA, then centrifuged at 3000 g for 5 min and the plasma transferred to a new tube and kept frozen at –80°C until analysis. Fish were euthanised by overdose in anaesthetic Aqui-S® (0·2 ml/l) before liver samples were collected, snap frozen on dry ice and then stored at –80°C prior to further RNA and protein analyses.
Table 1. Formulation, proximate composition and digestible protein and energy parameters of the diets*

* All values are g/kg DM basis unless otherwise shown.
† Peruvian anchoveta fishmeal and fish oil: Skretting Australia.
‡ Wheat gluten and pre-gelatinised wheat starch: Manildra.
§ Cellulose and vitamin-free casein: Sigma.
‖ Vitamin and mineral premix includes (g/kg of premix): vitamin A, 0·75 g; vitamin D3, 6·3 mg; vitamin E, 16·7 g; vitamin K, 3, 1·7 g; vitamin B1, 2·5 g; vitamin B2, 4·2 g; vitamin B3, 25 g; vitamin B5, 8·3; vitamin B6, 2·0 g; vitamin B9, 0·8; vitamin B12, 0·005 g; biotin, 0·17 g; vitamin C, 75 g; choline, 166·7 g; inositol, 58·3 g; ethoxyquin, 20·8 g; Cu, 2·5 g; ferrous Fe, 10·0 g; Mg, 16·6 g; Mn, 15·0 g; Zn, 25·0 g.
Fish Expt 2 – metabolic labelling with 2H2O
The greatest differences in growth performance were observed in fish fed a high proportion of dietary energy in the form of protein or starch; therefore, these two diets were further investigated through a metabolic tracer experiment, meaning that fish were fed their respective diets in the presence of deuterated (heavy) water (2H2O). Initially, two treatment groups of thirty fish each (initial 51·3 (se 0·5) g) were housed in two independent 200 litre recirculated seawater systems maintained at 29·7 (se 0·7) or 29·8 (se 0·8)°C, respectively, and dissolved O2 of 6·4 (se 1·0) or 6·8 (se 0·8) mg/l, respectively. Each group was assigned to one of the two diets protein or starch (Table 1) and fed once daily (09.00 hours) to apparent satiety for 21 d. The fish were sequentially transferred into a separate 200 litre tank enriched with about 3·5 % 2H2O and fed to apparent satiety once per d for 5 d and sampled on day 6, 24 h after their last meal. This 2H2O tank was maintained with an independent closed filtering system but had similar characteristics to each of the holding tanks used during the feeding period in terms of size, volume of water (200 litres), opacity, filtering material and water parameters (28·0 (se 0·6)°C and dissolved O2 of 6·6 (se 1·6) mg/l). Seawater was enriched by the addition of 99·9 % 2H2O (Sigma catalogue no. 151882) as described previously(Reference Viegas, Mendes and Leston28). Fish were sedated using 0·02 ml/l of anaesthetic Aqui-S®, then measured, weighed and sampled for blood from the caudal vein with heparinised syringes. Approximately 100 µl aliquot was centrifuged (3000 g, 10 min), and plasma was stored for quantification of body water (BW) 2H-enrichments. Fish were then euthanised by an overdose in anaesthetic Aqui-S® (0·2 ml/l) before livers were excised, weighed and stored at –80°C until further analysis.
Metabolite assays
Plasma glucose levels (n 12) were measured using an AccuCheckPerforma glucose meter (Roche). Plasma TAG levels (n 12) were determined using a colorimetric commercial kit adapted to microplates (Biomerieux). Plasma free amino acid levels (n 12) were determined using a fluorometric detection method(Reference Fisher, Arias and Quesada32) and using Amino Acid Standard H (Pierce no. 20088) as a reference.
Lipid quantification
The determination of the fatty acid (FA) profile of diets and liver utilised an adapted protocol described by Coutteau & Sorgeloos(Reference Coutteau and Sorgeloos33). Lipids were esterified by an acid-catalysed methylation and to each sample was added 0·3 mg of an internal standard (21 : 0 Supelco). The FA methyl esters were separated by GC using an Agilent Technologies 6890N GC system (Agilent Technologies) fitted with a DB-23 capillary column. The carrier gas used was H2 at a flow rate of 40 ml/min. The GC was programmed with the following temperature, 50–175°C at 25°C min then 175–230°C at 2·5°C min. FA methyl esters were detected by a flame ionization detector with the injector and detector temperatures being set at 250 and 320°C, respectively. The FA methyl esters were detected by comparing peak retention times to known standards (37 Comp. FAME mix, Supelco).
