A physiological rate of programmed death, or eryptosis( Reference Lang, Gulbins and Lerche 1 ), of erythrocytes allows clearance of aged cells; however, an increased number of circulating eryptotic erythrocytes, as observed in various diseases( Reference Bonomini, Sirolli and Settefrati 2 – Reference Calderon-Salinas, Munoz-Reyes and Guerrero-Romero 5 ), is dangerous and leads to complications. Because of phosphatidylserine (PS) exposed at the erythrocyte surface, eryptotic cells may activate coagulant enzymes( Reference Chung, Bae and Lim 6 ), causing thrombosis and thrombo-occlusive disease( Reference Wood, Gibson and Tait 3 , Reference Chung, Bae and Lim 6 – Reference Pandolfi, Di Pietro and Sirolli 9 ), and adhere to endothelial cells( Reference Closse, Dachary-Prigent and Boisseau 8 , Reference Borst, Abed and Alesutan 10 ), promoting vascular damage. Our recent studies have for the first time demonstrated that a mixture of oxysterols, known to be elevated in the serum of hypercholesterolaemic subjects( Reference Addis, Emanuel and Bergmann 11 – Reference Chang, Abdalla and Sevanian 13 ), exerts a remarkable eryptotic activity compared with healthy erythrocytes( Reference Tesoriere, Attanzio and Allegra 14 ), an event that enhances the pathogenic potential of these cholesterol derivatives in the initiation and promotion of atherogenic processes( Reference Poli, Biasi and Leonarduzzi 15 , Reference Brown and Jessup 16 ).
Evidence on the impact of dietary vegetable intake on the prevention of chronic diseases, including cancer, neurodegenerative disorders and atherosclerosis, has emerged from various studies( Reference Slavin and Lloyd 17 ). Polyphenol phytochemicals have long been considered as protective agents; however, recent attention has been focused on betalains( Reference Stintzing and Carle 18 ), a group of pigments sharing the common structure of betalamic acid. Indicaxanthin (Ind; Fig. 1), the characteristic yellow pigment of cactus pear (Opuntia ficus-indica) fruit, is the immonium derivative of proline with betalamic acid( Reference Piattelli and Conn 19 ). Ind is a reducing and amphipathic molecule, interacts with and partitions in membranes, enters various cells, including erythrocytes, and counteracts oxidative damage induced by various agents( Reference Butera, Tesoriere and Di Gaudio 20 – Reference Tesoriere, Allegra and Butera 26 ). Moreover, it has been shown to modulate specific redox-driven signalling pathways involved in the inflammatory response in cultured endothelial and intestinal cells, and interfere with molecular mechanisms involved in the apoptotic activity of 7-ketocholesterol (7-KC) in a human monocyte/macrophage cell line( Reference Gentile, Tesoriere and Allegra 27 – 29 ). Dietary Ind that may be absorbed by passive diffusion through the intestinal epithelium has been found to be unmodified in human blood and exhibits high bioavailability, reaching plasma concentrations of several micromolars after a cactus pear fruit meal( Reference Tesoriere, Allegra and Butera 30 – Reference Tesoriere, Gentile and Angileri 32 ). Therefore, the aim of the present study was to investigate whether dietary Ind affected oxysterol-induced eryptosis in human erythrocytes. For this purpose, a mixture of oxysterols in hypercholesterolaemia-relevant proportions( Reference Addis, Emanuel and Bergmann 11 – Reference Chang, Abdalla and Sevanian 13 ) and Ind concentrations consistent with the plasma levels achieved after a dietary intake of cactus pear fruits( Reference Tesoriere, Gentile and Angileri 32 ) were considered. The study was performed with either isolated erythrocytes or after ex vivo spiking of whole blood with oxysterols in the presence or absence of Ind. Finally, the effect of dietary Ind on the adherence of oxysterol-treated erythrocytes to endothelial cell monolayers (human umbilical vein endothelial cells; HUVEC) was explored.
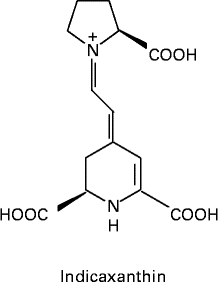
Fig. 1 Molecular structure of indicaxanthin.
