Brain serotonin (5-hydroxytryptamine; 5-HT) is involved in a wide spectrum of physiological and behavioural processes. Moreover, variation in central 5-HT transmission is associated with susceptibility to different mental pathologies, such as depression, anxiety, eating disorders, insomnia and drug abuse( Reference Murphy, Lerner and Rudnick 1 , Reference Russo, Kema and Bosker 2 ).
Dietary manipulations have been used as tool to investigate the involvement of brain 5-HT in behaviour, mood and cognition in humans( Reference Young and Leyton 3 ). Such manipulations have also been shown to affect the endocrine and behavioural response to stress in comparative vertebrate models( Reference Höglund, Bakke and Øverli 4 – Reference Lepage, Vílchez and Pottinger 8 ). Many of these effects have been attributed to the immediate precursor of 5-HT, the essential amino acid tryptophan (Trp), which enters the brain from the circulation in competition with other large amino acids (LNAA)( Reference Fernstrom and Wurtman 9 ).
In the teleost homologue of the hypothalamic–pituitary–adrenal axis (HPA axis), the hypothalamic–pituitary–interrenal axis (HPI axis), the corticotropin-releasing factor (CRF) controls the release of adrenocorticotropic hormone from the pituitary, which in turn stimulates synthesis and release of cortisol, the principal glucocorticoid in teleosts( Reference Bonga 10 ). Moreover, 5-HT plays a central role in the regulation of the HPA/I axis by affecting hypothalamic CRF( Reference Dinan 11 – Reference Winberg, Nilsson and Hylland 14 ). However, recent fish studies suggest long-term effects of dietary Trp on stress responsiveness which are independent of hypothalamic 5-HT( Reference Basic, Krogdahl and Schjolden 15 , Reference Basic, Schjolden and Krogdahl 16 ).
In addition to hypothalamic control of the HPI-axis activity, 5-HT in the limbic system in the telencephalon takes part in the regulation of the HPI/A axis( Reference Silva, Martins and Khan 17 ). Moreover, associations between telencephalic 5-HT and HPI-axis activity( Reference Höglund, Sørensen and Bakke 5 , Reference Hoglund, Balm and Winberg 12 , Reference Winberg, Nilsson and Hylland 14 , Reference Silva, Martins and Khan 17 – Reference Øverli, Winberg and Pottinger 20 ) lend further support to similar extrahypothalamic HPI-axis regulation as observed in mammals( Reference De Kloet, Joëls and Holsboer 21 ). Still, studies of the long-term effects of dietary Trp treatment in fish have so far only included neurochemical changes in the hypothalamus, and effects on telencephalic 5-HT neurochemistry might reveal extrahypothalamic mechanisms involved in the regulation of HPI-axis activity.
On this basis, the aims of the current study were to investigate if dietary Trp supplementation resulted in long-term effects on stress responsiveness, and if 5-HT in the hypothalamus or telencencephalon were related to these changes. To do this, Atlantic salmon were given feed containing one, two or three times the Trp content in normal feed for 1 week, a treatment period that has repeatedly been shown to induce behavioural and neuroendocrine changes in several fish species( Reference Höglund, Bakke and Øverli 4 – Reference Lepage, Vílchez and Pottinger 8 , Reference Basic, Krogdahl and Schjolden 15 , Reference Basic, Schjolden and Krogdahl 16 ). Following TRP treatment, the effects on plasma cortisol and 5-HT in the hypothalamus and telencephalon were investigated (8 and 21 d post Trp treatment) in response to standardised acute crowding stress.