Quantitative real-time RT-PCR
Total RNA was extracted using Trizol reagent (Invitrogen), according to the manufacturer’s instructions, and precipitated by adding 0·5 volumes of isopropyl alcohol and 0·5 volumes of RNA precipitation solution for purity improvement(Reference Green and Sambrook34). Total RNA was DNase digested with the Turbo DNA-free kit (Applied Biosystems). RNA quantity was assessed on a NanoDrop spectrophotometer (NanoDrop Technologies), and RNA quality was assessed using a Bioanalyser (Agilent Technologies) and RNA nanochips (Agilent no. 5067-1511). All RNA samples were diluted to 200 ng/µl. Reverse transcription was performed on 1 µg of total RNA using Superscript III (Invitrogen) with 25 µm oligo(dT)20, 25 µm random hexamers and 400 pg of luciferase RNA (Promega L4561) as an exogenous control gene.
Real-time PCR amplification using primers specific to each gene of interest (online Supplementary Table S1) was performed as previously described(Reference Wade, Skiba-Cassy and Dias24). Real-time PCR amplification reactions were carried out using 1X SYBR Green PCR Master Mix (Applied Biosystems); 0·2 µm of each primer and the equivalent of 7·5 ng of reverse-transcribed RNA. Amplification cycle conditions were 2 min at 50°C, 10 min at 95°C followed by forty cycles of 15 s at 95°C and 40 s at 60°C. Verification that there was no genomic DNA contamination was carried out by PCR amplification of a pool of DNAse-treated RNA samples using gene-specific primers. Normalisation was performed using the ΔCq method (where Cq is the quantification cycle), and expression levels of each gene relative to one another were determined by normalising the cycle threshold values for each gene to the endogenous control gene Ef1α and the exogenous Luciferase control, then to the average cycle threshold of each gene relative to the control diet. The variation in amplification of eef1a1 or luciferase across all samples was 0·63 and 0·10 cycles, respectively (data not presented).
Protein extraction and Western blotting
Frozen liver samples (about 100 mg) were extracted as previously described(Reference Wade, Skiba-Cassy and Dias24), and the resulting supernatants (n 9 for each treatment) were stored at –80°C until required. Protein concentrations were determined using the Bio-Rad Protein assay kit. Quantities of 20 µg protein per sample were separated by SDS-PAGE and analysed for the presence of specific proteins by Western blotting and using the appropriate antibodies. Primary antibodies for the analysis of signalling pathways were obtained from Cell Signaling Technologies (Akt-p no. 9272; Akt no. 9271; mTOR-p no. 2972; mTOR no. 2971; S6-p no. 4856; S6 no. 2217S; S6K1-p no. 9205; S6K1 no. 9202; FoxO1-p no. 9461; FoxO1-3-p no. 9464 and Tubulin no. 2775) or Epitomics (Labome FKHR (Fox-O1) no. 1874-1) and used at a dilution of 1:1000 as described previously(Reference Seiliez, Gabillard and Skiba-Cassy35). After incubation with a goat anti-rabbit IRDye infrared secondary antibody (LI-COR Inc. Biotechnology), bands were visualised and quantified by Infrared fluorescence using the Odyssey Imaging System (LI-COR Inc. Biotechnology).
Metabolite preparation
To obtain a sufficient amount of analytes for generating 2H NMR spectra with a high signal:noise ratio, the livers of five fish were pooled into six replicate groups (pooled analyses: n 6 per diet). Lipids were extracted from homogenised livers according to Matyash et al. (Reference Matyash, Liebisch and Kurzchalia36) using a mixture of methyl tert-butyl ether (Sigma) and methanol (Sigma). Briefly, homogenised livers were added to a mixture of methyl tert-butyl ether:methanol, and after phase separation, the upper lipid phase was carefully separated. The lipid extract was further fractionated into TAG and NEFA using solid-phase extraction (SPE) with prepacked 2 g cartridges (Discovery® DSC-NH2 52641-U, Supelco) according to Ruiz et al. (Reference Ruiz, Antequera and Andres37). Hepatic TAG and NEFA extracts were analysed separately by 2H NMR. Hepatic TAG quantifications were performed in a fully-automated analyzer Miura 200 (I.S.E. S.r.l.) using a dedicated TAG reagent kit (ref. A-R0100000901; n 5).