Materials and methods
7-KC, cholestan-3β,5α,6β-triol (TRIOL), 5α,6α-epoxycholesterol (α-epox), 5β,6β-epoxy-cholesterol (β-epox), 7α-hydroxy-cholesterol (7α-OH) and 7β-hydroxy-cholesterol (7β-OH) were obtained from Avanti Polar Lipids, Inc. All other reagents and chemicals were obtained from Sigma Chemical Co., unless otherwise indicated.
Preparation of indicaxanthin
Ind was isolated from cactus pear (Opuntia ficus-indica L. Mill) fruit pulp (yellow cultivar) by methanol extraction, purified and quantified as reported previously( 29 ).
Cells and incubation conditions
Erythrocytes collected from the blood of healthy volunteers (n 5; age 25–68 years; normal BMI range), with informed consent, were isolated by centrifugation (2000 g , 4°C, 20 min) on a Ficoll gradient (Biochrom KG). Erythrocytes (0·4 % haematocrit) were incubated at 37°C, 5 % CO2 and 90 % humidity in Ringer solution containing 125 mm-NaCl, 5 mm-KCl, 1 mm-MgSO4, 32 mm-N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES)/NaOH, 5 mm-glucose, 1 mm-CaCl2 (pH 7·4). The cells were pre-incubated for 1 h in the absence or presence of Ind before adding a mixture of oxysterols, followed by incubation for 48 h. The mixture of oxysterols at final concentrations of 7 μm-7-KC, 2 μm-TRIOL, 4 μm-α-epox, 1 μm-7α-OH, 2 μm-7β-OH and 4 μm-β-epox (20 μmof total oxysterols) was added to the cells using tetrahydrofuran at a 0·1 % (v/v) final concentration. Preliminary experiments showed that under these conditions, tetrahydrofuran did not have any effect on erythrocytes; therefore, erythrocytes incubated with the solvent were used as the control.
Measurement of phosphatidylserine externalisation and forward scatter
Erythrocytes washed in Ringer solution were adjusted at 1·0 × 106 cells/ml with the combining buffer according to the manufacturer's protocol (eBioscience, Inc.). Cell suspension (100 μl) was added to a new tube, and incubated with 5 μl Annexin V-FITC at room temperature for 15 min in the dark. Fluorescence-activated cell sorting (FACS) analysis was carried out using an Epics XL™ flow cytometer with Expo32 software (Beckman Coulter). Cells were analysed by forward scatter, and annexin V fluorescence intensity was measured in the fluorescence channel FL-1 with an excitation wavelength of 488 nm and an emission wavelength of 530 nm. At least 1 × 104 cells were examined.
Measurement of cytosolic calcium
Intracellular Ca2+ concentration was measured by the cell-permeable probe fluo-3 AM, whose fluorescence directly represents the ion concentration, as reported previously( Reference Tesoriere, Attanzio and Allegra 14 ). Fluorescent intensities were analysed by FACS analysis. Ca2+-dependent fluorescence intensity was measured by FACS analysis in the fluorescence channel FL-1 with an excitation wavelength of 488 nm and an emission wavelength of 530 nm. At least 1 × 104 cells were examined.
Measurement of PGE2
After incubation, as indicated above, PGE2 released (in pg/ml) from erythrocytes (1 × 109 cells/ml) was quantified using a Prostaglandin E2 Enzyme Immunoassay Kit (Cayman Chemical Corporation, Inc.) in accordance with the manufacturer's protocol. Briefly, after incubation, the cells were pelleted by centrifugation at 4°C, for 5 min, at 450 g . Samples of the supernatant were diluted at 1:2·5 with assay buffer. Then, a 100 μl sample, a 50 μl alkaline phosphatase PGE2 conjugate and a 50 μl monoclonal anti-PGE2 EIA antibody were applied to goat anti-mouse IgG microtitre plates, and incubated at room temperature for 2 h. After washing, 200 μl of p-nitrophenyl phosphate substrate solution were added and incubated at room temperature for 45 min. Finally, optical density at 405 nm was measured on a microplate reader. PGE2 concentrations in the samples were calculated from a PGE2 standard curve (25–5000 pg/ml), which was run in parallel.
