Fe is required for a wide spectrum of biological functions. The expression of several Fe-transport proteins is up regulated in Fe deficiencyReference Collins1. Ferric iron (Fe3+) is reduced to a ferrous (Fe2+) ion by duodenal cytochrome b reductase (Dcytb), an enzyme on the enterocyte's apical surfaceReference Mackenzie and Garrick2. Fe2+ is transported into the enterocyte by the divalent metal transporter 1 (DMT1). Fe may then be stored as ferritin or transferred across the basolateral membrane into the plasma by ferroportinReference McKie, Marciani and Rolfs3. Hephaestin, a multi-copper oxidase, is required for this transport, since it catalyses the oxidation of Fe2+ to Fe3+. Fe depletion increases DMT1 mRNA expression, but not ferroportin mRNA expressionReference Collins1. Further, Dcytb expression is stimulated by hypoxia and Fe deficiency, both of which increase Fe absorptionReference Latunde-Dada, Van der Westhuizen, Vulpe, Anderson, Simpson and McKie4. Dcytb mRNA is expressed at higher levels in the duodenum than in the large intestine, which is compatible with the observation that Fe absorption rates are highest in the proximal small intestine and diminish as digesta moves distallyReference Takeuchi, Bjarnason, Laftah, Latunde-Dada, Simpson and McKie5. Previous studies have clarified that Dcytb plays a pivotal role in Fe absorptionReference Latunde-Dada, Van der Westhuizen, Vulpe, Anderson, Simpson and McKie4. However, until now, sequences of the genes encoding for DMT1 and Dcytb in the pig small intestine have not been published and their expression regulation has not been studied.
Recently, efforts have been made to study how different nutrients might increase the dietary bioavailability of minerals. Prebiotics have been shown to increase mineral absorption. Of all the possible prebiotics, inulin and inulin-type fructans have been investigated the most. The average Western dietary intake of inulin by humans is estimated to be 1–4 g/dReference van Loo, Coussement, de Leenheer, Hoebregs and Smits6. Inulin as fructo-oligosaccharide (FOS) is composed of linear chains of repeating fructose monomers containing a terminal glucose residue, with chain lengths varying between three and sixty-five monomers. Dietary inulin or FOS increased intestinal Ca absorption in vitro Reference Bougle, Vaghefi-Vaezzadeh, Roland, Bouvard, Arhan, Bureau, Neuville and Maubois7, Reference Bosscher, Van Caillie-Bertrand, Van Cauwenbergh and Deelstra8 and in human subjectsReference van den Heuvel, Muys, van Dokkum and Schaafsma9, Reference Scholz-Ahrens and Schrezenmeir10. Also, in a study with gastrectomised rats, FOS enhanced intestinal Ca absorption and increased levels of calbindin-9 in the large intestine. This finding suggests that dietary FOS may be useful for improving intestinal Ca absorptionReference Ohta, Motohashi, Sakai, Hirayama, Adachi and Sakuma11. We previously showed that 4 % dietary inulin improved the utilisation of intrinsic Fe in a maize–soyabean meal diet by young anaemic pigs, and this benefit was associated with soluble Fe in the digesta. Those fed 4 % inulin demonstrated a 28 % improvement (P < 0·01) in Hb repletion efficiencyReference Yasuda, Roneker, Miller, Welch and Lei12. However, the underlying cellular and molecular mechanisms by which dietary FOS or inulin affects the absorption and transport of Fe and other mineral cations are not well understood.
In addition to enhancing intestinal mineral polyvalent cation absorption, inulin affects intestinal microbiota. Inulin-type fructans are not digested by enzymes secreted in the small intestineReference Ellegard, Andersson and Bosaeus13 but are hydrolysed and fermented by the microflora in the large intestineReference Gibson, Beatty, Wang and Cummings14. In the colon, prebiotics are fermented to produce SCFA; therefore, Bifidobacteria (and possibly other genera) are preferentially stimulated to grow, thus causing changes in the populations of the gut microflora by increasing the number of potentially health-promoting bacteria and reducing the number of potentially harmful species. Symbiosis between intestinal microflora and host can be optimised by prebioticsReference Bakker-Zierikzee, Alles, Knol, Kok, Tolboom and Bindels15–Reference Broekaert and Walker17. The importance of microbiota within the gastrointestinal tract has become evident from studies showing the role of intestinal micro-organisms in the synthesis of fermentation products that provide energy to the colon epitheliumReference Cummings, Pomare, Branch, Naylor and Macfarlane18, stimulation of the gut immune systemReference Salminen, Bouley, Boutron-Ruault, Cummings, Franck, Gibson, Isolauri, Moreau, Roberfroid and Rowland19, synthesis of vitamin KReference Conly and Stein20, and resistance to colonisation of exogenous bacterial pathogensReference Hopkins and Macfarlane21. Since dietary prebiotics modulate the intestinal microbial community towards a more beneficial array of organisms, they have gained research attentionReference Collins and Gibson22. The 16S ribosomal RNA (rRNA) technique offers an appropriate alternative to conventional culture methods and has been successfully applied for quantifying the relative ratio of colonic mucosa-associated bacteriaReference Zhu, Zhong, Pandya and Joerger23, Reference Smirnov, Perez, Amit-Romach, Sklan and Uni24, faecal bacteriaReference Apajalahti, Kettunen, Kettunen, Holben, Nurminen, Rautonen and Mutanen25, Reference de Wiele, Boon, Possemiers, Jacobs and Verstraete26 and in assessing the effects of functional foods on intestinal microbiotaReference Nelson, Thonney, Woolston, Zinder and Pell27, Reference Harmsen, Gibson, Elfferich, Raangs, Wildeboer-Veloo, Argaiz, Roberfroid and Welling28.