1H and 2H NMR analysis of lipids
Tank water and fish BW 2H-enrichments were analysed in duplicate from 10 µl samples of water and plasma by 2H NMR as described previously(Reference Jones, Merritt and Malloy38). Tank and plasma water content was assumed to be 96·5 % (35‰ salinity) and 92·0 %(Reference Krebs39) of total sample, respectively. NMR spectra of TAG and NEFA samples were obtained at 25°C with a Bruker Avance III HD system with an UltraShield Plus magnet (11·7 T, 2H operating frequency 500 MHz) equipped with a 5-mm 2H-selective probe with 19F lock and 1H-decoupling coil. Lipids were reconstituted in chloroform containing a pyrazine standard as previously described(Reference Viegas, Araujo and Rocha40) generating well-resolved spectra (online Supplementary Fig. S1). As control for the TAG extraction, a FA:glycerol ratio was calculated from the area of all FA α protons (online Supplementary Fig. S1(a)); A) times 2, divided by TAG-glycerol sn-1,3 protons (online Supplementary Fig. S1(a)); L). If successful, a TAG-only extraction theoretical FA:glycerol ratio should be approximately 3(Reference Duarte, Carvalho and Pearson41). As control for the NEFA extraction, all spectra were confirmed for absent glycerol sn-1,3 proton signals. The FA profile (in percentage) for SFA and unsaturated FA, both PUFA and MUFA were estimated by 2H NMR according to Viegas et al. (Reference Viegas, Jarak and Rito31).
Positional 2H-enrichments were quantified from the 1H and 2H NMR spectra by measuring the 1H and 2H intensities of selected signals relative to the 1H and 2H intensities of a pyrazine standard, after correction for linoleic acid contribution according to Duarte et al. (Reference Duarte, Carvalho and Pearson41). Briefly, this involved, (1) the determination of the 2H-enrichment in the FA terminal methyl group for both TAG-bound FA and NEFA derived from lipogenesis (online Supplementary Fig. S1; A); (2) the determination of the 2H-enrichment in the sn-1,3 glycerol site for newly synthesised or cycled TAG-bound glycerol (online Supplementary Fig. S1; L); (3) the determination of the 2H-enrichment in the MUFA’s allylic protons for desaturation of SFA (online Supplementary Fig. S1; F). Moreover, while the terminal methyl group is enriched with 2H during the first round of FA synthesis (thus indicative of DNL), the α protons (online Supplementary Fig. S1; H) incorporate 2H in the last round of elongation. Therefore, if elongation occurs on pre-existing (unlabeled) FA, the α- and methyl protons will be differentially labelled and will inform of the fractional contribution of elongation to lipid synthesis.
Fractional synthetic rates (FSR; in % per d) from (1) lipogenesis; (2) newly synthesised/cycled glycerol; (3) desaturation and (4) elongation rates (% per d) were estimated by dividing the respective positional 2H-enrichments by that of BW. 2H-enrichments were calculated after systematic subtraction of the values with 0·015 %, taken as the mean background 2H-enrichment(Reference Duarte, Carvalho and Pearson41). If the values were below zero, these were considered as 0·0 for FSR calculation purposes.
Statistical analyses
For comparison of the relationships between blood chemistry, gene expression and signalling pathways, each value was normalised to the average of the entire group and then log2-transformed. Prior to statistical comparison, measured values were assessed for normality using a Kolmogorov–Smirnov test. Where comparison between individual measurements was required, statistical significance was assessed by t test analysis of means allowing 5 % error. Statistical analyses were performed using a combination of StatPlus:Mac 2009 (AnalystSoft Inc.), Statistica (StatSoft) or R-software packages (R-Core Team). For 1H and 2H NMR analysis of lipids, Student’s two-tailed unpaired t test was used to compare means between dietary treatments. Analyses were performed in GraphPad Prism software (GraphPad Software). Differences were considered statistically significant at P < 0·05.
Results
Plasma metabolites
Levels of plasma metabolites were largely unaffected by the proportion of dietary energy supplied as different macronutrients at the sample time assessed. No significant differences were observed between the diets for plasma glucose, TAG levels or free amino acid (Table 2).
Table 2. Plasma and liver metabolites
(Mean values with their standard errors)
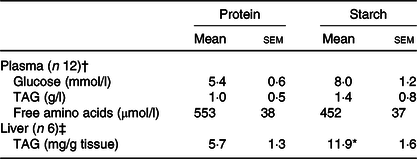
Significant difference between diets (t test; * P < 0·05).
† From fish Expt 1 – macronutrient source, 2 h post-feeding.
‡ From fish Expt 2 – metabolic labelling with 2H2O.
Liver lipids
When the FA content of the liver of animals fed the starch diet was compared with the protein diet, liver TAG levels were significantly elevated (Table 2). After separation of liver FA by GC, there was a significant elevation in the proportion of SFA (38·8–41·3 %) and MUFA (29·5–33·9 %), along with a significant decrease in PUFA (24·9–18·8 %), in particular n-3 PUFA (online Supplementary Table S2). Differences were predominantly caused by changes in 16 : 0 and 18 : 1n-9 cis FA. Decreased proportions were recorded for EPA (7·8–4·2 %), DPA (2·7–1·8 %) and DHA (6·3–4·5 %). The n-3:n-6 ratio found in the liver was significantly lower in starch fed fish compared with protein fed fish (2·9–1·6; online Supplementary Table S2). There was also a shift in the proportion of FA in the liver compared with the levels of FA in the diets, dependent upon the dietary treatment. Compared with the levels in the diet, liver SFA significantly increased by 10·8 % in animals fed the starch diet, along with a 7·9 % increase in MUFA. This caused the greatest relative reduction in liver PUFA in the starch diet fed animals, with 24·7 % less PUFA recorded in the liver than in the diet.