Measurement of intracellular reactive oxygen species
Reactive oxygen species (ROS) production was monitored by measuring fluorescence changes arising from intracellular oxidation of dichloro-dihydro-fluorescein diacetate (DCFDA, 10 μm). A final concentration was added to the cell medium 30 min before the end of the treatment. Erythrocytes were collected by centrifugation (5 min, 2000 rpm, 4°C), washed, suspended in PBS and subjected to FACS analysis. At least 1 × 104 cells were examined.
Measurement of glutathione
Butylated hydroxytoluene (40 mm, 25 μl) was added to 3·0 ml of incubation mixture to stop oxidative reactions. Then, the cells were precipitated and lysed with 0·5 ml H2O, and proteins were precipitated by the addition of 0·5 ml of metaphosphoric acid solution (1·67 g metaphosphoric acid, 0·20 g EDTA and 30 g NaCl in 100 ml H2O). After centrifugation (3000 g , 10 min), glutathione (GSH) was determined by titration with 5,5'-dithiobis-(2-nitrobenzoic acid) and spectrophotometric quantification at 412 nm using a molar extinction coefficient of 13 600 cm− 1, as reported previously( Reference Hu and Packer 33 ).
Partition of indicaxanthin in erythrocytes
Erythrocytes (2 ml of incubation mixture) were extracted with three volumes of chloroform–methanol (2:1, v/v). The methanol phase was dried under N2, resuspended in 1 % acetic acid in water, and analysed by HPLC using a Gilson modular liquid chromatography system (Gilson, Inc.) equipped with M 302 and 305 pumps and an injector (Model 77-25; Rheodyne) with a 20 μl injector loop and an M 802 manometric module. The chromatographic column was a Varian Microsorb C-18 column (250 × 4·6 mm inner diameter, 5 mm; Varian, Inc.) with a guard cartridge (5 × 3·9 mm; Varian, Inc.). Elution was performed with a 10 min linear gradient of solvent A (1 % acetic acid in water) to 10 % solvent B (1 % acetic acid in acetonitrile), at a flow of 1·5 ml/min. Detection was performed at 482 nm using an M 118 UV–Vis detector with the Gilson 712 HPLC system controller software. Sensitivity was 0·05 % absorbance units. The retention time of Ind was 4·2 min. Ind concentration was quantified by reference to standard curves constructed with 1–50 ng of the purified compound, and by relating the amount of the compound under analysis to the peak area.
Ex vivo spiking of blood with oxysterols and/or indicaxanthin
Whole blood collected from healthy volunteers (n 5, 39–45 % haematocrit) after an overnight fast was individually incubated with the oxysterol mixture either in the absence or presence of 5 μm-Ind (37°C, 5 % CO2, 95 % humidity, 48 h). Erythrocytes were isolated by centrifugation on a Ficoll gradient, washed and resuspended in PBS to obtain a haematocrit of 0·4 %. Cytofluorometric determination of PS exposure and ROS production were as described previously. The experiments were replicated two times. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki.
Endothelial cell culture and gravity adherence assays
HUVEC (American Type Culture Collection) were only used for up to eight additional passages. Cells were grown in 75 mm2 flasks with endothelial cell basal medium MV2 (PromoCell) containing 100 pg/ml of heparin, 10 % fetal bovine serum (GIBCO), penicillin/streptomycin (120 U/ml (72 μg/ml) and 120 pg/ml) and 30 pg/ml of endothelial cell growth supplement. At 85 % confluence, the cells were subcultured in ninety-six-well flat-bottom Costar plates, and used for adherence assays within 24 h. The adherence of erythrocytes to HUVEC was measured according to the method of Sugihara et al. ( Reference Sugihara, Sugihara and Mohandas 34 ), with some modification. Briefly, erythrocytes in Ringer solution (300 μl, 0·4 % haematocrit) were layered on confluent HUVEC that were previously washed with the same solution, and incubated at 37°C for 30 min. The plate was sealed with a packing tape, and inverted at 37°C for an additional 30 min. While still inverted, the occlusive sheet was removed, bulk fluid was taken out, and the sides and rim of each well were quickly swabbed to eliminate any fluid without disturbing the endothelial layer. SDS (0·5 %, 200 μl) was added and Hb in each well was determined at 405 nm, after incubation for 1 h at room temperature with stirring. Adherence was expressed as the percentage of erythrocytes adhering to HUVEC, calculated from the ratio of Hb absorbance after incubation to Hb absorbance of erythrocytes layered on HUVEC (average Hb absorbance of replicate wells), with correction for the absorbance of wells containing only HUVEC.
