The occurrence of overweight and its consequences are global health concerns of increasing severity. At present, at least one billion adults are estimated to be overweight and – depending on the criteria – another 475 to 600 million individuals are estimated to be obese(Reference Taskforce1). Body weight increases result from a surplus of energy intake relative to energy expenditure. This makes early meal termination and satiety enhancement by specific ingredients a sensible approach in weight management. Increasing dietary levels of soluble or insoluble fibre has been proposed as an avenue towards weight management(Reference Slavin and Green2).
Galacto-oligosaccharides (GOS) are carbohydrates consisting of chains that include between two and seven galactose units, as well as a terminal glucose unit(Reference Nauta, Bakker-Zierikzee, Schoterman, Cho and Finocchiaro3, Reference Boehm and Stahl4). After ingestion by human subjects and non-ruminant mammals, GOS escape digestion and absorption due to lack of the appropriate digestive enzymes in the small intestine. After arriving in the colon, GOS are metabolised by resident microbial species into lactate, SCFA and other volatile compounds. The SCFA provide fuel for colonic epithelial cells or may enter the circulation with various effects on, e.g. lipid metabolism(Reference Williams and Jackson5). GOS are enzymatically produced from lactose by the food industry. They are widely used in infant nutrition formulations to mimic the biological functions of human milk oligosaccharides, such as effects on gut microbiota and the immune system. GOS are also applied in dairy products, beverages and nutritional bars(Reference Nauta, Bakker-Zierikzee, Schoterman, Cho and Finocchiaro3). Given the known potential of other plant-derived oligosaccharides (e.g. fructo-oligosaccharides and inulin) to affect satiety and energy intake, GOS-enriched products might be useful for dietary weight management purposes as well.
The effects of oligosaccharides on satiety and energy intake may occur via a number of mechanisms, including delayed gastric emptying, lowering of energy density of diets(Reference Keenan, Zhou and McCutcheon6), enhanced colonic production and action of SCFA, and through SCFA receptors (e.g. ffar2 and ffar3) increased levels of gastrointestinal peptides associated with satiety(Reference Cani, Dewever and Delzenne7, Reference Tolhurst, Heffron and Lam8). Efficacy to reduce energy intake has been demonstrated for plant-derived oligosaccharides such as fructo-oligosaccharides and inulin(Reference Keenan, Zhou and McCutcheon6, Reference Cani, Dewever and Delzenne7, Reference Cani, Neyrinck and Maton9–Reference Gee and Johnson11), but not yet systematically for dairy-derived GOS.
In the present study, long-term (3-week) and acute (4 h) effects of dietary enrichment with GOS (Vivinal® GOS, a commercially available GOS preparation) on multiple parameters related to energy balance were evaluated in young adult male rats. In line with existing literature, we hypothesised the following effects of GOS: moderation of energy intake, attenuated body weight gain and fat-pad weight during growth, an increase in fermentation metabolite in colonic contents, decreased gene expression of ghrelin, the appetite-related stomach hormone, and increased colonic gene expression of peptide YY (PYY) and glucagon-like peptide-1 (GLP-1; pro-glucagon), two satiety-related intestinal hormones(Reference Cummings and Overduin12). Regarding acute meal-related effects of GOS, we hypothesised an enhanced suppression of the appetite-related hormone ghrelin and stimulation of the satiety-related peptides PYY and GLP-1.
When evaluating selected health effects of any dietary ingredient, one should consider relevant interactions with other diet components to safeguard both the ecological and physiological validity of results. For prebiotic oligosaccharides such as GOS, interactions with dietary Ca levels are especially relevant. Dietary Ca may influence colonic microbial composition and fermentation processes, colonic epithelial functioning and immune parameters(Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink13–Reference Schepens, Ten Bruggencate and Schonewille16), all of which may affect gastrointestinal signals that regulate appetite and food intake. Furthermore, dietary Ca may interact with fat metabolism by affecting fat absorption and lipolysis in ways that can promote weight loss(Reference Zemel17). For these reasons, we evaluated the effects of GOS enrichment in the context of two dietary Ca levels, with demonstrated effects on fermentation of other oligosaccharides(Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink13, Reference Remesy, Levrat and Gamet14). These levels correspond with lower and higher ends of Ca intake, as observed in human population studies(Reference Ross, Manson and Abrams18, Reference Gao, Wilde and Lichtenstein19).
Materials and methods
Animals
A total of forty-three male specific pathogen-free Wistar rats, aged 9 weeks at study onset, served as subjects, based on our previous observation of colonic fermentation effects of dietary oligosaccharide and Ca, with eight animals per condition. The animals were individually housed and exposed to a 12 h light–12 h dark cycle, with lights on at 06.00 hours. They had free access to water and purified diets as specified by the recommendations of the American Institute of Nutrition(Reference Reeves, Nielsen and Fahey20). All animal procedures were approved by the institutional animal use and care committee of Wageningen University.
