Population ageing is accelerating all over the world(1). Maintaining functional ability is the core of healthy ageing(Reference Beard, Officer and de Carvalho2). Skeletal muscle is required for physical performance in daily life(Reference Buchner, Larson and Wagner3). The decline in skeletal muscle mass (SMM) begins as early as 35 years of age and accelerates after 60 years(Reference Frontera, Hughes and Fielding4). Age-related loss of skeletal muscle, which leads to the onset of sarcopenia in severe cases, can increase the risk of functional impairment and mortality(Reference Brooks and Faulkner5–Reference Bales and Ritchie7). It is worthy to note that muscle mass reduction is an important feature of ‘pre-sarcopenia’(Reference Cruz-Jentoft, Baeyens and Bauer8). A life-course model of preventing sarcopenia(Reference Sayer, Syddall and Martin9) focuses on maximising peak muscle mass and strength in early life, maintaining peak muscle mass and strength in adult life and minimising muscle loss in older life(Reference Cruz-Jentoft, Bahat and Bauer10), which means early prevention is important and necessary for the prevention of sarcopenia. In addition to physical inactivity and inadequate dietary protein(Reference Cruz-Jentoft, Baeyens and Bauer8), few modifiable factors have been identified.
Betaine serves as an important methyl donor (Reference Craig11) in the transformation of homocysteine into methionine(Reference Obeid12), which could promote muscle protein synthesis by decreasing homocysteine thiolactone and thus activating the insulin-like growth factor-1 pathway(Reference Najib and Sanchez-Margalet13). Betaine could improve lean meat percentage of pig carcasses when restricting energy and protein intake(Reference Matthews, Southern and Bidner14–Reference Fernandez-Figares, Wray-Cahen and Steele16), and thus is widely added to animal feeds to increase lean meat in livestock(Reference Rojas-Cano, Lara and Lachica17–Reference Wang, Xu and Feng19). In recent years, the role of betaine in maintaining muscle mass in humans has been a focus of attention. Our previous cross-sectional study found that middle-aged and older men with higher serum betaine levels had a higher percentage of lean mass and a lower likelihood of low lean mass(Reference Huang, Zhu and Tan20). Consistently, cross-sectional studies from Newfoundland(Reference Gao, Randell and Zhou21) found that higher circulating betaine was associated with greater lean body mass. Given that the existing observational studies are primarily cross-sectional and only consider circulating betaine, further longitudinal studies are still needed to clarify the relationship between dietary betaine intake and skeletal muscle loss in middle-aged adults.
Therefore, this prospective cohort study aimed to investigate whether dietary betaine intake was associated with SMM change after 3 years of follow-up in community-dwelling middle-aged Chinese adults. We hypothesised that higher dietary betaine intake may be beneficial for maintaining SMM among middle-aged adults.
Methods
Study population
Data for this analysis are from the Guangzhou Nutrition and Health Study (GNHS), an ongoing, community-based prospective cohort study investigating the determinants of cardiometabolic outcomes and osteoporosis in middle-aged and older Chinese adults(Reference Liu, Xu and Wen22). The GNHS was established during 2008–2010, enrolling 3169 apparently healthy men and women aged 40–80 years living in the communities of Guangzhou, China, for at least 10 years. Of them, 2520 participants completed the first follow-up during 2011–2013. Meanwhile, another 879 participants were newly recruited in 2013. Therefore, a total of 3399 participants took part in the GNHS 2011–2013. After excluding 1920 older adults (aged ≥60 years), 1479 middle-aged adults (aged 41–60 years) were assessed for eligibility in this analysis and 1401 of whom completed the second follow-up occurring between the years 2014 and 2017. Face-to-face interviews were conducted to collect information on sociodemographic characteristics, lifestyles and food consumption in GNHS 2008–2010 and GNHS 2011–2013. Body composition measurements by whole-body dual-energy X-ray absorptiometry were performed in GNHS 2011–2013 and GNHS 2014–2017. Written informed consent was provided by all participants. All protocols were approved by the Ethics Committee of the School of Public Health at Sun Yat-sen University.