Statistical analysis
Data are presented as means and standard deviations, with independent experiments being carried out in triplicate. Statistical comparisons were made by the one-way ANOVA test followed by Bonferroni's correction for multiple comparisons using Instat-3 Statistical Software (GraphPad Software, Inc.). In all cases, significance was accepted if the null hypothesis was rejected at the P< 0·05 level.
Results
Indicaxanthin and oxysterol-induced eryptosis in isolated cells
Eryptosis was stimulated by a 48 h treatment of isolated erythrocytes with a mixture of oxysterols mimicking their plasma combination in hypercholesterolaemia (final concentration 20 μm)( Reference Addis, Emanuel and Bergmann 11 – Reference Chang, Abdalla and Sevanian 13 ). Hallmarks of eryptosis, including erythrocyte membrane scrambling with PS exposure and cell shrinkage, were investigated by cytofluorometry. Pre-incubation of erythrocytes with 1 to 5 μm-Ind decreased the oxysterol-induced increase in annexin V-binding fluorescent cells (42·9 % v. 2·0 % of the control) in a dose-dependent manner, with total protection conferred at the concentration of 5 μm. This concentration did not show any appreciable effect in the absence of oxysterols (Fig. 2(a) and (b)). Dietary Ind (5 μm) also prevented the loss of cell volume, estimated from forward scatter (Fig. 2(c) and (d)). Eryptosis is associated with Ca2+ entry and cytosolic Ca activity( Reference Lang, Gulbins and Lerche 1 ). Cytosolic Ca2+ was evaluated by a fluorescent probe. Dietary Ind dose-dependently reduced the oxysterol-induced increase in Fluo3-AM fluorescence intensity, returning to control values at the concentration of 5 μm (Fig. 3(a)). Formation of PGE2 controls the opening of Ca channels after treatment of erythrocytes with oxysterols( Reference Lang, Kempe and Myssina 35 ). Immunodetection measurements showed that Ind dose-dependently reduced the release of PGE2 in the medium (Fig. 3(b)). Ind alone did not modify cytosolic Ca levels or influence the release of PGE2 (Fig. 3(a) and (b)). Oxysterol-induced eryptosis is controlled by intracellular ROS generation and accompanied by the depletion of GSH( Reference Tesoriere, Attanzio and Allegra 14 ). Cytofluorometric analysis with DCFDA showed that 1 to 5 μm-Ind inhibited ROS production in a dose-dependent manner (Fig. 4(a) and (b)). The depletion of GSH was also prevented by Ind in a dose-dependent manner (Fig. 4(c)). Ind alone did not have any effect on ROS production or modify the redox tone of the cells at the concentration of 5 μm (Fig. 4(a) and (c)).

Fig. 2 Inhibition of oxysterol-induced eryptosis by indicaxanthin (Ind). (a) Percentage of phosphatidylserine-exposing erythrocytes or (c) their forward scatter, after 48 h incubation with the oxysterol mixture in the presence of 0–5 μm-Ind. Histograms of (b) annexin V-binding cells or (d) their forward scatter in a typical experiment in the absence (![]() ) or presence (
) or presence (![]() ) of 5 μm-Ind. Cells incubated with vehicle were used as the control. Values are means of six independent experiments carried out in triplicate, with their standard deviations represented by vertical bars. *Each value was significantly different from that of the control (P< 0·0001; ANOVA with Bonferroni's test). † Each value was significantly different from that of the oxysterol mixture in the absence of indicaxanthin (P< 0·05; ANOVA with Bonferroni's test).