Methods
Experimental fish and facilities
The experiment was conducted at the Danish Technical University of Denmark, National Institute of Aquatic Resources research (DTU Aqua) facility located in Hirtshals, Denmark. All animal procedures used in this study followed the policy and ethics as described by Federation of European Laboratory Animal Science Associations. Smoltified Atlantic salmon were obtained from Fister Smolt AS/Marine Harvest and transported to the experimental facility at DTU Aqua. Fish with a mean weight of 125 (sd 33) g were equally distributed in twelve circular tanks (diameter 95 cm and 119 cm height) at an initial density of 22 kg/m3. Each tank had a central column with a diameter of 0·3 m, and a net volume of 600 litres. Water supply to each tank was derived from the reservoir of a central recirculating system. Tanks were fitted with a central bottom drain, connected to a whirl separator fitted to each tank, from which faeces and uneaten pellets could be collected and quantified. A series of apertures on the vertical inlet pipe generated a high velocity in the incoming water, and water flow was adjusted to achieve a water current of approximately 0·9 body lengths/s, or 0·2 m/s, in each tank. Fish were fed ad libitum a diet consisting of dry pellets from Biomar at a feeding ratio of 1·75 % of their body mass. Automated clock belt feeders were used to distribute the feed from 08.00 to 14.30 hours each day. Salinity was maintained at approximately 3·5 % , the photoperiod was 14 h light–10 h dark and the water temperature was 12·3–13·1°C. Water-quality parameters were monitored daily and were as follows: NO3 −<100 mg/l, NO2 −=0–1 mg/l, total ammonia NH3/NH4 +=0–1 mg/l, average water pH had a mean value of 7·53 (sd 0·18).
Experimental design
Three different types of experimental feed were prepared by BioMar AS. These feeds contained different levels of Trp in relation to the total amount of other LNAA. The feeds, specifically developed for Atlantic salmon, were identical in energetic value and differed only in the amount of Trp; the control feed contained 0·44 % Trp (1×Trp), whereas the experimental feeds contained 0·85 % (2×Trp) and 1·2 % Trp (3×Trp). After the acclimation period, the fish were randomly distributed between twelve tanks, four replicate tanks for each of the three diets, and fed for a period of 7 d. Thereafter the fish were fed control feed for 21 d.
After the Trp treatment period, the fish were exposed to standardised crowding stress (500 kg/m) for 1 h by lowering the water level in the rearing tanks( Reference McKenzie, Höglund and Dupont-Prinet 22 ) at days 8 and 21. These sampling times were chosen to investigate the duration of the suppression of the HPI-axis reactivity, appearing between 2 and 10 d after dietary Trp treatment in Atlantic salmon( Reference Basic, Krogdahl and Schjolden 15 ), and to investigate the involvement of hypothalamic and telencephalic 5-HT in these changes. Three individuals from each tank were sampled just after the crowding stress. Sampled fish were anaesthetised with a lethal dose of MS 222 and blood samples were withdrawn. The blood samples were then centrifuged at 15 000 rpm for 5 min and the plasma was separated into 1 ml Eppendorf tubes and frozen at −80°C for later analysis. Moreover, the hypothalamus and telencephalon were dissected out and were frozen in N2, following which they were stored at −80°C for later 5-HT neurochemical analyses.
Analysis of serotonin neurochemistry and plasma cortisol
Brain concentrations of 5-HT and its catabolite 5-hydroxyindoleacetic acid (5-HIAA) were analysed using a HPLC system with an electrochemical detection system. This system consisted of a solvent-delivery pump (LC-10AD; Shimadzu), an auto injector (Spark; Famod), a reverse-phase column (4·6, 100 mm, C18, 3·5 mm; Hichrom) and an ESA Couochen 2 detector (ESA) with two electrodes at −40 and +320 mV. A conditioning electrode with a potential of +40 mV was employed before using the analytical electrodes to oxidise any contaminants. The mobile phase (HPLC buffer solution) consisted of 10·35 g/l monosodium dihydrogen phosphate (NaH2PO4), 0·3252 g/l sodium octyl sulphate, 0·0037 g/l EDTA and 700 ml/l Acetonitril. pH was adjusted to 3·1 by adding concentrated phosphoric acid (H3PO4).
The telencephalon was weighed and homogenised using an ultrasonic disintegrator in a homogenising reagent (4 % perchloric acid containing 0·2 % EDTA and 40 ng/ml dihydroxybenzylamine hydroxide solution). The samples were then centrifuged at 21 000 rpm for 10 min at 4°C.