The liver lipid analysis as obtained by 1H NMR for the 2H2O experiment corroborated this profile and statistical changes for the protein and starch diets (online Supplementary Table S3). TAG 1H NMR analysis revealed a consistent FA:glycerol ratio of approximately 3 for protein-fed fish (3·1 (se 0·1)) and starch-fed fish (3·0 (se 0·1)), while NEFA 1H NMR spectra showed no presence of glycerol peaks (online Supplementary Fig. S1(a) (inlet at 4·14 parts per million)). Finally, after the metabolic tracer experiment, hepatic TAG levels were significantly elevated in fish fed with the starch diet (Table 2).
Gene expression
Gene expression changes at 2 h after feeding were generally small, but the expression of several genes was significantly affected by varying the dietary macronutrient source. Compared with the protein diet, barramundi fed the starch diet showed significantly decreased expression of pk and fbp1 but increased expression of pck2. None of the genes regulating FA synthesis or breakdown was affected by diet.
Signalling pathways
There were very few significant changes in the phosphorylation of various signalling molecules in response to different dietary macronutrients. At 2 h after feeding, there was a significant down-regulation in the level of phosphorylated mTOR in fish fed the starch diet (Fig. 2). A similar trend was observed in the average phosphorylation status of S6, S6K1 and FoxO1-3 in starch-fed fish, although this decrease was not significant (Fig. 2). Phosphorylation of FoxO1 tended to increase in response to a starch diet, but again was not considered significant.
Lipogenic flux from 2H2O
Equilibration of BW and tank water was confirmed, with no apparent differences being observed on either diet (protein-fed fish: BW 3·25 (se 0·09) v. tank water 3·46 (se 0·19); starch-fed fish: BW 3·74 (se 0·12) v. tank water 3·79 (se 0·04); 2H-enrichment in %). Separate estimations for lipogenesis revealed differential fluxes for hepatic lipids. The FSR for TAG-bound FA was significantly increased in the starch-fed fish (starch 0·6 (se 0·1) % per d; protein 0·4 (se 0·1) % per d; P = 0·035), while the FSR for NEFA was unaffected by diet (starch 0·9 (se 0·1) % per d; protein 0·8 (se 0·3) % per d; P > 0·05) (Fig. 3; Table 3). The FSR for newly synthesised/cycled TAG-bound glycerol was similarly unaffected by diet (starch-fed fish 2·8 (se 0·3) % per d; protein-fed fish 3·4 (se 0·3) % per d; P > 0·05), even though the FSR of TAG-bound glycerol was found to be 5–10-fold higher than the FSR for TAG-bound FA (Table 3). Estimations for modifications of FA revealed different dynamics (Table 3). For TAG-bound FA, the rate of elongation was significantly lower in starch-fed fish, while the rate of desaturation showed no statistical difference, even if slightly elevated in starch-fed fish compared with protein-fed fish. For NEFA, elongation rates were calculated in nine out of the twelve samples. These revealed no statistical difference between diets. Peaks for calculating desaturation rates on the other hand were not detected in all samples (online Supplementary Fig. S1(b) inlet). In agreement with the increased FSR for TAG-bound FA, there was a significant increase in total hepatic TAG in starch-fed fish (protein 5·7 (se 1·3) mg/g tissue; starch 11·9 (se 1·6) mg/g tissue).
Table 3. Fractional synthetic rate (FSR) for hepatic NEFA and TAG-bound fatty acids (FA) and glycerol (expressed as percentage of newly synthesised FA from lipogenesis per d; FSR in % per d) and modification (elongation and desaturation) rates (expressed as % of FA) in barramundi (Lates calcarifer) fed with protein or starch diet, after a 6 d residence in a tank with approximately 3·5 % 2H-enriched water
(Mean values with their standard errors; n 6 unless indicated)

ND, not detected.
Significant differences between diets (t test; *P < 0·05, **P < 0·01).
† n 5 (1 ND).
‡ n 4 (2 ND).