Because dual-energy X-ray absorptiometry scans were first administered in GNHS 2011–2013, that visit served as the study baseline for this analysis, and only those participants who attended both GNHS 2011–2013 and GNHS 2014–2017 were eligible for inclusion (n 1401). In the present study, 159 participants were excluded due to (1) severe metabolic diseases, including malignant neoplasms, liver cirrhosis, renal insufficiency, diabetes and chronic hepatitis; (2) missing values for body composition measurements in either GNHS 2011–2013 or GNHS 2014–2017; (3) missing dietary data or covariates (e.g. physical activity, age); (4) extreme intake of energy (men, <3347 kJ/d (<800 kcal/d) or >16 736 kJ/d (>4000 kcal/d); women, <2092 kJ/d (<500 kcal/d) or >14 644 kJ/d (>3500 kcal/d)) or dietary betaine (the top and bottom 0·5 % of the betaine intake) and (5) abnormal weight or weight change >15 % over 3 years of follow-up. Finally, 1242 participants were included for analysis (Fig. 1). All participation was voluntary, and all of them provided written informed consent. The study was approved by the Ethics Committee of the School of Public Health at Sun Yat-sen University and was conducted according to the Declaration of Helsinki.
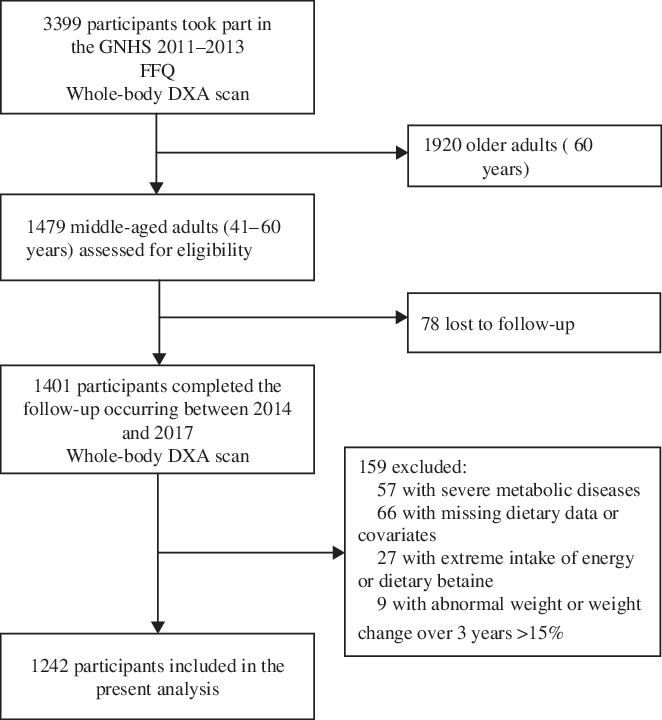
Fig. 1. Flow chart of selection of participants from the Guangzhou Nutrition and Health Study (GNHS) for the analysis of dietary betaine intake and changes in skeletal muscle mass over 3 years. DXA, dual-energy X-ray absorptiometry.
Skeletal muscle mass
Body composition was assessed in GNHS 2011–2013 and GNHS 2014–2017 by using dual-energy X-ray absorptiometry (Discovery W; Hologic Inc) according to standard procedures. Dual-energy X-ray absorptiometry calibration was performed daily with a standard phantom. Lean mass, fat mass and bone mass of the whole body, trunk, arms and legs were analysed using the Hologic Discovery software version 3.2. We assumed that all non-fat and non-bone tissue were skeletal muscle in arms and legs, thus SMM of the arms and legs was determined by subtracting bone mineral content from the total lean mass. SMM of the limbs was calculated as the sum of the SMM in arms and legs. The methods and validation data (the intraclass correlation coefficient for the test-retest reliability of the appendicular SMM measurement was 0·98 (95 % CI 0·96, 0·99)) have been reported previously(Reference Li, Fang and Ma23). The appendicular skeletal mass index (ASMI, kg/m2) was defined by dividing SMM of the limbs in kg by the square of height in metres(Reference Kim, Yang and Yoo24). Relative changes in SMM of arms, legs and limbs or ASMI were calculated following the formula:
Relative change in SMM or ASMI = ((SMM or ASMI in GNHS 2014–2017) – (SMM or ASMI in GNHS 2011–2013))/(SMM or ASMI in GNHS 2011–2013) × 100 %.
Dietary assessment
A paper-based seventy-nine-item semi-quantitative FFQ(Reference Zhang and Ho25) was used to collect dietary information in GNHS 2011–2013. For each food item, frequency (i.e. never, per year, per month, per week or per d) and amount of food consumption (servings or portion sizes) were recorded. Pictures of common foods and serving sizes were provided to help participants estimate their usual food consumption in the previous year. The participants’ daily nutrient and energy intake were calculated according to the China Food Composition Table 2004(Reference Yang, Wang and Pan26). Betaine comes mainly from plant foods such as beets, wheat bran, wheat germ, spinach, pretzels, wheat bread and crackers. Dietary betaine intake was determined according to the food composition data obtained from Zeisel et al.(Reference Zeisel, Mar and Howe27) and the US Department of Agriculture Database(28). Nutrient intake was adjusted for total energy intake using the residual method proposed by Willett & Stampfer(Reference Willett and Stampfer29).