Telencephalic and hypothalamic 5-HT and 5-HIAA were quantified by comparing sample peak areas with standard solutions of known concentrations and corrected for recovery of the internal standard using HPLC software (CSW; DataApex Ltd). 5-HTergic turnover was quantified as the ratio between concentrations of 5-HIAA and 5-HT.
Plasma cortisol was analysed using a RIA. Plasma samples were diluted in the ratio 1:2 in RIA buffer (containing 0·05 % NaN3), followed by heat treatment for 1 h at 80°C in order to denature proteins. After cooling for 1 h, samples were centrifuged at 1500 rpm for 20 min at 4°C, following which the supernatant containing cortisol was collected and stored at 4°C. Samples were assayed in duplicate, with all tubes containing 100 µl of plasma sample, 200 μl of anti-cortisol antibody (ab1949; Abcam) and 50 μl of hydrocortisone (1, 2, 6, 7-3 H (N); Perkin Elmer). Plasma concentration of cortisol was measured by specific RIA following the procedure described by Mayer et al.( Reference Mayer, Berglund and Rydevik 23 ), which included a comprehensive validation of the steroid RIA for Atlantic salmon plasma, including a comparison between the above heat-treatment method with extraction followed by thin-layer chromatographic separation of the steroids. As there was no significant difference between the two methods, the heat-treatment method was chosen. The intra- and inter-assay CV for the cortisol assay were 6·3 and 12·1 %, respectively.
Statistical methods
Effects of dietary TRP content and sampling time on cortisol and 5-HT neurochemistry were investigated by two-way ANOVA with sampling time and dietary treatment as independent factors. ANOVA was followed by the HSD post hoc test to investigate significant differences between treatment groups. All analyses were performed using the software Statistica version 13 (Dell Software).
Results
Cortisol response to crowding stress
Dietary Trp treatment affected post-stress plasma levels of cortisol significantly (P<0·001; Fig. 1). Cortisol values in fish treated with 3×Trp feed were significantly lower compared with those of fish treated with 1×Trp (P<0·005) and 2×Trp (P<0·0005) feeds (Fig. 1). However, there were no significant differences in plasma cortisol between 1×Trp- or 2×Trp-treated fish (P<0·22; Fig. 1). These effects were independent of being sampled 8 or 21 d post Trp treatment (P=0·11; Fig. 1), and there were no significant interaction effects between sampling time and Trp treatment (P=0·38; Fig. 1).

Fig. 1 Plasma cortisol concentrations, 8 and 21 d after termination of a 7-d dietary tryptophan (Trp) treatment period in smoltified Atlantic salmon exposed to a standardised crowding stress for 1 h. Treatment feed contained 1 (1×TRP), 2 (2×TRP) or 3 (3×TRP) times the Trp content in normal food. Values are means (n), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P<0·05). Significant effects in the two-way ANOVA are as follows. Diet: F 2,64=15, P<0·00001; days after treatment: F 1,64=2·6, P=0.11; diet×days after treatment: F 2,64=0·97, P=0·38.
Serotonin neurochemistry
Dietary Trp affected telencephalic 5-HT turnover significantly (P=0·028; Table 1). Fish treated with 3×Trp had significantly lower 5-HT turnover compared with fish treated with 2×TRP (P=0·023; Fig. 2). However, 5-HT turnover in fish treated with 1×Trp did not differ significantly from that of fish treated with 2×Trp (P=0·23) or 3×Trp (P=0·53) feed (Fig. 2). These effects were independent of being sampled 8 or 21 d post Trp treatment (P=0·28; Table 1), and there were no significant interaction effects between Trp treatment and sampling time (P=0·62; Table 1). Moreover, there were no significant effects of dietary Trp (P=0·14), sampling time (P=0·08) or interactions between these factors on 5-HIAA (P=0·33) (Table 1), nor did dietary Trp content (P=0·26), sampling time (P=0·89) or interaction between these factors (0·44) affect 5-HT concentrations in the telencephalon (Table 1).