Discussion
Presently, there is no understanding of the molecular mechanisms that regulate intermediary metabolism in barramundi, a highly valued species of global importance, in response to feeding different dietary energy sources. This study combined gene expression and signalling cascades with a metabolic labelling approach to define the hepatic mechanisms by which barramundi assimilate and store excess dietary CHO energy. These changes underlie the significantly reduced growth performance (protein 3·72 g/d, starch 3·32 g/d)(Reference Glencross, Blyth and Irvin9), increased lipid deposition efficiency (protein 77·3 %, starch 182·8 %)(Reference Glencross, Blyth and Irvin9) and reduced protein energy utilisation coefficient (protein θk PE = 0·557, starch θk PE = 0·412)(Reference Glencross, Blyth and Bourne22) observed in barramundi attributed to the replacement of dietary protein with plant-based raw materials that contain high levels of digestible CHO.
Plasma metabolites and hepatic gene expression
Most carnivorous fish display an elevation of blood glucose levels and prolonged periods of hyperglycaemia after consuming a CHO-rich diet, often associated with increased glycolytic and lipogenic enzyme activity and gene expression(Reference Polakof, Panserat and Soengas10,Reference Moon11,Reference Enes, Panserat and Kaushik42) . Similar to most carnivorous fish, certain forms of starch are highly digested by barramundi(Reference Glencross, Blyth and Tabrett43,Reference Allan, Booth and Stone44) , including >85 % digestibility of the pre-gelatinised starch in this study(Reference Glencross, Blyth and Irvin9). However, 2 h after consuming a high-starch meal, barramundi showed no significant elevation of plasma glucose, TAG or free amino acid compared with protein-fed fish (Table 2). The expression of genes regulating key metabolic pathways was also largely unaffected, with the exception of pk, fbp1 and pck2 (Fig. 1). The lack of increased acyl or fas expression do not suggest that lipogenesis was affected, although strong evidence demonstrating this role was defined in the metabolic labelling experiment, as is further discussed below. This is despite evidence that many barramundi genes displayed significant postprandial regulation, including gk, g6pca, acyl and fas (Reference Wade, Skiba-Cassy and Dias24), which implies the expression of these genes is regulated in a coordinated way after feeding.

Fig. 1. Change in liver expression of genes regulating glucose metabolism (A) or fatty acid metabolism (B) in fish fed a diet that differed in protein or starch macronutrient composition. Expression values are shown as log2-fold change of each gene relative to the expression in the protein diet. a,b Unlike letters indicate significant (P < 0·05) differences between the different diets; nsd denotes no significant difference.
In rainbow trout Oncorhynchus mykiss, elevated dietary starch levels of more than 20 % have been shown to significantly elevate both plasma glucose and hepatic gk gene expression and enzyme activity(Reference Panserat, Médale and Blin45–Reference Capilla, Médale and Navarro47). Barramundi are most closely related to European sea bass Dicentrarchus labrax, common dentex Dentex dentex and gilthead sea bream Sparus aurata, yet the response to dietary starch of these species was similar to that reported in rainbow trout(Reference Enes, Panserat and Kaushik48–Reference Caseras, Meton and Fernandez50), through stimulation of glycolysis rather than inhibition of gluconeogenesis. The inability to down-regulate gluconeogenesis has been suggested as a cause of the deficiencies in post-prandial glucose regulation in fish(Reference Polakof, Panserat and Soengas10,Reference Enes, Panserat and Kaushik42,Reference Skiba-Cassy, Polakof, Seiliez, Saroglia and Liu51) . Gluconeogenesis was down-regulated in carp Cyprinus carpio and sea bream fed 20 % starch(Reference Panserat, Plagnes-Juan and Kaushik46), through reduced gck1 and g6pca expression, respectively, suggesting these fish repress glucose production when not required. The expression of g6pca was down-regulated after feeding in both barramundi(Reference Wade, Skiba-Cassy and Dias24) and sea bream(Reference Caseras, Meton and Vives52). However, sea bass and sea bream fed with high levels of gelatinised starch showed no effect on g6pca mRNA abundance and/or enzyme activity(Reference Viegas, Rito and Jarak29,Reference Meton, Caseras and Fernandez49,Reference Caseras, Meton and Vives52) , with a similar result observed in the present study (Fig. 2). These data suggest that glucose metabolism is not directly regulated by dietary starch/glucose levels in these fish, and the underlying metabolic regulators have not been identified.