The objectives of the present study were to assess the intestinal tissue response to inulin supplementation by evaluating the gene expressions of Fe transporters, enzymes and binding protein (DMT1, Dcytb, ferritin, ferroportin and transferrin receptor (TfR)). A PCR-based method was used to detect and quantify the caecal 16S ribosomal DNA (rDNA) of Lactobacillus, Bifidobacterium, Escherichia coli and Clostridium. Since inulin has been found to up regulate mucin secretionReference Kleessen, Hartmann and Blaut29, and mucin–bacteria interactions are relevant to the integrity of the intestinal mucous barrier, mucin gene expression was also studied. The mineral transporters and Fe-binding-protein gene expression data may provide evidence for mechanisms operating to enhance Fe absorption.
Materials and methods
Diets
The control diet consisted of maize and soyabean meal (Table 1), and contained adequate concentrations of all nutrients30 except for Fe (non-inorganic Fe was added). The actual concentrations of Fe in diets were analysed using an inductively coupled Ar plasma emission spectrometer (ICAP 61E Trace Analyzer; Thermo Electron, Waltham, MA, USA)Reference Eppard, Bauman, Bitman, Wood, Akers and House31. The actual concentration of inulin in the experimental diet was determined using the method described by Yasuda et al. Reference Yasuda, Roneker, Miller, Welch and Lei12 (Table 1). Synergy 1 (ORAFTI, Malvern, PA, USA) was used as the source of inulin, replacing maize starch in the control diet. This product was a mixture of α-d-glucopyranosyl-(β-d-fructofuranosyl)n − 1 β-d-fructofuranosides (n 10–60, mean 25), and oligofructose,α-d-fructofuranosyl-(β-d-fructofuranosyl)n − 1 β-d-fructofuranosides (n 2–7, mean 4).
Table 1 Composition of the experimental diets
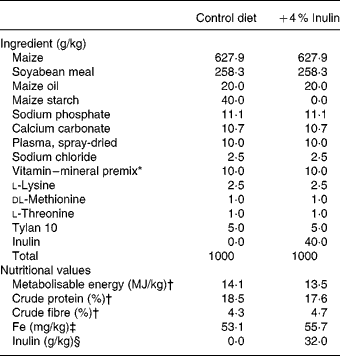
* Vitamin and mineral premix provided (per kg diet): retinyl palmitate, 1208 μg; ergocalciferol, 5·5 μg; dl-α-tocopheryl acetate, 10·72 mg; menadione, 0·5 mg; d-biotin, 0·05 mg; choline chloride, 0·5 g; folic acid, 0·3 mg; niacin, 15 mg; Ca-D pantothenate, 10 mg; riboflavin, 3·5 mg; thiamin, 1 mg; pyridoxine, 1·5 mg; cyanocobalamin, 17·5 μg; CuSO4.5H2O, 6 mg; C2H8N22HI (ethylene diamine dihydroiodine), 0·14 mg; MnO, 4 mg; Na2SeO3, 0·3 mg; ZnO, 100 mg.
† Calculated based on National Research Council values30.
‡ Analysed using an inductively coupled Ar plasma emission spectrometer (ICAP 61E Trace Analyzer; Thermo Electron, Waltham, MA, USA)Reference Eppard, Bauman, Bitman, Wood, Akers and House31.
§ Synergy 1 (ORAFTI, Malvern, PA, USA) was the source of inulin.