Dietary intervention
The composition of the four test diets (Table 1), which were made in-house and presented in powdered form, differed in levels of GOS (0 or 6 % of dry weight; Vivinal® GOS; typical composition 59 % GOS, 21 % lactose, 19 % glucose and 1 % galactose on DM; Friesland-Campina Domo) and Ca (30 or 100 mmol/kg calcium monophosphate; Sigma-Aldrich). The dietary level of GOS reflects common dietary intake of oligosaccharides in human subjects and is selected based on previous studies on rats demonstrating effects of colonic fermentation(Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink13, Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink21, Reference Coussement22). The control, non-GOS diet formulations contained 6 wt% of cellulose instead of GOS. For rats, cellulose is metabolically inert, i.e. a non-digestible, non-fermentable glucose polymer (Arbocel type B800, JRS). For calcium phosphate, the lower dose (30 mmol/kg) reflected 600 mg daily Ca intake in human subjects (assuming 500 g dry weight food intake per d), which is lower than that of the recommended daily allowance RDA for Ca(Reference Ross, Manson and Abrams18), but is common among several age, sex and ethnic subgroups of the population(Reference Park, Yetley and Calco23–Reference Jackson and Savaiano25). The high Ca level (100 mmol/kg) was previously validated to affect fermentation processes via its buffering action against acidification within the gastrointestinal lumen(Reference Schepens, Ten Bruggencate and Schonewille16). For human subjects, these doses would reflect Ca intakes well above the recommended daily intake (RDI) (1000 or 1300 mg, depending on conditions(Reference Ross, Manson and Abrams18), but attainable when adding supplemental Ca-rich food items(Reference Gao, Wilde and Lichtenstein19); for a more detailed description of the used diets see Schepens et al. (Reference Schepens, Ten Bruggencate and Schonewille16)). Energy densities were calculated from energy value of individual ingredients(Reference Garrow and James26), assuming 8 kJ/g = 1·9 kcal/g for GOS(Reference Cummings, Roberfroid and Andersson27–Reference Sako, Matsumoto and Tanaka29).
Table 1 Characteristics of experimental diets (wt%) and number of animals tested
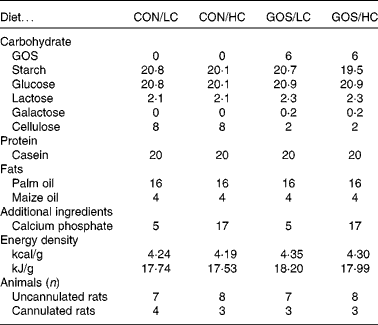
CON, control; LC, low-Ca; HC, high-Ca; GOS, galacto-oligosaccharides.
The time line of the present study was as follows (Fig. 1): in the week preceding dietary intervention, all rats were habituated to the test diet variant without GOS, with the lower level of Ca. This run-in period was followed by the dietary intervention, which lasted for 3 weeks in total, during which rats received ad libitum access to one of the four experimental diets as the single food source. Food intake was measured daily, with special caution to detect food spills in the cage. Allocation of rats to the diets was arranged so that average daily energy intake during the run-in period was equivalent across conditions. Water was freely available throughout the dietary intervention.

Fig. 1 Time line showing the several phases of the study.
Meal test
Meal presentation and blood sampling
At 17 d after the start of the dietary intervention, acute meal-induced hormonal responses were measured in all rats. Blood parameters were analysed in pre- and (4 h) post-meal samples collected from thirty rats (n 7 or 8 per dietary condition) via an incision in the tail vein. Meal presentation was at 10.30 hours and rats had been food deprived for 18 h to obtain appetite and stabilise basal hormone levels. The examined hormones were the gastrically produced hormone, ghrelin, whose blood levels correlate with hunger, and the small-intestinal peptides GLP-1 and PYY, which are released postprandially in association with satiety. All rats remained in their home cage except during blood collection. Blood was collected from an incision in the tail vein into EDTA-containing tubes with protease inhibitors, aprotinin (0·6 trypsin-inhibiting units (TIU)/ml of blood; Phoenix Europe GmbH) and dipeptidyl peptidase inhibitor (2·5 μl; Millipore). Blood samples were immediately put on ice, and centrifuged at 1600 g and 4°C to isolate plasma for storage at − 80°C.
Baseline blood samples were taken 10 min before meal presentation. For all conditions, 5 g, isoenergetic meals were formulated by mixing 53·1 kJ (12·7 kcal; about 3 g) of the powder diet with the remaining weight (about 2 g) of water. All animals completed their 5 g mash meal within 10 min after presentation. Drinking-water and regular food were removed from the rats' cages until the final blood samples were taken. The post-meal blood samples were collected 240 min after meal onset.
Cannulated rats
The time course of meal-induced ghrelin, GLP-1 and PYY responses depends on gastrointestinal digestion and transit of the food bolus, as well as the sites from which nutrients induce hormone secretion(Reference Cummings and Overduin12). For example, PYY and GLP-1 release can be biphasic, consisting of an early release triggered by macronutrients in the duodenum and a later release triggered by fermentation-derived metabolites in the ileum and colon(Reference Anini, Fu-Cheng and Cuber30, Reference Lim and Brubaker31). To obtain a more detailed time course of GOS-induced gut-hormone responses, thirteen rats were fitted with a surgically implanted chronic jugular vein cannula(Reference Remie, Van Dongen, Rensema, Remie, van Dongen, Rensema and van Wunnik32) and allocated to one of the dietary conditions. Dietary interventions, post-mortem tissue collection and acute meal test were the same as those performed with uncannulated rats. During the meal test, 2 h before food presentation, a syringe connected to an external polyethylene line was hooked up to the catheter exit. Rats remained in their home cage while blood samples were taken just before and at 20, 40, 60, 120 and 240 min after test meal presentation. Because of the particular construction of the cage needed for blood sampling, no daily food intake was measured from these cannulated rats.