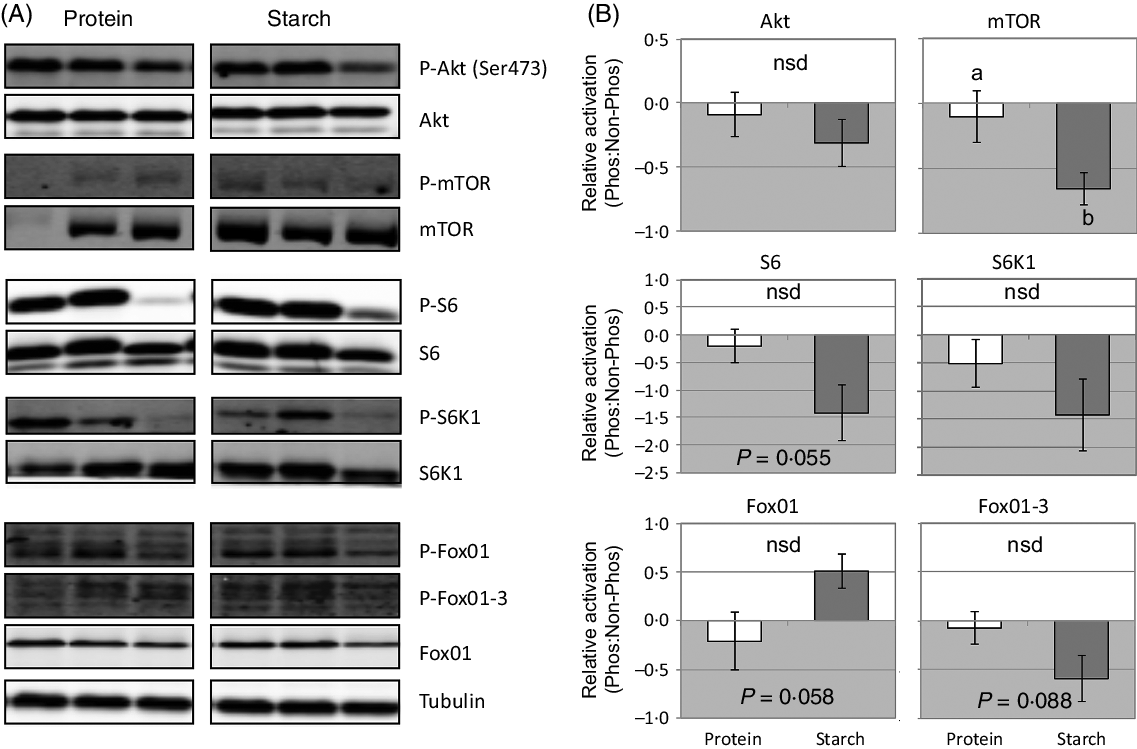
Fig. 2. Change in liver activation of signalling cascades that regulate growth and metabolism in fish fed a diet that differed in protein or starch macronutrient composition. (A) Raw gel images used for quantification of band intensity of active (phosphoryated) to total (phosphorylated and unphosphorylated forms) of each signalling protein, with tubulin used to demonstrate equal protein loading. (B) Mean values with their standard errors (log2-fold change) of the proportion of active to total forms of each signalling protein relative to the activation in the protein diet. a,b Unlike letters indicate significant (P < 0·05) differences between the different diets; nsd denotes no significant difference.
The phosphofructokinase/fructose-1,6-bisphosphatase substrate cycle is another important regulatory locus for glucose metabolism and has been hypothesised as a potential mechanism for the poor regulation of blood glucose levels in fish. The expected shift towards glycolysis over gluconeogenesis in response to the more abundant dietary CHO, through reduction of fbp1 expression or activity, has been observed in sea bass(Reference Garcia-Rejon, Sanchez-Muros and Cerda53), sea bream(Reference Panserat, Plagnes-Juan and Kaushik46,Reference Bonamusa, De Frutos and Fernandes54–Reference Enes, Panserat and Kaushik56) and trout(Reference Skiba-Cassy, Panserat and Larquier23,Reference Kirchner, Kaushik and Panserat57) . The present study in barramundi showed a similar significant down-regulation of fbp1 expression 2 h after feeding as part of their response to dietary CHO (Fig. 2). However, although endogenous gluconeogenesis was also significantly repressed in sea bass fed 30 % dietary starch(Reference Viegas, Rito and Jarak29), half the circulating glucose in starch-fed fish was still derived from gluconeogenic pathways. Gluconeogenesis remains a major contributor of hepatic glucose-6-phosphate synthesis in carnivorous fish(Reference Viegas, Rito and González58), yet the precise contribution of gluconeogenesis in barramundi glucose utilisation remains to be determined. Combined, molecular data suggest that there is at least one component of the gluconeogenic pathway strongly decreased (phosphoenolpyruvate carboxykinase, cytosolic form (pck1), g6pca or fbp1), and/or at least one component of the glycolytic pathway strongly increased (gk, pfkl or pk). These responses form part of a variety of species-specific mechanisms involved in fish metabolism of dietary CHO, although that utilisation remains universally poor in most carnivorous fish(Reference Skiba-Cassy, Polakof, Seiliez, Saroglia and Liu51).