) of 5 μm-Ind. Cells incubated with vehicle were used as the control. Values are means of six independent experiments carried out in triplicate, with their standard deviations represented by vertical bars. *Each value was significantly different from that of the control (P< 0·0001; ANOVA with Bonferroni's test). † Each value was significantly different from that of the oxysterol mixture in the absence of indicaxanthin (P< 0·05; ANOVA with Bonferroni's test).

Fig. 3 Inhibition of oxysterol-induced (a) Ca2+ entry and (b) PGE2 release by indicaxanthin (Ind) in human erythrocytes. Cells were incubated in the absence or presence of 0–5 μm-Ind for 48 h. Cells incubated with vehicle were used as the control. Values are means of six independent experiments carried out in triplicate, with their standard deviations represented by vertical bars. * Each value was significantly different from that of the control (P< 0·0001; ANOVA with Bonferroni's test). † Each value was significantly different from that of the oxysterol mixture in the absence of indicaxanthin (P< 0·05; ANOVA with Bonferroni's test).

Fig. 4 Inhibition of oxysterol-induced (a, b) reactive oxygen species (ROS) production and (c) glutathione (GSH) depletion by indicaxanthin (Ind) in human erythrocytes. (a) Dichloro-dihydro-fluorescein diacetate (DCFDA)-associated mean fluorescence intensity (MFI) and (c) GSH levels, after 48 h incubation of erythrocytes with the oxysterol mixture in the absence or presence of 0–5 μm-Ind. (b) Histogram of DCFDA-stained cells in a typical experiment without (![]() ) or with (
) or with (![]() ) 5 μm-Ind. Cells incubated with vehicle were used as the control. Values are means of six independent experiments carried out in triplicate, with their standard deviations represented by vertical bars. * Each value was significantly different from that of the control (P< 0·0001; ANOVA with Bonferroni's test). † Each value was significantly different from that of the oxysterol mixture in the absence of indicaxanthin (P< 0·05; ANOVA with Bonferroni's test).
) 5 μm-Ind. Cells incubated with vehicle were used as the control. Values are means of six independent experiments carried out in triplicate, with their standard deviations represented by vertical bars. * Each value was significantly different from that of the control (P< 0·0001; ANOVA with Bonferroni's test). † Each value was significantly different from that of the oxysterol mixture in the absence of indicaxanthin (P< 0·05; ANOVA with Bonferroni's test).
Previous studies have indicated that ROS generation controlled the upstream production of PGE2, followed by the opening of PGE2-dependent Ca channels and Ca2+ entry, in the oxysterol-induced eryptotic signalling axis( Reference Tesoriere, Attanzio and Allegra 14 ). The intracellular ROS level was monitored from 60 min to 48 h in erythrocytes incubated with a mixture of oxysterols, either in the absence or presence of 5 μm-Ind. ROS were rapidly generated within 60 min; thereafter, a steady-state level was maintained until the end of the observation at 48 h, in the absence of Ind. ROS production appeared to be completely prevented during the entire observation time following 1 h pre-incubation with Ind (Fig. 5). Separate experiments were carried out to investigate the incorporation and fate of Ind in oxysterol-treated erythrocytes. Ind extracted from erythrocytes after 1 h pre-incubation amounted to 0·5 nmol/107 cells, and a progressive decline in the level was observed 1 h after the addition of oxysterols, with disappearance being observed at 12 h (Fig. 5). Taken together, the data suggest an early activity of Ind against ROS production, resulting in a long-lasting protection of the cell redox environment, even after Ind consumption.