Experimental animals and protocols
Twelve weanling Yorkshire × Hampshire × Landrace cross-bred pigs from the Cornell University Swine Farm were used for the present experiment. All animal and experiment protocols were approved by the University Institutional Care and Use Committee. Experimental pigs were selected from litters that were injected with only half of the normal Fe dose (50 mg Fe as Fe-dextran) at birth and were allocated to two treatment groups (n 6) based on body weight (mean 7·70 ± 0·19 kg), litter, sex and Hb concentrations. Pigs were housed individually in pens with concrete floors in a temperature-controlled barn (22 to about 25°C) with a 12 h light–12 h dark cycle, given free access to feed and water, and checked daily. Pigs were fed with the experimental diets for 6 weeks. Before the beginning of the experiment, all pigs were fed the control diet for 2 weeks to adjust their body Fe stores.
Blood samples of all individual pigs (fasted overnight for 8 h) were collected weekly from the anterior vena cava using 5 ml heparin syringes to assay for blood Hb. All pigs were killed by electrical stunning and exsanguination. Pigs were first fasted for 8 h and then were given free access to feed for 10 h before slaughter to enable us to collect comparable and sufficient digesta samples from the caecum. The digestive tracts were quickly removed from the carcass and separated into various sections for tissue (about 5 cm) and digesta (about 5 g) sampling. The samples were immediately frozen in liquid N2, and then stored in a − 80°C freezer until analysis.
Blood sample analysis
Blood Hb concentrations were measured spectrophotometrically using the cyanomethaemoglobin method following the manufacturer's instructions (Pointe Scientific, Inc., Canton, MI, USA). Hb repletion efficiency values are as described by Yasuda et al. Reference Yasuda, Roneker, Miller, Welch and Lei12.
Total RNA isolation and reverse transcription
Intestinal duodenal and colon cross-sections were homogenised in TRI-REAGENT (Molecular Research Center, Cincinnati, OH, USA) to isolate total RNA and reverse-transcribed using oligo (dT) and Superscript II reverse transcriptase (MBI Fermentas, Burlington, ON, Canada).
Isolation of pig intestinal divalent metal transporter 1 and duodenal cytochrome b reductase gene fragment
Primers were designed to correspond to nucleotides 2041–2066 (5′-ccc aag ggc cag ctc agt tta tcc t-3′) and 2367–2392 (5′-agt taa gag ggg tga gaa aac aaa g-3′) of the previously published human small-intestinal DMT1 sequenceReference Sharp, Tandy, Yamaji, Tennant, Williams and Singh Srai32 and 361–386 (5′-gcc tgc aca gct ggg ttg gac tga c-3′) and 696–721 (5′-cca ggc tct gtc cct ctc cag cta a-3′) of the previously published house mouse small-intestinal Dcytb sequenceReference McKie, Barrow and Latunde-Dada33. Total RNA was amplified using the Promega Access RT-PCR System (Promega Corporation, Madison, WI, USA). The program was as follows: 2 min at 94°C, 1 min at 48°C, 2 min at 68°C for forty cycles followed by 7 min at 68°C. The RT-PCR products were separated on a 1·5 % agarose gel, visualised by staining with ethidium bromide, excised from the gel and purified using a High Pure PCR Product Purification kit (Quagen, Hilden, Germany). The pig duodenal DMT1 and Dcytb cDNA fragments were subjected to automated sequencing using an Applied Biosystems 373A DNA sequencer (Applied Biosystems, Foster City, CA, USA). Nucleic acid sequences were analysed and homology between pig and other DMT1 and Dcytb sequences were calculated using DNAMAN version 4 software (Lynnon Biosoft, Vaudreuil-Dorion, QC, Canada).
Divalent metal transporter 1, duodenal cytochrome b reductase, ferritin, ferroportin, transferrin receptor and mucin gene expression analysis
First strand cDNA were synthesised from 5 μg of total RNA from each pig using oligo (dT)18 as primers in the presence of Moloney murine leukaemia virus RT (Fermentase, MD, USA), for 1 h at 42°C. PCR was carried out with primers chosen from the fragment of the pig duodenal DMT1 gene (forward: 5′-GGT GTT GTG CTG GGA TGT TA-3′; reverse: 5′-AGT ACA TAT TGA TGG AAC AG-3′), Dcytb gene (forward: 5′-CCA TGG GCT CCG CCC TCT CTC CGG G-3′; reverse: 5′-TAG GGC GTT TCC ATT GGG GCC TGG T-3′), ferritin gene (forward: 5′-GAG GGG ACA TGC TGA GAA ACT GAT G-3′; reverse: 5′-CAC TGC TCC CCA GGG TGT GCT TGT C-3′), mucin gene (forward: 5′-AGG CCA GGA TCT GTA CTG GTA GAG C-3′; reverse: 5′-TGG TAG GTG GGG TAC TCG CTC ATA G-3′), ferroportin gene (forward: 5′-CCC ATT AGG TGA GTT CCC TCC AAA GG-3′; reverse: 5′-GGT TTA CCA GAA GAC CCC TGC TCT GC-3′) and TfR gene (forward: 5′-ATG ATG AAC ATT TTG TTA AGA TTC A-3′; reverse: 5′-AAC TGG AAC ATC TGC ATT GAT GAT A-3′). Ribosomal 18S was used to normalise the