Assays
Plasma levels of the gastrointestinal hormones ghrelin, GLP-1 and PYY were determined. Ghrelin is produced primarily in the stomach; its plasma levels correlate with hunger and are suppressed after meals. GLP-1 and PYY are produced in the small and large intestines, their blood levels correlate with satiation and rise after meal consumption in response to the intestinal presence of macronutrients and microbial metabolites such as SCFA(Reference Gee and Johnson11, Reference Cummings and Overduin12, Reference Delzenne, Cani and Neyrinck33). All hormones were determined by ELISA kits according to the standard directions from the manufacturer (Phoenix Europe GmbH). Plasma ghrelin was measured by kit EK-031-31, which detects total ghrelin, i.e. the combined octanoylated (bioactive) and des-octanoylated forms(Reference Foster-Schubert, Overduin and Prudom34), in rats (lower detection limit: 0·12 ng/ml; intra-assay CV: 6·9 %). GLP-1 plasma levels were determined with RIA FEK-028-11 (lower detection limit 27·6 pg/ml; CV: 4·6 %), which detects the main gastrointestinally secreted, bioactive form of GLP-1 (GLP-1 (7-36) amide) as well as its primary, inactive metabolite (GLP-1 (9-36)amide), a commonly used marker of GLP-1 secretion by virtue of its slightly longer half-life in plasma(Reference Deacon35, Reference Holst36).
PYY was measured by kit FEK-059-03 (lower detection limit 16·2 pg/ml; CV: 5·3 %), which detects PYY(1-36) and PYY(3-36), the two main circulating, bioactive forms in rats and other mammals(Reference Keire, Whitelegge and Souda37).
Post-mortem collection and analysis of tissue and colonic contents
Following the meal test on day 17, rats were fed with their specific test diet for an additional 4 d, after which they were euthanised by exposure to a carbon dioxide–oxygen mixture (ratio 2:1). Epididymal fat pads, caecal tissue, proximal colonic luminal contents and stomach and colonic mucosa scrapings were collected and analysed as follows.
Epididymal fat pads were removed and weighed on laboratory scales with 0·1 g accuracy to provide a marker of total and abdominal body fat(Reference Cani, Dewever and Delzenne7). The caecum was clamped with haemostats and then severed at the ileocaecal and caeco-colonic junctions. Then, the caecal tissue was thoroughly rinsed with saline, dried on filter paper and immediately weighed with 0·1 g resolution.
Luminal contents were collected from the proximal 3 cm of the opened colon, packed in foil and immediately snap-frozen in liquid N2 for later analysis of lactate and SCFA. Lactate levels (comprising d- and l-lactate) were determined by colorimetric detection at 340 nm of NADH, after oxidation to pyruvate by NAD in the presence of lactate dehydrogenase (Raisio Diagnostics Spa). The SCFA (acetic, propionic and butyric acids) in proximal colonic contents were determined by GC, as described elsewhere(Reference Tangerman and Nagengast38), using a Carlo-Erba GC-8000 series chromatograph with a capillary column CP-FFAP CB for NEFA (dimensions: 25 m (length) × 0·53 mm (inner diameter) × 0·70 mm (outer diameter); film thickness 1 μm) with a cold on-column injector and flame ionisation detection.
The dissected gastric and colonic tissues were thoroughly rinsed with saline, and the mucosal layers from stomach-fundus and proximal colonic sections were scraped off using a spatula and snap frozen in liquid N2 for later determination of selected gastroinstestinal peptide mRNA levels.
Mucosal scrapings were ground in liquid N2 using a mortar and pestle cooled with liquid N2. Total RNA was isolated from these homogenates using Trizol reagent (Invitrogen) according to the manufacturer's instructions. The concentration and quality of isolated RNA were assessed by spectrophotometry at 260 and 280 nm (NanoDrop ND-1000, NanoDrop Technologies, Inc.). RNA cleanup was performed using the RNeasy Mini protocol (Qiagen).
To eliminate remaining genomic DNA contamination, a second DNase treatment (DNase I; Invitrogen) was included for all RNA samples before first-strand complementary DNA synthesis. Reverse transcription was carried out according to the manufacturer's protocol (Applied Biosystems) using 1 μg of RNA per sample per reaction. The resulting complementary DNA samples were stored at − 20°C until use. Real-time PCR amplification was performed in ninety-six-well plates on an ABI Prism 7500 Fast Real-Time PCR system (Applied Biosystems), using primers and probe sets specific to the preproghrelin gene for ghrelin(Reference Beck, Richy and Stricker-Krongrad39), proglucagon gene for GLP-1(Reference Cani, Neyrinck and Maton9, Reference Zhou, Hegsted and McCutcheon40) and the PYY gene(Reference Keenan, Zhou and McCutcheon6). Taqman probes, containing a 5′ carboxy fluorescein (FAM) fluorescent reporter and a 3′ tetramethylrhodamin (TAMRA) or minor grove binder (MBG) quencher dye, were synthesised by PE Applied Biosystems Custom Oligonucleotide Synthesis Services. The gene expression data for animals in the different conditions were compared to average expression for the housekeeping gene, β-actin, in animals that had received the control/low-Ca diet. The PCR primers were obtained from Sigma-Aldrich. The used primer sequences for the preproghrelin gene were: 5′-GCTTCTTGAGCCCAGAGCAC-3′ (forward) and 5′-GTGGCTGCAGTTTAGCTGGTG-3′ (reverse) for the preproghrelin gene; 5′-GTAATGCTGGTACAAGGCAG-3′ (forward) and 5′-TTGATGAAGTCTCTGGTGGCA-3′ (reverse) for the proglucagon gene; and 5′-GCAGCGGTATGGGAAAAGAG-3′ (forward) and 5′-CTTCTGGCCGAGACCTGA-3′ (reverse) for the PYY gene. For β-actin, the primer sequences used were 5′-CTTTCTACAATGAGCTGCGTGTG-3′ (forward) and 5′-GTCAGGATCTTCATGAGGTAGTCTGTC-3′ (reverse).