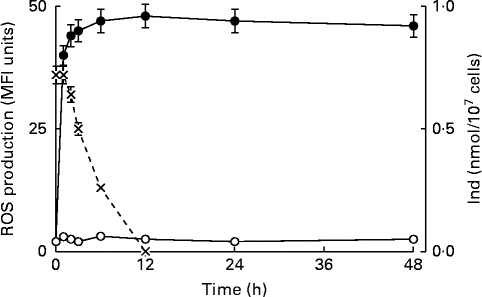
Fig. 5 Time-dependent reactive oxygen species (ROS) production in erythrocytes treated with the oxysterol mixture in the absence (![]() ) or presence (
) or presence (![]() ) of 5 μm-indicaxanthin (Ind) and incorporation of the phytochemical in the cells. Dichloro-dihydro-fluorescein diacetate-associated mean fluorescence intensity (MFI). Values are means of six independent experiments carried out in triplicate, with their standard deviations represented by vertical bars.
) of 5 μm-indicaxanthin (Ind) and incorporation of the phytochemical in the cells. Dichloro-dihydro-fluorescein diacetate-associated mean fluorescence intensity (MFI). Values are means of six independent experiments carried out in triplicate, with their standard deviations represented by vertical bars. ![]() , ROS production (MFI);
, ROS production (MFI); ![]() , Ind (nmol/107 cells).
, Ind (nmol/107 cells).
Indicaxanthin and oxysterol-induced eryptosis ex vivo
Ex vivo spiking of blood with the oxysterol mixture was carried out to simulate a pathophysiological condition of hypercholesterolaemia. Whole blood from healthy human subjects was incubated with a mixture of oxysterols, either in the absence or presence of 5 μm-Ind, and then erythrocytes were isolated and subjected to annexin V-binding and DCFDA-associated fluorescence measurements. With respect to the cells isolated from homologous blood incubated in the absence of Ind, oxysterols induced an increase in PS exposure (Fig. 6(a)) and intracellular ROS production (Fig. 6(b)), with the depletion of cellular GSH content (Fig. 6(c)). All these effects were completely prevented by Ind (Fig. 6(a)–(c)). PS-exposing erythrocytes have been shown to adhere to the cells of the vascular wall, playing a role in vaso-occlusive events( Reference Chung, Bae and Lim 6 , Reference Andrews and Low 7 ). Additional experiments were performed to investigate the activity of Ind against the adherence of oxysterol-treated erythrocytes to endothelial cells. HUVEC monolayers were exposed to erythrocytes, isolated after the ex vivo spiking of blood with a mixture of oxysterols in the absence or presence of 5 μm-Ind for 48 h. After gentle washing of wells and treatment with SDS, the adherence of erythrocytes was evaluated from the ratio of Hb of lysed HUVEC to Hb of erythrocytes applied to the wells. Following incubation with a mixture of oxysterols, Ind completely prevented the adhesion of erythrocytes to HUVEC isolated after the ex vivo spiking of blood (Fig. 7). Ex vivo spiking with 5 μm-Ind alone did not affect the adherence of erythrocytes (Fig. 7).
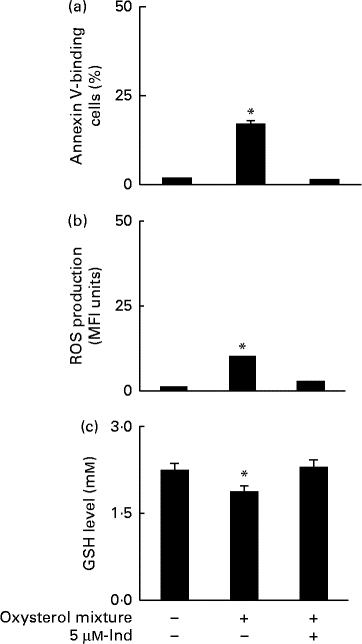
Fig. 6 (a) Phosphatidylserine exposure, (b) reactive oxygen species (ROS) production and (c) glutathione (GSH) level in erythrocytes isolated after a 48 h ex vivo spiking of fresh human blood with the oxysterol mixture in the absence or presence of 5 μm-indicaxanthin (Ind). Cells isolated from homologous blood incubated in the presence of vehicle were used as the control. Values are means of independent experiments carried out in duplicate with blood collected from five volunteers (each contributing two samples (n 10)), with their standard deviations represented by vertical bars. * Each value was significantly different from that of the control (P< 0·0001; ANOVA with Bonferroni's test).