results, with primers from the pig small intestine ribosomal 18S mRNA (GI 37956930) (forward: 5′-GAA CTA CGA CGG TAT CTG ATC GTC T-3′; reverse: 5′-CCG CCC GTC CCC GCC GGT TGC CTC T-3′). Determination of the linear phase of the PCR amplification was performed with thermus filiformis (Tfi)-DNA polymerase (Access RT-PCR system; Promega Corporation) with pooled samples removed at fifteen, twenty, twenty-five, thirty, thirty-five, forty, forty-five, fifty and fifty-five cycles. Amplification of the pig duodenal DMT1, Dcytb, ferritin, mucin, ferroportin and TfR genes was performed for thirty-two, thirty-five, thirty-three, thirty, thirty-four and thirty-five cycles respectively, which consisted of denaturation (95°C, 30 s), annealing (48°C, 1 min) and extension (72°C, 1 min); ribosomal 18S was amplified at thirty-two cycles under identical conditions in a different tube. Ribosomal 18S (350 bp) and pig duodenal DMT1 and Dcytb (220 bp) PCR products were separated by electrophoresis on 2 % agarose gel, stained with ethidium bromide, and quantified using a Gel-Pro analyser version 3.0 (Media Cybernetics LP, Silver Spring, MD, USA).
Collection of microbial samples and DNA isolation
After killing the animals, the caecum was removed and treated as described by Zhu et al. Reference Zhu, Zhong, Pandya and Joerger23. The contents of the caecum were squeezed out into a sterile 50 ml tube containing 9 ml sterile PBS, and homogenised by vortexing with glass beads (3 mm diameter) for 3 min. Debris was removed by centrifugation at 700 g for 1 min, and the supernatant fraction was collected and centrifuged at 12 000 g for 5 min. The pellet was washed twice with PBS and stored at − 20°C until DNA extraction. For DNA purification, the pellet was re-suspended in 50 mm-EDTA and treated with lysozyme (Sigma Aldrich Co., St Louis, MO, USA) (final concentration of 10 mg/ml) for 45 min at 37°C. The bacterial genomic DNA was isolated using a Wizard Genomic DNA purification kit (Promega Corporation). DNA concentration was determined spectrophotometrically.
Primer design and polymerase chain reaction amplification of bacterial 16S rDNA
Primers for Lactobacillus, Clostridium, E. coli and Bifidobacteria were designed according to previously published dataReference Zhu, Zhong, Pandya and Joerger23, Reference Langendijk, Schut, Jansen, Raangs, Kamphuis, Wilkinson and Welling34, Reference Amit-Romach, Sklan and Uni35. The primers used in the present study are shown in Table 2. Universal primers identifying all known bacteria were designed using the invariant region in the 16S rDNA of the bacteria. The universal primer set was used for determining the total microflora population. For PCR amplification of the bacterial targets from caecal contents, 5 μl of DNA extract was added to 45 μl of PCR mixture containing 27·5 μl of nuclease-free water, 5 μl of each primer (10 μg/ml), 1·5 μl of nucleotide (dNTP) mix, 5 μl of PCR buffer and 1 μl of Taq polymerase (Go-Taq; Promega Corporation). The PCR thermal conditions were as follow: one cycle of 94°C for 3 min, thirty-five cycles of 94°C for 30 s, 60°C for 1 min and 68°C for 2 min and finally one cycle of 68°C for 7 min. The PCR reaction was run with different numbers of cycles (twenty-five, thirty, thirty-five, forty, forty-five or fifty) for each primer set and thirty-five cycles was in the centre of the exponential increase in PCR products. PCR products were separated by electrophoresis on 2 % agarose gel, stained with ethidium bromide and quantified using a Gel-Pro analyser version 3.0 (Media Cybernetics LP). To evaluate the relative proportion of each examined bacteria, all products were expressed relative to the content of the universal primer product and proportions of each bacterial group are presented where the total of the examined bacteria was set at 100 %. Results are presented as mean values with their standard errors.
Table 2 Microbial polymerase chain reaction primers used

Statistical analysis
Results were analysed by ANOVA using the general linear model method with SAS software (SAS Institute, Inc., Cary, NC, USA). Differences between treatments were compared by Tukey's test and values were considered statistically different at P ≤ 0·05 unless otherwise stated.
Results
Growth performance and haemoglobin
There was no difference in overall growth performance between pigs fed the control diet and 4 % inulin. Pigs fed 4 % inulin had 14 % higher (94·4 (se 4·5) v. 107·8 (se 3·7) g/l; P = 0·06) blood concentrations at week 6, and 22 % higher (20·4 (se 1·4) v. 24·9 (se 0·7) %; P < 0·05) overall Hb repletion efficiency than pigs fed the control diet. The changes in Hb concentrations over the 6-week period were greater (P < 0·05) in pigs fed 4 % inulin than those fed the control diet.