Statistical analysis
Statistical tests were conducted using Microsoft Excel and SYSTAT 11.0 software (Systat). Effects of dietary manipulations were evaluated with 2 (control/GOS) × 2 (low-/high-Ca) between-group ANOVA followed by post hoc Scheffé tests in case of significance (P< 0·05). Following the run-in period, at the start of the dietary intervention, a small, but statistically significant, difference of 4·5 % in body weight existed between animals in the low-Ca conditions. To control for possible confounding influences on results of the dietary intervention, initial body weight was therefore included as a covariate in all ANOVA analyses. Data on fat-pad weight, tissue gene expression and colonic contents of cannulated and uncannulated animals were pooled. Hormonal responses during acute meal tests were analysed separately for cannulated and uncannulated rats. The intensity of hormonal responses was indexed by the difference between pre-meal and 4 h post-meal levels in uncannulated rats and 4 h AUC, using the trapezoid rule for cannulated rats. To analyse associations between energy intake and weight gain, and PYY/GLP-1 plasma levels and tissue gene expression, non-parametric Spearman correlation coefficients were calculated based on the assumption of non-normality of energy intake and hormonal data for the combined dietary conditions. Overall, P values of 0·05 and below for two-sided tests were considered to be statistically significant.
Results
Energy intake, body weight and epididymal fat pad and caecal weight
Mean daily energy intake during the 3 d preceding dietary intervention did not differ between the four conditions (Table 2). For the 17 d dietary intervention period before the meal tests, a main effect of dietary GOS level was found reflecting decreased daily energy intake (average: 14 %; F(1,24) = 39·5; P< 0·001; Table 3; Figs. 2 and 3). A main effect for Ca was also found (F(1,24) = 6·5, P= 0·018), reflecting higher intakes (average: 4·5 %) at high than at low Ca levels. However, no GOS × Ca statistical interaction effect was found, indicating similar GOS-related suppression of food intake at both dietary Ca levels. The day-to-day energy intake records (Figs. 2 and 3) show that GOS-related differences in energy intake occurred most clearly during the first 6 d of dietary intervention, GOS-related reduced intakes were not followed by rebound hyperphagia on subsequent days. The cumulative energy intake of GOS-enriched diets remained consistently lower throughout the entire dietary intervention period.
Table 2 Average body weight and daily energy intake of rats in different conditions before the dietary intervention (Mean values with their standard errors)
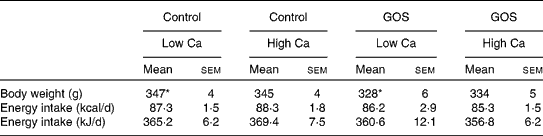
GOS, galacto-oligosaccharides.
* Energy intake did not differ between conditions, but body weight differed between the two low-Ca conditions (pair-wise Scheffé test, P= 0·04). Body weight was therefore used as a covariate in ANOVA of dietary intervention effects.
Table 3 Effects of experimental diets on parameters of energy balance‡ (Mean values with their standard errors)

GOS, galacto-oligosaccharides; GLP-1, glucagon-like peptide-1; PYY, peptide YY.
Significant, pair-wise between-condition differences (per Scheffé test) are indicated as follows: effect of GOS, *P< 0·05, **P< 0·01 and ***P< 0·001; effect of calcium, †P< 0·05 ††P< 0·01 and †††P< 0·001.
‡ Δ Energy intake indicates the average change in daily intake during the first 16 d of dietary intervention relative to average daily intake during the 3 d before dietary intervention; body weight gain was measured over the first 16 d of the dietary intervention; caecal and epididymal fat-pad weight, lactate levels in colonic contents, stomach mucosal expression of the appetite-related peptide ghrelin and colonic mucosal expression of satiety-related hormones GLP-1 and PYY were measured at the end of the study, after 3 weeks of dietary intervention.

Fig. 2 Daily energy intake during the 16 d dietary intervention (d1–d16). Values are means, with their standard errors represented by vertical bars. ‘Run in’ denotes the last 3 d of the 7 d habituation period for all animals to the low-calcium, no galacto-oligosaccharides (GOS) powder diet. ![]() , Control, low-calcium (n 7);
, Control, low-calcium (n 7); ![]() , control, high-calcium (n 8);
, control, high-calcium (n 8); ![]() , GOS, low-calcium (n 5);
, GOS, low-calcium (n 5); ![]() , GOS, high-calcium (n 8).
, GOS, high-calcium (n 8).
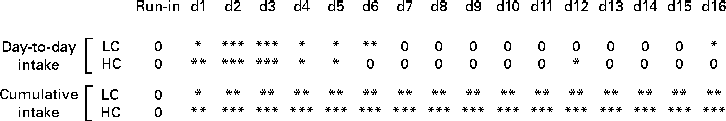
Fig. 3 Statistical significance of galacto-oligosaccharide (GOS) effects on daily and cumulative energy intake during dietary intervention. Results of pair-wise comparisons, by Scheffé tests, of control v. GOS-enriched diets, analysed separately for low-calcium (LC) and high-calcium (HC) diets (* P≤ 0·05; ** P≤ 0·01; *** P≤ 0·001; 0 = no difference) are shown. GOS reduced energy intake significantly during the first week of the dietary intervention, while not causing compensatory overeating on subsequent days. Accordingly, cumulative energy intake with GOS-enriched diets was reduced throughout the 16 d dietary intervention, amounting to an average 14 % reduction of energy intake over the period as a whole.