Covariates
Weight and height were measured with the participant’s barefoot and wearing light clothes. BMI (kg/m2) was calculated as body weight in kilograms divided by the square of height in metres. Waist circumference was measured twice, and the average value was used for subsequent analyses. An interviewer-administered questionnaire was used to collect the following information in GNHS 2011–2013: sociodemographic characteristics (e.g. age, sex); general lifestyles and chronic disease history and medicine use. Daily physical activity(Reference Ainsworth, Haskell and Herrmann30), including occupation, transportation, exercise, leisure-time sedentary activity and housework, was estimated using a nineteen-item questionnaire and was classified into three categories according to tertiles of daily total metabolic equivalents: low (<0·05 metabolic equivalents × h/d), medium (0·05–3·86 metabolic equivalents × h/d) and high (>3·86 metabolic equivalents × h/d) intensity.
Statistical analysis
The interactions between betaine intake and sex on relative changes in SMM were not significant (all P > 0·05). Therefore, all analyses were carried out in the total population. Baseline characteristics of the study population were compared across tertiles (T) of energy-adjusted dietary betaine intake by using one-way ANOVA or Pearson’s χ 2 test, where appropriate. Continuous data are summarised as mean values and standard deviations. Categorical data are reported as frequencies and percentages. Energy-adjusted dietary betaine intake was evaluated as both a continuous and a categorical variable by using tertiles (medians: T1, 145·8 (range 49·7–195·6) mg/d; T2, 241·4 (range 196·5–285·5) mg/d; T3, 361·3 (range 286·8–759·3) mg/d). Multiple linear regression models and ANCOVA were used to examine the associations between dietary betaine intake and relative changes in SMM of arms, legs, limbs and ASMI, respectively. Model 1 was adjusted for age, sex, energy intake, baseline SMM or ASMI, waist circumference and height. Model 2 was adjusted additionally for physical activity level, protein intake, choline intake and use of multivitamins. Post hoc comparisons were conducted by using Bonferroni corrections. Tests for a linear trend across tertiles of dietary betaine intake were conducted by using the median value in each tertile as a continuous variable in the linear regression models.
All tests were two-sided, and P values <0·05 were considered significant. All analyses were performed using SPSS version 23.0 (IBM Corp.)(31).
Results
Participant characteristics
A total of 294 men and 948 women were included in this analysis. The mean age of the participants was 56·1 (sd 3·1) years. Mean energy and energy-adjusted dietary betaine intake were 7594 (sd 2033) kJ/d (1815 (sd 486) kcal/d) and 259·0 (sd 120·1) mg/d.
Table 1 presents the descriptive characteristics of the study population by tertiles of energy-adjusted dietary betaine intake in the GNHS 2011–2013. There was a similar proportion of men and women in each tertile of betaine intake. Although there were no significant differences in body weight, height and waist circumference across tertiles of dietary betaine intake, middle-aged adults with a higher dietary betaine intake had a higher BMI (P = 0·039). Daily dietary intake of energy and protein, as well as physical activity level, did not differ significantly between participants with different dietary betaine intakes in middle-aged adults. Nevertheless, a higher dietary choline intake was observed to be associated with a higher betaine intake. SMM of arms, legs, limbs and ASMI in the GNHS 2011–2013 was comparable across the tertiles of betaine intake, and all of them have no significant difference.
Table 1. Descriptive characteristics of 1242 middle-aged adults (41–60 years) by tertiles (T) of energy-adjusted dietary betaine intake in the Guangzhou Nutrition and Health Study 2011–2013
(Mean values and standard deviations; numbers and percentages)
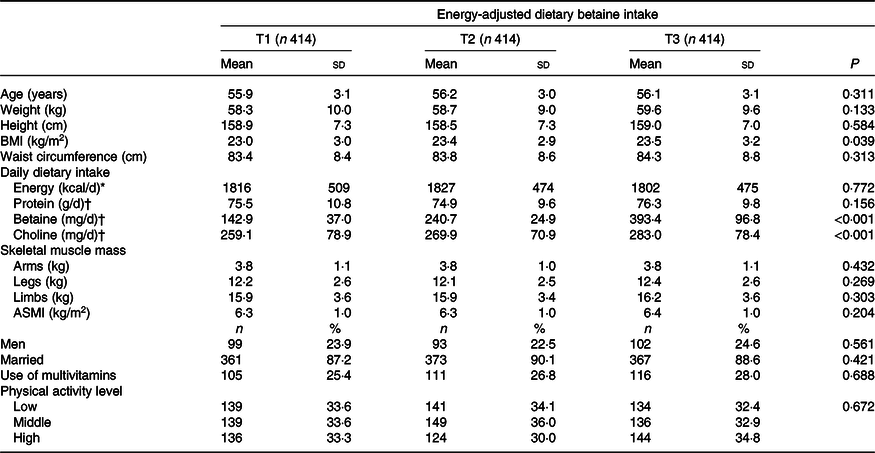
ASMI, appendicular skeletal muscle index.