Fig. 2 Telencephalic serotonergic turnover 5-hydroxyindoleacetic acid (5-HIAA)/serotonin (5-HT) (A), and concentrations of 5-HIAA (B), 5-HT (C), 8 and 21 d after termination of a 7-d dietary tryptophan (Trp) treatment period in smoltified Atlantic salmon exposed to a standardised crowding stress for 1 h. Treatment feed contained 1 (1×TRP), 2 (2×TRP) or 3 (3×TRP) times the Trp content in normal food. Values are means (n), with their standard errors represented by vertical bars. ![]() , 8 d after Trp treatment;
, 8 d after Trp treatment; ![]() , 21 d after Trp treatment. a,b Mean values with unlike letters that are independent of days after termination of Trp treatment were significantly different (P<0·05). Two-way ANOVA values are presented in Table 1.
, 21 d after Trp treatment. a,b Mean values with unlike letters that are independent of days after termination of Trp treatment were significantly different (P<0·05). Two-way ANOVA values are presented in Table 1.
Table 1 Effects of dietary tryptophan (Trp) content, time (8 or 21 d) after a 7-d period of dietary Trp and interactions between these factors on serotonin (5-HT) turnover (the ratio between 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA)) and concentrations of 5-HIAA and 5-HT in Atlantic salmon exposed to crowding stress for 1 h
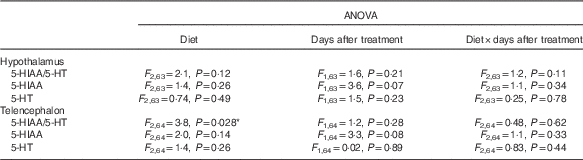
* P<0.05.
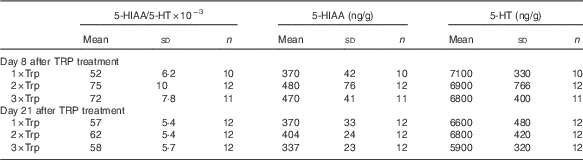
There were no significant effects of dietary Trp treatment on hypothalamic 5-HT turnover that were independent of being sampled 8 or 21 d after Trp treatment in the hypothalamus (P=0·12; Tables 1 and 2). Furthermore, there were no significant effects of being sampled 8 or 21 d after Trp treatment that were independent of sampling time (P=0·21; Tables 1 and 2), and there were no significant interaction effects between dietary Trp content and sampling time (P=0·62; Tables 1 and 2). Moreover, there were no significant effects of dietary Trp (P=0·26), sampling time (P=0·07) or interactions between these factors on 5-HIAA (P=0·34), nor did dietary Trp content (P=0·49), sampling time (P=0·23) or interaction between these factors (0·78) affect 5-HT concentrations in the hypothalamus (Tables 1 and 2).
Table 2 Serotonin (5-HT) turnover (the ratio between 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA)) and concentrations of 5-HIAA and 5-HT in hypothalamus of Atlantic salmon exposed for standardised crowding stress for 1 hFootnote * (Mean values with their standard errors)
Trp, tryptophan.
* Fish were treated with feed containing 1 (1×Trp), 2 (2×Trp) or 3 (3×Trp) times the Trp content in normal feed for period of 7 d, 8 or 21 d before the crowding stress.
Discussion
This study indicates that a 7-d treatment with Trp-supplemented feed resulted in dose-dependent effects on HPI-axis reactivity, observed at both 8 and 21 d post TRP treatment, in seawater-reared Atlantic salmon. Dietary treatment with 3×Trp but not with 2×Trp suppressed post-stress plasma cortisol levels. Moreover, these effects were reflected in 5-HT neurochemistry in the telenecephalon but not in the hypothalamus.