Growth signalling cascades
Previous work has shown that the starch-fed fish used in this study had approximately 11 % reduced growth than the protein-fed fish(Reference Glencross, Blyth and Irvin9) and that this was potentially caused by a reduction in the utilisation of total energy (k E protein = 0·715, starch = 0·481) mostly due to reduced protein energy utilisation (θk PE protein = 0·557, starch = 0·412)(Reference Glencross, Blyth and Bourne22). Therefore, analysis of the activation of the mTOR signalling cascade in barramundi provides a potential mechanism to explain the poor growth performance observed in fish-fed high-starch diets. Changes in phosphorylation status were subtle, and only significant in mTOR and S6, but consistent with the peak of activation of these signalling proteins at the time of sampling(Reference Wade, Skiba-Cassy and Dias24) and their relative position within the signalling cascade(Reference Dai, Panserat and Mennigen25,Reference Lansard, Panserat and Seiliez27) . The role of mTOR as a regulator of growth and whole-body metabolism(Reference Polak and Hall59) is consistent with evidence in barramundi that suggests that as starch levels increase, there is a direct negative effect on growth and energy utilisation, particularly that of protein(Reference Glencross, Blyth and Bourne22). However, past work in trout has shown that dietary fishmeal replacement with plant ingredients (maize and wheat gluten) did not induce differences in the Akt-mTOR signalling pathway(Reference Lansard, Panserat and Seiliez27). In addition, trout Akt-mTOR activation has been shown to be a key regulator of hepatic lipogenesis through stimulation of srebf1, fas and gk expression(Reference Dai, Panserat and Mennigen25), which was not evident in barramundi. Meanwhile, 2 h after a meal of a high-lipid diet, Senegalese sole (Solea senegalensis) displayed prolonged hyperglycaemia and down-regulated the Akt-mTOR signalling pathway, while a high-CHO diet had no effect(Reference Borges, Valente and Veron60). The results of this study combined with past work favours the notion that species-specific mechanisms are central to glucose homoeostasis in carnivorous fish, but may provide a potential basis for the ineffective utilisation of this macronutrient as a direct energy source for growth in barramundi.
Hepatic lipids and lipogenic flux
Tracer methods provide direct measurements of lipogenic fluxes(Reference Ekmann, Dalsgaard and Holm61–Reference Magnoni, Vaillancourt and Weber66), but have still not been widely applied in fish. 2H2O in particular rapidly equilibrates with fish BW(Reference Viegas, Mendes and Leston28) and gets incorporated into newly synthesised or exchanged metabolites as described for glucose and glycogen synthesis in sea bass(Reference Viegas, Mendes and Leston28–Reference Viegas, Rito and Jarak30), lipogenesis of hepatic(Reference Viegas, Jarak and Rito31) and extrahepatic(Reference Viegas, Trenkner and Rito12) TAG, hepatic alanine metabolism in gilthead sea bream(Reference Gonzalez, Caballero and Viegas67), and muscle protein synthesis in catfish (Ictalurus punctatus)(Reference Gasier, Previs and Pohlenz68). In barramundi, hepatic lipogenesis was significantly increased (Fig. 3), which provides direct support for the accumulation of hepatic TAG (Table 2) and FA (online Supplementary Table S2) and increased lipid retention and deposition from high-starch diets(Reference Glencross, Blyth and Irvin9,Reference Glencross, Blyth and Bourne22) . Dietary lipid levels were reduced in the starch diet, leading to significantly reduced gross lipid intake (protein 31·0; starch 19·6 g per fish) in starch-fed fish despite a slight increase in feed intake(Reference Glencross, Blyth and Irvin9). This reduced intake may partially explain the approximately 25 % improved lipid retention but cannot account for the 182 % lipid deposition that is demonstrated here to be a direct result of increased lipogenesis.
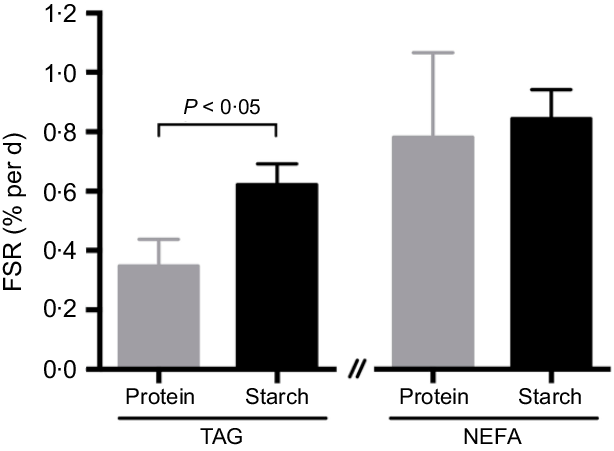
Fig. 3. Fractional synthetic rate (FSR) for TAG-bound fatty acid (FA) and NEFA, expressed as percentage of newly synthesised FA from lipogenesis per d in hepatic lipids of barramundi (Lates calcarifer) fed with a protein or starch diet, after a 6-d residence in a tank with approximately 3·5 % 2H-enriched water. Mean values with their standard errors are presented (n 6). A significant difference between diets is indicated (t test; P < 0·05).