Isolation and sequencing of partial pig small-intestinal divalent metal transporter 1 and duodenal cytochrome b reductase cDNA
A 372 bp fragment of the pig intestinal DMT1 gene and a 220 bp fragment of the pig intestinal Dcytb gene were isolated by RT-PCR and were sequenced. They were 89 and 83 % homologous to human and 87 and 79 % homologous to rat genes, respectively. The cDNA sequences of the pig intestinal DMT1 and Dcytb were entered into the EMBL nucleotide Sequence Database under accession number GI|86197473| and GI|105295521| respectively. The predicted amino acid sequence of the DMT1 fragment resulted in a predicted translation product of 124 amino acids. This amino acid sequence was 95 % homologous to Homo sapiens DMT1 and 96 % homologous to Rattus novergicus DMT1. The predicted amino acid sequence of Dcytb resulted in a predicted translation of seventy-two amino acids. This amino acid sequence was 80 % homologous to H. sapiens Dcytb and 79 % homologous to both R. novergicus and Mus musculus.
Gene expression of iron enzymes, receptors and binding protein in the duodenum
Semi-quantitative RT-PCR analysis revealed significantly (P ≤ 0·05) increased mRNA expression in the small intestine duodenal segments of ferritin, DMT1, ferroportin, Dcytb and the TfR (an elevation of 100, 300, 150, 70 and 100 % respectively) in the inulin group compared with the control (Fig. 1).
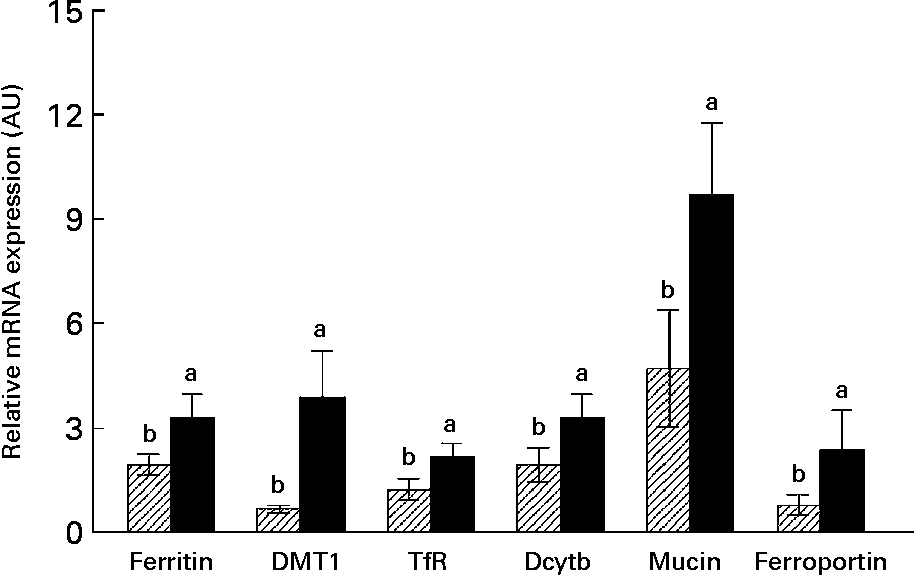
Fig. 1 Pig duodenum intestinal mRNA expression in control (▨) and dietary inulin-supplemented animals (4 % inulin; ■). Changes in mRNA expression were measured by semi-quantitative RT-PCR and expressed relative to the expression of 18S rRNA in arbitrary units (AU). DMT1, divalent metal transporter 1; TfR, transferrin receptor; Dcytb, duodenal cytochrome b reductase. Values are means (n 6), with their standard errors represented by vertical bars. a,b Mean values within genes tested with unlike letters were significantly different (P ≤ 0·05).
Gene expression of iron enzymes, receptors and binding protein in the colon
Gene expression in the colon increased by 120, 70 and 20 % for DMT1, Dcytb and ferritin mRNA levels respectively (P < 0·05) in the inulin group (Fig. 2). No significant differences were detected in the other tested genes in the colon.

Fig. 2 Pig colon intestinal mRNA expression in control (▨) and dietary inulin-supplemented animals (4 % inulin; ■). Changes in mRNA expression were measured by semi-quantitative RT-PCR and expressed relative to the expression of 18S rRNA in arbitrary units (AU). DMT1, divalent metal transporter 1; TfR, transferrin receptor. Values are means (n 6), with their standard errors represented by vertical bars. a,b Mean values within genes tested with unlike letters were significantly different (P ≤ 0·05).