Most studies considering the effects of dietary Trp supplementation report suppressive effects on the behavioural and neueroendocrine stress response. Reduced post-stress plasma cortisol levels have been observed in rainbow trout the day after 7-d treatment with Trp-supplemented feed( Reference Lepage, Larson and Mayer 6 – Reference Lepage, Vílchez and Pottinger 8 ). Studies in brown trout (Salmo trutta)( Reference Höglund, Sørensen and Bakke 5 ), cod (Gadus morhua)( Reference Basic, Schjolden and Krogdahl 16 ) and Atlantic salmon( Reference Basic, Krogdahl and Schjolden 15 ) confirm the stress-reducing effects observed the day after a 7-d Trp treatment period. Still, there are a few studies in which no effect on post-stress plasma cortisol levels could be detected 1 d post Trp treatment( Reference Basic, Krogdahl and Schjolden 15 , Reference Basic, Schjolden and Krogdahl 16 , Reference Martins, Silva and Costas 24 ). In the study performed by Basic et al. ( Reference Basic, Krogdahl and Schjolden 15 ), the suppressive effect on post-stress plasma cortisol appeared between 2 and 10 d after treatment with 3×Trp, suggesting that the effect of Trp can appear later than 1 d after treatment. In agreement with the latter study, suppressive effects of 3×Trp supplementation on post-stress plasma cortisol levels was present 8 d post TRP treatment in our study. Moreover, these effects were present throughout the 21 d post the Trp treatment period, suggesting the involvement of long-term slow-acting processes in Trp-induced alteration of HPI-axis reactivity.
Generally, dietary Trp supplementation seems to enhance brain 5-HT turnover, and several studies indicate that this effect is specific to certain brain regions. For example, dietary Trp affected 5-HT turnover significantly in the hypothalamus( Reference Lepage, Tottmar and Winberg 7 ) and optic tectum( Reference Lepage, Vílchez and Pottinger 8 ) 1 d post Trp treatment, whereas this effect of dietary Trp was expressed less in the brain stem and telencephalon. Moreover, Basic et al.( Reference Basic, Krogdahl and Schjolden 15 ) showed that Trp treatment tended to enhance hypothalamic 5-HT 1 d post Trp, and that this effect was not present 10 d post Trp treatment. The latter finding is in line with the results from our study showing clear long-term effects of dietary Trp on 5-HT turnover in the telencephalon but not in the hypothalamus. One reason for the variable sensitivity to dietary Trp in different brain regions could be the fact that tryptophan hydroxylase (THP), the rate-limiting enzyme in 5-HT production, occurs in two different isoforms: THP1 and 2( Reference Lillesaar 25 ), and that they show different expression patterns in the brain. The Km value of THP2 is in the range of the free Trp concentration in the brain( Reference McKinney, Knappskog and Haavik 26 , Reference McKinney, Teigen and Frøystein 27 ) and is expressed in the raphe, whereas TPH1 dominates in diencephalic 5-HT cells( Reference Lillesaar 25 ). Thus, the 5-HT synthesis in the raphe is limited by Trp availability, whereas 5-HT synthesis in other 5-HT neuronal populations is not( Reference Winberg, Höglund and Øverli 28 ). Moreover, raphe 5-HT neurons show an extremely divergent projection pattern, including projections to forebrain areas. One possible explanation for higher dietary Trp sensitivity in the telencephalon could be that it is only enervated by the 5-HT-producing cells in the raphe. The hypothalamus, on the other hand, contains cells where TPH1 is involved in 5-HT production, limiting the impact of the Trp-sensitive 5-HT-producing cells projecting from the raphe.
In this study, telencephalic 5-HT turnover showed the same general pattern as plasma cortisol levels. This is in accordance with the general consensus that 5-HT is involved in the regulation of the neuroendocrine stress response in vertebrates( Reference Dinan 11 , Reference Winberg and Nilsson 19 , Reference Chaouloff 29 ), and with previous studies in teleosts showing a positive relationship between 5-HT turnover in the telencephalon and plasma cortisol( Reference Hoglund, Balm and Winberg 12 , Reference Winberg, Nilsson and Hylland 14 , Reference Silva, Martins and Khan 17 , Reference Höglund, Kolm and Winberg 30 ). Moreover, in a recent study it was demonstrated that chronic stress induced changes in stress responsiveness and concomitant 5-HT turnover in the telencephalon in rainbow trout, indicating similar extrahypothalmic control of the neuroendocrine stress axis in fish and in mammals( Reference Moltesen, Laursen and Thörnqvist 31 ). Our study, showing that dietary Trp content affects stress responsiveness and 5-HT in the same manner, lends further support to the fact that the involvement of the telencephalon in regulation of the stress response is conserved within the vertebrate lineage.