Overall, daily energy intake and body weight gain during the dietary intervention correlated significantly (Spearman ρ = 0·58; P< 0·01). Average body weight gain in GOS-fed rats was 5 g less than in control rats, but this difference was not statistically significant (Table 3). The GOS diets were associated with decreased epididymal fat-pad weight (F(1,37) = 12·1, P= 0·001; Table 3). For Ca, no significant main or interaction effects were seen on body weight gain and on body and fat-pad weights.
Finally, caecal weight was increased in rats on GOS diets (F(1,37) = 155; P< 0·001). Ca moderated caecal weight (F(1,37) = 7·4; P< 0·01) by interacting with GOS (F(1,36) = 11·5; P= 0·002), as confirmed by Scheffé post hoc tests (Table 3).
SCFA and lactate in proximal colonic contents (Table 3)
Statistical main effects for GOS and Ca (F(1,36) = 7·2, P< 0·002 and F(1,36) = 8·0; P< 0·01), as well as a GOS × Ca interaction effect, were observed on propionic acid levels (F(1,36) = 16; P< 0·001). Post hoc Scheffé tests showed that GOS increased propionate at high, but not low, Ca levels. Overall, butyrate levels were lowered in GOS-containing diets (F(1,36) = 42; P< 0·001). While a main effect of Ca was found reflecting increased butyrate levels (main effect for Ca, F(1,36) = 6·1; P< 0·02), pair-wise comparisons by post hoc tests did not reveal Ca effects at fixed GOS levels (Table 3). Acetic acid levels did not show any effects of GOS or Ca.
Lactate level was elevated at high levels of GOS (F(1,37) = 8·1, P= 0·007) and low levels of Ca (F(1,37) = 6·3, P= 0·017; Table 3). In addition, a statistical GOS × Ca level interaction (F(1,37) = 6·1, P= 0·018) was found. The post hoc test showed that lactate levels were significantly elevated in the GOS/low-Ca conditions compared to all other conditions.
Plasma hormone responses during meal tests
Uncannulated rats (Fig. 4)

Fig. 4 Plasma levels of the satiety-related intestinal peptides peptide YY (PYY) (a) and glucagon-like peptide-1 (GLP-1) (b) and the appetite-related gastric peptide ghrelin (c) before and 4 h after consumption of isocaloric experimental meals in uncannulated rats. Values are means, with their standard errors represented by vertical bars. Measurements were made in tail-vein blood sampled just before and at 4 h after meal presentation. Significant differences between galacto-oligosaccharides (GOS)-fed rats and their control group counterparts are indicated; *** Mean values were significantly different for low-calcium diets (P< 0·001). ††† Mean values were significantly different for high-calcium diets (P= 0·001). ![]() , Control, low-calcium (n 7);
, Control, low-calcium (n 7); ![]() , control, high-calcium (n 8);
, control, high-calcium (n 8); ![]() , GOS, low-calcium (n 7);
, GOS, low-calcium (n 7); ![]() , GOS, high-calcium (n 8).
, GOS, high-calcium (n 8).
Dietary GOS stimulated the pre-meal baseline plasma levels of PYY, as shown by a main effect of GOS (F(1,25) = 22·0, P< 0·001). Also, a main effect of Ca (F(1,25) = 15·3) was found (P= 0·001) and a Ca × GOS interaction effect (F(1,25) = 8·0, P= 0·009), indicative of attenuation of PYY plasma levels by Ca. Post hoc Scheffé tests confirmed higher baseline PYY levels in the GOS/low-Ca than in the GOS/high-Ca condition. The 4 h post-meal elevations of PYY showed a main (stimulatory) effect of GOS (F(1,25) = 8·73, P= 0·007) and a main (moderating) effect of Ca (F(1,25) = 4·2, P= 0·05). Post hoc tests indicated increased 4 h PYY elevations in both GOS conditions, regardless of Ca level. Pre-meal basal levels and 4 h post-meal responses of GLP-1 were equal across conditions. Similarly, neither basal nor meal-induced ghrelin responses showed significant effects of dietary GOS or Ca.
Cannulated rats (Fig. 5)

Fig. 5 Plasma levels of gastrointestinal peptides (peptide YY (PYY) (a), glucagon-like peptide-1 (GLP-1) (b) and ghrelin (c)) before and after isoenergetic experimental meals in cannulated rats. Values are means, with their standard errors represented by vertical bars. Response patterns in different dietary conditions resembled those observed in uncannulated rats. Mean values were significantly different between galacto-oligosaccharides (GOS)-fed rats and their control group counterparts, which are indicated as follows: *** P< 0·001 for low-calcium meals; ††† P= 0·001 and ‡ P= 0·009 for high-calcium meals. ![]() , Control, low-calcium (n 4);
, Control, low-calcium (n 4); ![]() , control, high-calcium (n 3);
, control, high-calcium (n 3); ![]() , GOS, low-calcium (n 2);
, GOS, low-calcium (n 2); ![]() , GOS, high-calcium (n 3).