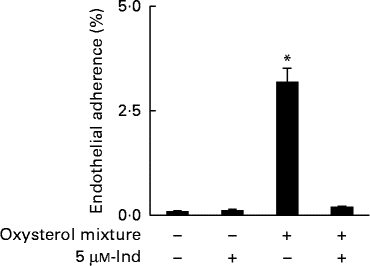
Fig. 7 Adherence of erythrocytes to human umbilical vein endothelial cell monolayers isolated after a 48 h ex vivo spiking of fresh human blood with the oxysterol mixture in the absence or presence of 5 μm-indicaxanthin (Ind). Cells isolated from homologous blood incubated in the presence of vehicle were used as the control. Values are means of independent experiments carried out in duplicate with blood collected from five volunteers (each contributing two samples (n 10)), with their standard deviations represented by vertical bars. * Each value was significantly different from that of the control (P< 0·0001; ANOVA with Bonferroni's test).
Discussion
Oxysterols of pathophysiological interest have recently been revealed as powerful inducers of suicidal erythrocyte death or eryptosis( Reference Tesoriere, Attanzio and Allegra 14 ). The present study investigating for the first time the anti-eryptotic activity of a dietary phytochemical shows that Ind from cactus pear (Opuntia ficus-indica) fruit, in a concentration range including the amounts measured in human plasma after a fruit meal( Reference Tesoriere, Allegra and Butera 30 ), prevents eryptosis of healthy human erythrocytes exposed to a mixture of oxysterols mimicking the plasma composition of subjects with hypercholesterolaemia( Reference Addis, Emanuel and Bergmann 11 – Reference Chang, Abdalla and Sevanian 13 ). The effect was observed in either isolated cells or after the ex vivo spiking of whole blood.
Mechanistic aspects of eryptosis promoted by various oxysterols, including the mixture used in the present study, have for the first time been investigated in our laboratory( Reference Tesoriere, Attanzio and Allegra 14 ). The process that appeared entirely controlled by early ROS generation was totally abrogated by antioxidants and substantially independent of Ca ions. A number of studies have characterised Ind as a radical scavenger( Reference Butera, Tesoriere and Di Gaudio 20 , Reference Tesoriere, Allegra and Butera 22 ), and antioxidant activity and protective effects of the pigment against chemically induced oxidative stress have been shown in biological environments( Reference Tesoriere, Allegra and Butera 26 , Reference Tesoriere, Butera and D'Arpa 36 ). It has also been observed that erythrocytes of healthy human subjects incorporate Ind after consumption of a cactus pear fruit meal, which results in the resistance of erythrocytes to an oxidative stress induced ex vivo ( Reference Tesoriere, Butera and Allegra 25 ). Given the pivotal role of ROS and oxidative stress in oxysterol-induced eryptosis( Reference Tesoriere, Attanzio and Allegra 14 ), the fact that Ind prevents ROS production and preserves the cell redox balance could easily explain its anti-eryptotic effect. Our kinetic study monitoring the level of ROS generated in oxysterol-treated erythrocytes and the level of incorporated Ind, between 1 and 48 h, suggests the activity of Ind occurring at a very early step, preventing the incidence of the eryptotic signal and downstream events. Apparently, this is enough to definitely inhibit the eryptotic cascade even after consumption of Ind. ROS-controlled PGE2 formation followed by the opening of plasma membrane Ca channels are the characteristic features of oxysterol-induced eryptosis( Reference Tesoriere, Attanzio and Allegra 14 ). Indeed, we observed that while preventing ROS production, Ind also prevented PGE2 release and Ca2+ entry, which supports its primary activity at the level of ROS scavenging and/or production. Our experiments were designed to mimic a human pathophysiological condition of hypercholesterolaemia. In this context, eventual activity of Ind in the absence of Ca was not investigated.