Bacterial populations in the pig caecum contents
Analysis of caecal contents showed different relative proportions of the bacteria examined (Fig. 3 A). Relative amounts of Lactobacillus and Bifidobacterium in the caecum were increased by 200 and 100 % respectively in the inulin group compared with the control group (Fig. 3 B). No significant differences in E. coli and Clostridium relative amounts were detected.

Fig. 3 (A) The effect of dietary inulin supplementation (4 % inulin; ■) compared with control (▨) on the proportion of Lactobacillus, Clostridium, Escherichia coli and Bifidobacterium species in caecum contents expressed in arbitrary units (AU). Values are means (n 6), with their standard errors represented by vertical bars. a,b Mean values within each bacterial species tested with unlike letters were significantly different (P ≤ 0·05). (B) Representative PCR products of 16S rDNA of Lactobacillus, Clostridium, E. coli and Bifidobacterium species and 16S rDNA of invariant sequences of all known intestinal bacterial species (Universal) in the caecum of control (1) and inulin-treated animal groups. Each lane represents a different pig.
Discussion
The present results suggest that inulin supplementation to maize–soya swine diets lead to up regulation of the expression of genes encoding for Fe transporters, enzymes and ferritin in intestinal enterocytes. Also, inulin had a beneficial effect on the caecal microbial community (increasing the Bifidobacteria and Lactobacilli populations) and up regulated the expression of mucin. These results may point to possible mechanisms whereby inulin enhances Fe absorption, since, as we have previously shown, 4 % dietary inulin improved intrinsic Fe utilisation and bioavailability in a maize–soyabean meal diet by young anaemic pigletsReference Yasuda, Roneker, Miller, Welch and Lei12.
Fe absorption is regulated, in part, by intracellular Fe concentrations in enterocytesReference Ludwiczek, Theurl, Artner-Dworzak, Chorney and Weiss36. In general, Fe ions (Fe3+) reach the duodenal brush-border membrane and then are reduced by Dcytb to Fe2+, which is then transported into the enterocyte by DMT1. Within the cell, Fe is either stored as ferritin or trafficked to the basolateral membrane and exported into the circulation. Transport across the basolateral membrane is accomplished by the coordinated action of ferroportin, an Fe transporter, and hephaestin, an oxidase which oxidises Fe2+ to Fe3+. Fe3+ then binds to transferrin for distribution throughout the body via the plasma circulationReference Collins, Franck, Kowdley and Ghishan37. In Caco-2 cells, DMT1 protein in the plasma membrane was significantly decreased by exposure to high Fe for 24 h, in a concentration-dependent manner, whereas whole-cell DMT1 protein abundance was unaltered. This suggests that part of the response to high Fe involved redistribution of DMT1 between the cytosol and cell membrane. These events preceded changes in DMT1 mRNA, which was only decreased following 72 h exposure to high FeReference Sharp, Tandy, Yamaji, Tennant, Williams and Singh Srai32. Whilst under Fe deficiency or anaemic conditions, intracellular Fe concentration decreases, leading to increased gene expression of DMT1, Dcytb, ferroportin and hephaestinReference Anderson38. DMT1 is the main Fe transporter expressed at the apical surface of human intestinal cellular brush-border membrane (including in vitro cultured Caco-2 cells) where it mediates pH-dependent Fe2+ uptakeReference Sharp, Tandy, Yamaji, Tennant, Williams and Singh Srai32, Reference McKie, Barrow and Latunde-Dada33. DMT1 is responsible for the uptake of non-haeme Fe from the intestinal lumenReference Gunshin, Mackenzie, Berger, Gunshin, Romero, Boron, Nussberger, Gollan and Hediger40, Reference Fleming and Andrews41.
Since the pig's intestinal DMT1 and Dcytb gene sequences have not been published, their gene expression patterns have never been investigated. Therefore, we isolated and sequenced fragments of the pig's intestinal DMT1 and Dcytb genes. The DMT1 predicted amino acid sequence of the isolated gene fragment showed 95 % homology to human and 96 % homology to rat DMT1Reference Sharp, Tandy, Yamaji, Tennant, Williams and Singh Srai32, Reference McKie, Barrow and Latunde-Dada33. The Dcytb predicted amino acid sequence was 79 % homologous to both human and rat Dcytb genes.