, GOS, high-calcium (n 3).
The data obtained from blood samples from cannulated rats were in line with those from uncannulated rats. Pre-meal baseline PYY plasma levels were increased in GOS-fed rats (F(1,7) = 9·6, P< 0·02). The more detailed blood sampling data indicated that GOS-containing meals were followed by a biphasic PYY response, i.e. an immediate post-meal rise followed by a secondary rise 4 h after the meal. Post hoc Scheffé tests showed that PYY plasma levels at 4 h after the GOS-containing meals were higher at both low- and high-Ca levels (P= 0·009 and 0·018, respectively). Also, the area under the curve of the PYY response was higher after GOS than after control meals (F(1,7) = 12·1, P= 0·01). Ca attenuated post-meal PYY excursions (F(1,7) = 8·2, P= 0·025), with post hoc Scheffé tests showing larger PYY responses after GOS/low-Ca meals than after no-GOS/high-Ca meals. Rats fed on GOS diets had a slightly elevated pre-meal baseline levels of GLP-1 (F(1,7) = 8·6, P= 0·02). However, the GLP-1 data did not show the 4 h GOS fermentation-related rise in plasma levels seen with PYY. Instead, a post-meal rise to maximum at 2 h after test meal consumption was seen in all dietary conditions, with no further elevation at 4 h. Meal-induced ghrelin levels reached their nadir at the 90 min observation point in all conditions. Neither GOS nor Ca levels affected the size of ghrelin basal or post-ingestive levels in cannulated rats.
Peptide hormone gene expression in stomach and colon tissue
Gastrointestinal gene expression levels of PYY and proglucagon (the precursor of GLP-1; Table 3) were on average 1·7 and 3·5 times higher after being fed on GOS than after non-GOS diets (F(1,37) = 11·3, P= 0·002 and F(1,37) = 33·7, P< 0·001, respectively). PYY and proglucagon gene expression correlated strongly (ρ = 0·82, P< 0·001). Interestingly, PYY and proglucagon gene expression correlated significantly with pre-meal PYY plasma levels during meal tests (ρ = 0·57 and 0·70, P< 0·001 for both), but not with pre-meal GLP-1 plasma levels (ρ = 0·26, non-significant in both cases). Dietary GOS did not affect ghrelin gene expression in the stomach mucosa. No statistical main or interaction effects of dietary Ca were seen on any of the tissue gene expression data.
Discussion
In the present study, effects of a 3-week dietary enrichment with 6 % (w/w) GOS on parameters of satiety and energy balance were evaluated in rats. GOS were tested in combination with two dietary Ca levels (30 and 100 mmol/kg), known for their different impact on intestinal fermentation of oligo- and polysaccharides in the gastrointestinal tract and reflecting the lower and upper end of the Ca-intake distribution in human subjects. The main results were as follows: day-to-day records showed reduced energy intake of GOS-fed rats most prominently during the first 6 d of the dietary intervention. On subsequent days, GOS-fed rats did not show compensatory overeating, rendering their cumulative energy intake consistently lower (by an average of 14 %) than control rats not receiving GOS. GOS also increased colonic gene expression of the satiety-related peptide PYY (1·7 fold) and that of proglucagon (3·5 fold). Pre-meal and meal-induced plasma levels of PYY were increased in GOS-fed rats, which further showed a reduction of epididymal fat-pad weight, an increase of caecal tissue weight and elevated lactate levels in proximal colonic contents. Calcium phosphate supplementation was associated with a higher energy intake (by 4·5 %), regardless of GOS levels, and also attenuated the postprandial rise of plasma PYY. Furthermore, Ca affected GOS-induced colonic fermentation pattern: lactate elevation was abolished while propionic acid levels were increased. In spite of its impact on GOS fermentation pattern, calcium phosphate did not abolish the impact of GOS on key parameters of energy balance (i.e. food intake reduction and changes in tissue weight and gastrointestinal gene expression).
The reduction in energy intake in response to GOS diets is in line with results reported for other fermentable fibres, e.g. fructo-oligosaccharides, inulin and resistant starch(Reference Keenan, Zhou and McCutcheon6, Reference Cani, Dewever and Delzenne7). Satiety enhancement by fibre-rich diets has also been reported in human subjects(Reference Gee and Johnson11, Reference Delzenne, Cani and Neyrinck33, Reference Cani, Joly and Horsmans41). The acute reduction of food intake by GOS was probably not crucially dependent on fermentation-related acidification in the caecum and colon, because similar results were found when GOS was combined with calcium phosphate, which acts as a buffer against fibre-induced acidification(Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink13). A mechanism that may have contributed is the release of PYY and possibly GLP-1 by SCFA activation of ffar receptors on L-cells(Reference Tolhurst, Heffron and Lam8) within the distal small intestine, caecum and colon. At 1 week into the dietary intervention, tissue changes (i.e. up-regulation of PYY and GLP-1, caecal enlargement and proliferation of L-cells) may have started to contribute as well. It could be that these structural changes, which take several days to develop, may have played a more strategic role to prevent rebound overeating in GOS-fed rats. Therefore, it would be interesting to test to what extent discontinuation of dietary GOS enrichment at different time points would trigger increased consumption to compensate for the energy deficit of GOS-fed rats accrued during the dietary intervention period.