The molecular mechanism underlying the activity of Ind could not be assessed here; however, previous studies and present findings allow some speculation. Because of their chemico-physical characteristics, oxysterols and Ind may share the property of perturbing membrane arrangement( Reference Turco Liveri, Sciascia and Lombardo 23 , Reference Turco Liveri, Sciascia and Allegra 24 , Reference Olkkonen and Hynynen 37 , Reference Massey and Pownall 38 ) and possibly interfering with membrane lipids and functional proteins. The anti-apoptotic activity of Ind recently observed in human macrophages exposed to the highly toxic 7-KC( 29 ) has been accounted for by interference with early 7-KC-dependent activation of NADPH oxidase 4, which preceded Ca2+ entry and cytosolic Ca activity. The presence of NADPH oxidase at the erythrocyte membrane( Reference George, Pushkaran and Kostantinidis 39 ) and the observed inhibition of oxysterol-induced ROS production may suggest the occurrence of Ind activity at this level, which deserves to be investigated. Moreover, the activity of Ind as an oxyradical scavenger( Reference Butera, Tesoriere and Di Gaudio 20 , Reference Tesoriere, Allegra and Butera 22 , Reference Tesoriere, Allegra and Butera 26 , Reference Tesoriere, Butera and D'Arpa 36 ) cannot be ruled out.
According to current knowledge on the redox regulation of signalling pathways involved in cell survival and death( Reference Forman, Davies and Ursini 40 , Reference Leonarduzzi, Sottero and Poli 41 ), disturbance of the cellular redox homeostatic system and potentially harmful effects induced by redox-active phytochemicals may emerge in normal cells compared with, for example, tumour cells or cells under various pathophysiological conditions( Reference Nair, Li and Kong 42 ). It is important to mention that our experiments provide evidence that dietary-compatible concentrations of Ind that counteracted oxysterol activity did not modify the redox environment of erythrocytes in the absence of oxysterols, ruling out interference with redox-dependent physiological functions of healthy cells.
A pathophysiological condition of hypercholesterolaemia was simulated by treating whole blood with a mixture of oxysterols. A remarkable amount of eryptotic erythrocytes, ROS formation and depletion of cellular GSH content were observed after 48 h, with all these effects being prevented by the concurrent exposure of erythrocytes to dietary-compatible amounts of Ind. Finally, PS exposure in eryptotic erythrocytes is a key event that promotes the adherence of erythrocytes to endothelial cells( Reference Borst, Abed and Alesutan 10 ). We observed that Ind completely inhibited the adhesion of erythrocytes to HUVEC monolayers, isolated after the spiking of blood with a mixture of oxysterols. Ind is a membrane-active compound that interacts with and is located in phospholipid bilayers( Reference Turco Liveri, Sciascia and Lombardo 23 , Reference Turco Liveri, Sciascia and Allegra 24 ). Our findings that Ind did not exhibit adhesive effects when alone indicated that while preventing oxysterol-promoted, PS exposure-dependent adhesive events, Ind did not create or unmask other adherence sites( Reference Borst, Abed and Alesutan 10 ).
Cytotoxic oxysterols are implicated in the pathophysiology of atherosclerosis, where they induce apoptosis in the cells of the vascular wall and in monocytes/macrophages( Reference Palozza, Serini and Verdecchia 43 – Reference Larsson, Baird and Nyhalah 45 ), thus contributing to the development of an atheromatous plaque( Reference Tabas 46 ). The eryptotic activity of these compounds in hypercholesterolaemia may cause additional injury because of the adhesion of eryptotic erythrocytes to endothelial cells and eventual thrombo-occlusive complications. Our previous( 29 ) and present findings showing that nutritionally relevant amounts of Ind protect against toxicity induced by oxysterols in immunocompetent cells and erythrocytes suggest the potential health benefits from consumption of cactus pear fruits in preventing atherogenesis-related pathologies.
Acknowledgements
The present study was supported by Grant from Italy's Ministry of University and Research, MIUR (University of Palermo, ex 60 %, 2012). The MIUR had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: L. T. and M. A. formulated the research questions and designed the study; M. A. L. coordinated the work and wrote the paper; A. A. carried out the experiments and analysed the data.
None of the authors has any conflict of interest to declare.