In the present study we investigated how supplementation of diets with inulin affects DMT1 and Dcytb gene expression in the intestines of anaemic pigs. When added to a diet, inulin increases intestinal polyvalent mineral absorptionReference Ohta, Motohashi, Sakai, Hirayama, Adachi and Sakuma11, Reference Coudray, Bellanger, Castiglia-Delavaud, Remesy, Vermorel and Rayssignuier42, Reference Raschka and Daniel43. DMT1 and Dcytb gene expressions were higher (P ≤ 0·05) in the inulin group in both the duodenum and colon. Up regulation of DMT1 and Dcytb possibly led to accumulation of intracellular Fe and thereby increased (P ≤ 0·05) cellular ferritin (the cell's Fe-storage protein) gene expression. Previously it was shown that in Fe deficiency, DMT1 protein is up regulated, while concentration of intracellular ferritin is unchangedReference Yeh, Yeh, Watkins, Rodriguez-Paris and Glass44 but that does not necessarily reflect the gene transcription regulation. In the present study, ferritin mRNA transcription was up regulated, but its translation may have been blocked since the animals were Fe deficient and the diets were low in Fe. These conditions would lead to low intracellular Fe concentrations resulting in increased binding of Fe-regulatory proteins (IRP) by Fe-responsive elements (IRE) on the mRNA. This binding to IRE located within the 5′-untranslated region is known to block translation of mRNA into proteinReference Ludwiczek, Theurl, Artner-Dworzak, Chorney and Weiss36. Presumably, low levels of intracellular ferritin would allow Fe taken up by the enterocytes to be transported into the circulation via ferroportin rather than being sequestered within the cell. In addition to ferritin, the IRE regulate several key proteins in Fe homeostasis, including DMT1, ferroportin and TfR. The IRP binding affinity for IRE is regulated by the concentration of Fe within the cell. In the case of TfR, reduced intracellular Fe concentrations (such as in anaemic conditions) activate IRP binding activity, thus stabilising mRNA which bear their IRE within the 3′-untranslated region, thereby increasing TfR gene expressionReference Ludwiczek, Theurl, Artner-Dworzak, Chorney and Weiss36. Further investigation including the development of antibodies specific for porcine DMT1, Dcytb, ferroportin, TfR and ferritin is required, that will allow the determination of the effect of inulin on protein concentrations.
One hypothesis for explaining the enhancing effect of inulin on Fe absorption is that fermentation products of inulin, for example, butyric acid, enhance Fe solubility in the intestinal lumen, thereby making more Fe available for uptake by enterocytes. If this is the case, one would expect an increase in intracellular Fe and this would be expected to down regulate, not up regulate, the expression of Fe-transporter genes. Since we observed the opposite, it appears that inulin influences the expression of these genes by some other mechanism independent of intracellular Fe concentration. Furthermore, the up regulation we observed in the duodenum suggests that inulin itself, not its fermentation products, may affect gene expression in enterocytes since, presumably, there is little if any fermentation in the duodenum. Moreover, the observed up regulation of the ferritin gene is difficult to explain since pigs in both groups were given low-Fe diets throughout the study and a positive effect of inulin on blood Hb values was only shown at week 6. However, Zn- and Fe-deficient rats had an increased ferritin mRNA expression after being fed my mouth with a low concentration (40 parts per million) Fe solutionReference Sreedhar and Nair45. We suggest that our findings may be consistent with this observation since ferritin gene expression was elevated in the inulin group. Again, some other mechanism may be involved. It was previously shown that inulin and, to a lesser extent, oligofructose chicory-derived fructans enhanced the levels of calbindin-9 K, a protein known to play an important role in Ca absorption, in the large intestine of ratsReference Nzeusseu, Dienst, Haufroid, Depresseux, Devogelaer and Manicourt46. Relative concentrations of calbindin-9 K in the intestine strongly correlate with the apparent Ca absorptionReference Staun and Jarnum47, Reference Ohta, Motohashi, Ohtsuki, Hirayama, Adachi and Sakuma48. Inulin may have a similar effect on ferritin, the intracellular Fe storage protein.