Epididymal fat-pad weight, a marker of abdominal fat mass, was distinctly lower in GOS-fed rats. This could be a general consequence of reduced energy intake and/or a specific, direct effect of GOS on fat metabolism, e.g. increased lipid oxidation(Reference Higgins, Higbee and Donahoo42). Assuming that fat-pad weight reflects total body adiposity, this might represent a far-reaching effect of GOS diets, because lean tissue (e.g. muscle) is metabolically more active than adipose tissue(Reference Schutz, Jequier, Bray and Bouchard43) and, in the longer term, might sustain energy expenditure in the face of energy restriction(Reference Westerterp-Plantenga, Smeets and Nieuwenhuizen44).
Total body weight gain in the still-growing rats was 5 g less on the GOS than on the non-GOS diets, but the difference did not reach statistical significance. A similar discrepancy between fibre-induced reductions of energy intake and observed weight loss has previously been reported for diets enriched in plant fibres(Reference Cani, Dewever and Delzenne7). The present study may have been insufficiently powered to pick up weight differences, in the face of several contributing factors. First, the dietary intervention period might have been insufficiently long to attenuate weight gain significantly (compare, for example, a study by Wang et al. (Reference Wang, Zuberi and Zhang45), reporting body weight effects after a 12-week dietary fibre supplementation). Second, GOS may have primarily reduced the less-dense adipose tissue (as shown by our fat-pad weight data), and less so in lean mass or water content. Third, fermentable-fibre-rich diets increase the weight of the filled gastrointestinal tract, which cannot be accounted for by tissue weight alone(Reference Keenan, Zhou and McCutcheon6). This effect, added to the observed increase in caecal tissue weight in GOS-fed rats, may have obscured effects of adipose weight loss from other organs, and decreased body weight differences between GOS-fed and control rats(Reference Cani, Dewever and Delzenne7, Reference Delzenne, Cani and Daubioul10). Finally, a theoretical possibility remains that GOS lowered energy expenditure or activity in rats, although the former effects have not been found for other fibres (i.e. inulin and fructo-oligosaccharides(Reference Parnell and Reimer46)).
Dietary calcium phosphate was associated with decreased test meal-induced PYY release and a small increase in daily energy intake, regardless of dietary GOS level. The latter effect of Ca is certainly at odds with studies showing its potentiation of weight loss(Reference Zemel17). Importantly, we selected doses of calcium phosphate based on their known impact on gastrointestinal fermentation and physiology(Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink13, Reference Remesy, Levrat and Gamet14). Because of this, other Ca-dependent mechanisms that affect energy balance, i.e. the formation of Ca soaps in the gastrointestinal tract leading to increased fat excretion, may not have been fully engaged, although we did not measure faecal fat excretion directly. Previous studies reporting this mechanism have typically used 2- to 6-fold higher Ca doses(Reference Lapre, De Vries and Koeman47–Reference Zemel, Shi and Greer49).
GOS activated potential gastrointestinal satiety mechanisms by increasing the colonic mucosal gene expression of satiety-related gastrointestinal peptides PYY (1·7 fold) and the GLP-1 precursor proglucagon (3·5 fold). Similar effects have been found in previous intervention studies of fermentable dietary fibres, in which reductions in body weight or food intake were also found(Reference Cani, Dewever and Delzenne7, Reference Zhou, Hegsted and McCutcheon40, Reference Wang, Zuberi and Zhang45). These gene expression effects may be based on increased mRNA levels in peptide-producing L-cells and/or increased L-cell proliferation within the colon, prompted by up-regulated differentiation factors such as neurogenin 3 and NeuroD(Reference Cani, Hoste and Guiot50). Increased PYY gene expression in GOS-fed rats is easily reconciled with its higher pre-meal plasma levels in the present study. Presumably by stimulating PYY levels, GOS triggered an anorexigenic mechanism contributing to effects on energy intake(Reference Cummings and Overduin12). For GLP-1, a different pattern of results emerged, despite extensive colocalisation of GLP-1 and PYY in L-cells(Reference Anini, Fu-Cheng and Cuber30). The GOS-related basal pre-meal plasma levels of GLP-1 were less clearly elevated, in spite of the clearly increased colonic gene expression of proglucagon. Anatomical and physiological factors might explain this. First, a significant proportion of GLP-1 is produced in cells in the proximal parts of small intestine(Reference Theodorakis, Carlson and Michopoulos51), which receive minimal exposure to colonic GOS fermentation metabolites. Thus, GOS-induced up-regulation of colonic proglucagon gene expression would affect only a part of the entire gastrointestinal GLP-1 production. Second, for GLP-1, discrepancies between tissue gene expression and plasma levels may occur because of fluctuations in many factors, including mRNA stability and translation rate, peptide release from L-cells, efficacy of hepatic and renal metabolism and degradation by plasma peptidases(Reference Cani, Dewever and Delzenne7, Reference Deacon35). The post-meal rise of GLP-1 was, somewhat surprisingly, similar after GOS and non-GOS meals, lacking the secondary elevation seen with PYY at 4 h post-meal, during colonic fermentation(Reference Zhou, Hegsted and McCutcheon40). It could be that our meals also triggered GLP-1 release by digestible, non-GOS nutrients acting at small-intestinal sites(Reference Lim and Brubaker31, Reference Theodorakis, Carlson and Michopoulos51). This early, small-intestinally triggered release of colonic GLP-1 could have obscured the later GLP-1 release stimulated locally by colonic SCFA.