Metabolic flux for NEFA revealed no lipogenic increase, no elongation rate alterations, and no evidence for any desaturation. In a similar feeding and tracer administration setting, sea bass revealed that TAG levels were significantly augmented in the liver in response to high-starch diets(Reference Viegas, Rito and Jarak29), but this feature could not be attributed to an increase in hepatic TAG-bound FA from lipogenesis. Meanwhile, muscle TAG levels remained unaltered, but TAG-bound FA from lipogenesis significantly increased in starch-fed fish, indicative of TAG-FA recycling in response to diet(Reference Viegas, Trenkner and Rito12). In barramundi, no enrichment of muscle TAG could be detected, but in visceral fat, TAG-bound FA and glycerol synthesis/cycling was elevated by starch diets(Reference Viegas, Trenkner and Rito12), although the rate of lipogenesis was approximately 6-fold higher rates in liver tissue. Changes in endogenous lipids and TAG consisted of increased SFA and MUFA at the expense of PUFA, with SFA accumulation mainly driven by an increase in palmitate (16 : 0), the end product of lipogenesis. Combined, the strong enhancement of visceral fat lipogenesis and high TAG-glycerol cycling in starch-fed fish(Reference Viegas, Trenkner and Rito12) further supports the accumulation of large amounts of visceral fat and modifications to whole-body composition observed in barramundi as a result of consuming high-starch diets(Reference Glencross, Blyth and Bourne22).
Observations from other carnivorous species further substantiate that the unaltered expression of lipid synthesis-related gene expression or enzymes 2 h after a meal should not be interpreted as lack of lipogenic potential from dietary starch per se (Reference Enes, Panserat and Kaushik42). In gilthead sea bream, dietary starch affected lipid absorption and transport, probably due to a delay in lipid absorption(Reference Castro, Corraze and Basto69), which would delay any potential response from FAS for several hours. As outlined above, studies in rainbow trout demonstrated that amino acids, and not CHO, are potent stimulators of lipogenesis(Reference Dai, Panserat and Plagnes-Juan70). While mRNA abundance and enzymatic rates involved in the metabolism of dietary CHO provide valuable insights, the 2H2O experiment captured metabolic changes of free-swimming fish fed over 6 d. Changes in endogenous TAG and glycerol synthesis rates provide a holistic lipogenic flux profile that is independent of short-term post-prandial, circadian or stress-induced enzymatic or gene expression fluctuations.
Conclusion
This study demonstrates that barramundi utilise a unique series of specific hepatic regulatory mechanisms to assimilate and store excess dietary CHO energy in the form of lipids, to the detriment of protein utilisation and growth. This study also highlights the potential advantages of using metabolic tracers to track dietary nutrient assimilation over several days of feeding, as this method overcomes many of the limitations of molecular-based studies that select a single time point (often 2, 6 or 24 h post-feeding) to draw conclusions. Although metabolic pathways are shared among carnivorous fish, results from hepatic enzyme assays, gene expression and signalling cascades, as well as lipogenic and metabolic flux analysis, support the notion that there is significant diversity in the underlying regulation of dietary nutrient assimilation in different fish species. This study provides a deeper understanding of the metabolic utilisation of feed ingredients in barramundi and therefore the ability to formulate advanced species-specific feeds.
Acknowledgements
The authors would like to thank Simon Tabrett, Natalie Habilay, Dean Musson, Nick Polymeris and Mike Anderson for assistance with trial maintenance, and Ben Maynard for assistance with trial sampling. The authors would like to thank João Rito and John Jones for assisting in the NMR analysis.
This work was supported by a grant from the Australian Centre for International Agricultural Research (ACIAR) project FIS-2006-141 and co-funded by CSIRO Agriculture and Food. In addition, this work was supported by Fundação para a Ciência e Tecnologia (FCT; Portugal) through national funds with the co-funding by the FEDER, within the PT2020 Partnership Agreement, and COMPETE 2020 (IV SFRH/BPD/90032/2012; MP Centro2020 - ReNATURE; Centro-01-0145-FEDER-000007). The authors also thank Artur Rombenso for considered revision of this manuscript.
Authors’ responsibilities were as follows: B. D. G., I. V. and N. M. W. designed experiments, D. B., S. I. and L. H. T. performed experiments, B. A., N. B., K. D., M. P., L. C. T., L. H. T., C. V., I. V. and N. M. W. performed laboratory analyses and analysed the data. N. M. W., B. D. G., I. V. and S. S. wrote the manuscript. All authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520001051