As mentioned earlier, one of the possible reasons for increased mineral bioavailability in the presence of inulin is that inulin-type fructans serve as a substrate for SCFA synthesis by different types of bacteria in the large intestineReference Hoverstad and Bjorneklett49–Reference Cummings and Macfarlane52. FOS and inulin enhance the proliferation of selected groups of the colonic microflora, thereby altering the composition toward a more beneficial communityReference Gibson, Beatty, Wang and Cummings14, Reference Tzortzis, Goulas, Gee and Gibson53. In vitro, inulin was found to selectively stimulate the growth of Bifidobacteria and Lactobacilli, genera considered beneficial to healthReference Gibson, Beatty, Wang and Cummings14, Reference Mentschel and Claus54. SCFA are absorbed by the human colon in a concentration-dependent mannerReference Ruppin, Bar-Meir, Soergel, Wood and Schmitt55 and are the major respiratory fuels for colonocytes, supplying up to 70 % of their energy needsReference Hoverstad, Fausa, Bjorneklett and Bohmer51, Reference Livesey56. SCFA may enhance the absorption of cations in the colon, such as Ca, Zn and Fe. It has been reported that Bifidobacteria have a nutritional advantage compared with other intestinal micro-organisms due to their β-1,2-glycosidase activityReference Cummings, Pomare, Branch, Naylor and Macfarlane18, Reference Conly and Stein20, Reference Hopkins and Macfarlane21, Reference de Wiele, Boon, Possemiers, Jacobs and Verstraete26, Reference Cummings and Englyst57. Human in vivo trials have established that the addition of FOS or inulin to the diet leads to an increase in faecal Bifidobacteria Reference Gibson, Beatty, Wang and Cummings14, Reference Kolida, Tuohy and Gibson58–Reference Bouhnik, Raskine, Simoneau, Vicaut, Neut, Flourie, Brouns and Bornet60, and several studies have described in vitro FOS fermentation in pure cultures of Bifidobacterium Reference Apajalahti, Kettunen, Kettunen, Holben, Nurminen, Rautonen and Mutanen25, Reference Nilsson and Bjorck61–Reference Uehara, Ohta, Sakai, Suzuki, Watanabe and Adlercreutz66. In the present study, we examined the effect inulin has on the relative amounts of E. coli, Clostridium, Bifidobacterium and Lactobacilli. Inulin had no effect on the relative amounts of both E. coli and Clostridium populations. However, the proportion of Bifidobacterium and Lactobacilli was significantly increased (P < 0·05) in the caecal contents of the inulin group. This bacterial response to dietary inulin probably affected SCFA concentrations in the lumen. One of the well-studied SFCA, butyrate, has been shown to regulate a number of genes associated with the processes of proliferation, differentiation and apoptosis of colonic epithelial cells, and hence, homeostasis of colonic tissueReference Mentschel and Claus54, Reference Daly, Cuff, Fung and Shirazi-Beechey67. It was previously demonstrated that butyrate has a trophic effect on the colon epithelium in vivo. It was reported that the lack of butyrate has led to mass apoptosis in the guinea-pig colonReference Hass, Busche, Luciano, Reale and Engelhardt68. In addition, feeding of butyrate to calves stimulated ruminal mucosa development mainly by inhibition of apoptosisReference Mentschel and Claus54. In a previous pig study, where raw potato starch was added to the diet, the systemic effects of butyrate were described. The resistant-type starch led to increased butyrate formation that was accompanied by reduced apoptosisReference Mentschel and Claus54.
SCFA play an important role in intestinal health by affecting the production and secretion of the mucus layer covering the gastrointestinal mucosa. This layer is considered the first line of defence against aggressions arising from the luminal contents. It is mainly composed of high-molecular-weight glycoproteins called mucins. Butyrate increased intestinal mucin secretion by up regulating mucin gene expressionReference Gaudier, Jarry, Blottiere, de Coppet, Buisine, Aubert, Laboisse, Cherbut and Hoebler69. In the present study, mucin gene expression was increased (P ≤ 0·05) in the duodenum in the inulin-treated group. In the Fe-deficient gut, large quantities of DMT1 have been reported in goblet cells (cells that produce and secrete mucins) and intraluminal mucins, suggesting that DMT1 is secreted with mucin into the intestinal lumen to bind Fe, facilitating uptake by the cellsReference Tandy, Williams, Leggett, Lopez-Jimenez, Dedes, Ramesh, Srai and Sharp39, Reference Simovich, Hainsworth, Fields, Umbreit and Conrad70. However, the mechanism by which inulin affects the gene expression regulation of enterocyte Fe-related transporters, enzymes and binding proteins remains vague. Previously it was shown that supplementing 4 % inulin improved the utilisation of intrinsic Fe in a maize and soyabean-meal diet by young anaemic pigs, and this benefit was associated with soluble Fe and sulfide concentrations but not pH or phytase activity in the digesta. Those fed 4 % inulin demonstrated a 28 % improvement (P < 0·01) in Hb repletion efficiency and 15 % (P < 0·01) improvement in the final blood Hb concentrationReference Yasuda, Roneker, Miller, Welch and Lei12.
We suggest that inulin may contribute to the regulation of Fe absorption either directly by affecting SCFA synthesis or through changes in bacterial population and/or by affecting mucin gene expression. It is possible that there are two pathways by which inulin might affect intestinal gene expression: (1) increased soluble Fe in the intestinal lumen and (2) increased SCFA production in the colon lumen leading to a possible systemic effect of butyrate on Fe metabolism.
In conclusion, dietary inulin alters the expression of genes encoding for Fe absorption in the duodenum as well as in the colon in Fe-deficient pigs. This up regulation of gene expression appears to be independent of Fe status and may explain the increase in Fe bioavailability from inulin-supplemented maize–soya diets we observed in these same pigs.
Acknowledgements
We thank HarvestPlus for supporting the present study.