These results demonstrate long-term effects of dietary Trp supplementation on telencepahalic 5-HT neurochemistry and HPI-axis reactivity. Both dietary Trp supplementation and serotonin reuptake inhibitors (SSRI) are expected to increase 5-HTergic synaptic transmission acutely and common long-term mechanisms, such as changes in 5-HTergic receptor expression/sensitivity, have been suggested for these two treatments( Reference Lepage, Larson and Mayer 32 ). Interestingly, similar results as in our study have been reported in rat models of the SSRI discontinuation syndrome, where 5-HIAA levels were elevated 14 d after a 21-d treatment period with the SSRI fluoxetine( Reference Trouvin, Gardier and Chanut 33 ). Moreover, desensitisation of 5-HT1A receptors have been reported to last up to 60 d after a 14-d treatment period with this SSRI( Reference Raap, Garcia and Muma 34 ). However, whether these effects of SSRI treatment periods are associated with persistent changes in the neuroendocrine stress response is, to our knowledge, unknown. In addition, mammalian studies show that the 5-HT system(s) is involved in the organisation and development of its own neural projection pattern( Reference Daubert and Condron 35 ). For example, treatment with SSRI early in development has been shown to affect 5-HT neurotransmission in adults( Reference Oberlander, Gingrich and Ansorge 36 ). Similarly, in rats, social isolation in the time window between post-weaning and adolescence has been demonstrated to have a persistent effect on behaviour, brain architecture and neurotransmission( Reference Fone and Porkess 37 , Reference Lukkes, Watt and Lowry 38 ). Some of these changes are associated with alterations in 5-HT release and activity in limbic structures in the telencephalon( Reference Andersen and Navalta 39 ). Likewise, in Atlantic salmon, a 2-week period with repeated stress during the freshwater phase, early in development, affects 5-HT neurochemistry 10 weeks later in the seawater phase( Reference Vindas, Madaro and Fraser 40 ). In contrast to mammals, teleost fish have a remarkable neurogenic and regenerative capacity throughout ontogeny. The above studies provide evidence suggesting a relationship between enhanced 5-HT release/turnover early in development and 5-HT neurotransmission later in ontogeny. In this context, it can be hypothesised that structural changes may underlie the long-lasting effects on telencephalic neurochemistry of dietary Trp in teleost fish. This calls for comparative studies of the mechanisms, such as changes in 5-HT1A receptor sensitivity and/or the 5-HTergic projection pattern, underlying long-term effects of a dietary Trp treatment period on the HPA/I-axis reactivity.
Conclusion
In this study, we present results showing long-lasting brain-part-specific effects of 1 week of dietary Trp treatment on 5-HT turnover and HPI-axis reactivity. A dietary supplementation with 3×TRP suppressed post-stress plasma cortisol levels, and 5-HT turnover followed the same general pattern. Moreover, these effects were present throughout the experimental period (21 d post Trp treatment) and were reflected in telenecephalic but not in hypothalamic 5-HT turnover. This suggests that trophic/structural effects in the telencephalon are involved in long-lasting Trp-induced alterations in 5-HT turnover and HPI-axis reactivity, and lends further support to the fact that the extrahypothalamic control of the neuroendocrine stress response is conserved within the vertebrate lineage.
Acknowledgements
This work was supported by the Norwegian Research Council and BioMar.
E. H. wrote substantial parts of the manuscript. Ø. Ø. was involved in interpretation of the results. E. H., M. Å. A., P. S. and C. L. were involved in carrying out the experimental work. M. M. performed the analysis of 5-HTergic neurochemistry. E. H., Å. K., J. S., S. W. and M. H. were involved in planning/designing the study. E. H., Å. K. and M. A. V. were involved in the analysis/interpretation of the results. Ø. Ø., M. Å. A., P. S., C. L., M. M., Å. K., J. S., S. W., M. H., M. A. V. and I. M. were involved in the writing the manuscript. I. M. carried out the cortisol analyses.
The authors declare that there are no conflicts of interest.