Enhanced suppression by GOS meals of plasma levels of the appetite-related hormone, ghrelin, was not observed; although theoretically, increased production of GLP-1 or PYY could affect ghrelin acutely(Reference Theodorakis, Carlson and Michopoulos51, Reference Dumoulin, Moro and Barcelo52). The finding that dietary GOS did not suppress ghrelin gene expression in the stomach is less surprising. Ghrelin cells and their progenitors are located in the proximal gastrointestinal tract, at a considerable distance from caecal and colonic sites of microbial fermentation and exposed to much weaker fluxes of GOS metabolites (SCFA and lactate) than are the colonic L-cells expressing PYY and GLP-1.
Fermentative production of lactic acid and SCFA by caecal and colonic microbiota is generally assumed to underlie the physiological and behavioural effects of dietary oligosaccharides such as GOS(Reference Anini, Fu-Cheng and Cuber30, Reference Zhou, Hegsted and McCutcheon40, Reference Cani, Hoste and Guiot50, Reference Dumoulin, Moro and Barcelo52). We observed that GOS most clearly increased colonic lactic acid level, and that addition of calcium phosphate to GOS diets abolished the elevated lactic acid levels, while increasing propionic acid levels. Comparable results are known for other fermentable fibres(Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink13, Reference Remesy, Levrat and Gamet14, Reference Younes, Coudray and Bellanger53). The present results could be explained by metabolic cross-feeding, a coordinated process performed by combined microbial species residing in the gastrointestinal tract(Reference Louis, Scott and Duncan15). The elevated lactic acid is a primary GOS metabolite from bifidobacteria and lactobacilli, species that become more abundant on diets containing prebiotics like GOS(Reference Holma, Juvonen and Asmawi54, Reference Le Blay, Michel and Blottiere55). Initial caecal conversion of GOS into lactic acid and other metabolites lowers luminal pH, thereby specifically inhibiting the activity of organisms that metabolise lactic acid, e.g. propionic acid-producing bacteria(Reference Cummings56, Reference Belenguer, Duncan and Holtrop57). The accumulating lactic acid has a poor absorbability(Reference Cummings56) and transits to the colon where fermentation proceeds at a lower pace. Dietary calcium phosphate probably influenced this fermentation by buffering against caecal acidification by lactate, thereby speeding up the conversion of caecal lactate to SCFA in the caecum. Incidentally, Ca's buffering action might explain the more moderate increases in caecal weight in combination with GOS. Evidently, the present data do not provide a comprehensive view of GOS fermentation. Colonic contents were collected at arbitrary times since the previous meal, so that substrate (GOS) levels were probably not maximal during measurement(Reference Bergman58). Also, the GOS-derived volatile SCFA are rapidly absorbed from the caecum, which is enlarged, exposed to increased blood flow and enriched in GOS-fermenting microbes in GOS-fed rats(Reference Remesy, Levrat and Gamet14, Reference Holma, Juvonen and Asmawi54, Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink59, Reference Butler, Topping and Illman60). Effective caecal extraction through absorption of GOS-derived SCFA might partly explain our unexpected observation of lower levels of colonic butyric acid in GOS-fed rats (for a recent counterexample see Walton et al. (Reference Walton, van den Heuvel and Kosters61)), while lactic acid, which is much less absorbable, may reach the colon in larger quantities. Finally, caecal and colonic samples of GOS-fed rats contain more fluid, especially in rats on low-Ca GOS diets, possibly lowering detected GOS metabolite concentrations(Reference Ten Bruggencate, Bovee-Oudenhoven and Lettink-Wissink59). Therefore, while propionic acid levels were the only measured SCFA to show elevated levels within the colon, it cannot be ruled out that GOS-derived, caecally released acetic acid and butyric acid also contributed to increased caecal tissue weight and proglucagon and PYY gene expression.
In sum, GOS-enriched diets not only reduced short-term energy intake acutely, but also induced sustained hormonal and tissue-related changes that could support negative energy balance and weight control. Although dietary calcium phosphate influenced patterns of GOS fermentation, it did not abrogate the key behavioural, hormonal and tissue-related effects triggered by GOS.
The effects of GOS on multiple parameters of energy balance merit replication in human subjects, who share many aspects of gastrointestinal physiology examined in the present study with rodents. Also, the exact relationship between rapid-onset anorexigenic actions of GOS and later structural changes in gastrointestinal and adipose tissue requires further clarification. Finally, there is a growing interest in diet-induced population shifts of gut microbiota, which can evolve within days(Reference Walker, Ince and Duncan62) and which may affect metabolic health more profoundly than previously thought(Reference Ley63). Measurement of these factors could further delineate the potential of GOS as a dietary tool supporting satiety and weight management.
Acknowledgements
The authors wish to thank the biotechnicians at the Small Animal Center of Wageningen University for expert assistance and Elly Lucas, Denise Jonker, Jan Hoolwerf and Rob Dekker for analysis of the biological samples. The present work was supported by a project grant provided by FrieslandCampina. During the study, M. H. C. S. and W. C. were both employed by FrieslandCampina, the company that has developed and markets Vivinal® GOS, the principal ingredient tested in the present study. J. O., A. J. S. and S. J. M. t. B. were employed by NIZO food research, an independent contract research company. J. O. drafted and finalised the manuscript, contributed to the concept, design and execution of the study and performed the data analysis. S. J. M. t. B. and M. H. C. S. conceptualised and designed the study and contributed to finalisation of the manuscript. A. J. S. was involved in the planning and execution of the study and contributed to the data analysis. W. C. contributed to the data analysis and finalisation of the manuscript.