* To convert energy values from kcal to kJ, multiply by 4·184.
† Nutrient intake was adjusted for energy intake using the residual method.
Table 2 shows crude changes in SMM and ASMI over 3 years. SMM of arms, legs, limbs and ASMI decreased with advancing age. Relative change in SMM of arms, legs, limbs and ASMI was –2·25 (sd 6·19), –1·28 (sd 5·70), –1·55 (sd 5·10) and –1·20 (sd 5·20) % over 3 years of follow-up.
Table 2. Crude changes in skeletal muscle mass (SMM) and appendicular skeletal mass index (ASMI) of 1242 middle-aged adults (41–60 years) between Guangzhou Nutrition and Health Study (GNHS) 2011–2013 and GNHS 2014–2017
(Mean values and standard deviations)

* Change = (SMM or ASMI in GNHS 2011–2014) – (SMM or ASMI in GNHS 2014–2017).
† Relative change = change/(SMM or ASMI in GNHS 2011–2014) × 100 %.
Associations between betaine intake and relative changes in skeletal muscle mass
The associations between dietary betaine intake and relative changes in SMM of arms, legs, limbs and ASMI were examined by using both continuous and categorical betaine variables. The results obtained from the multiple linear regression (continuous) and the ANCOVA (categorical) models were highly consistent.
Adjusted regression coefficients for relative changes in SMM of arms, legs, limbs and ASMI per 1 sd of energy-adjusted dietary betaine intake in middle-aged adults are summarised in Table 3. After adjustment for age, sex, energy intake, baseline SMM or ASMI, waist circumference and height, dietary betaine intake was significantly associated with relative changes in SMM of legs, limbs and ASMI over 3-year follow-up (β: 0·311 (se 0·155), 0·301 (se 0·140) and 0·301 (se 0·143) in model 1, respectively; all P < 0·05). The associations between dietary betaine intake and relative changes in SMM of legs, limbs and ASMI remained significant after additional adjustment for physical activity level, protein intake, choline intake and use of multivitamins (β: 0·322 (se 0·157), 0·309 (se 0·142) and 0·303 (se 0·145) in model 2, respectively; all P < 0·05). No significant associations were observed between dietary betaine intake and relative change in SMM of arms (P > 0·05).
Table 3. Relative change (Δ)* in skeletal muscle mass (SMM) and appendicular skeletal mass index (ASMI) per 1 SD of energy-adjusted dietary betaine intake in middle-aged adults from the Guangzhou Nutrition and Health Study (GNHS)
(Adjusted regression coefficients with their standard errors)
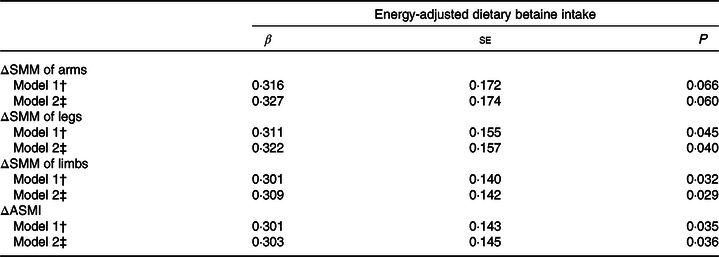
* Relative change (Δ) in SMM or ASMI = ((SMM or ASMI in GNHS 2014–2017) – (SMM or ASMI in GNHS 2011–2013))/(SMM or ASMI in GNHS 2011–2013) × 100 %.
† Model 1 was adjusted for age, sex, energy intake, baseline SMM or ASMI, waist circumference and height.
‡ Model 2 was adjusted for variables in model 1 + physical activity level, protein intake, choline intake and use of multivitamins.
Table 4 and Fig. 2 show adjusted means (SE or 95 % CI) of relative changes in SMM of arms, legs, limbs and ASMI by tertiles of dietary betaine intake, after adjustment for sex, age, energy intake, baseline SMM or ASMI, height, waist circumference, physical activity level, protein intake, choline intake and use of multivitamins. Participants in the highest tertile of dietary betaine intake had significantly less loss in SMM of limbs and ASMI and more increase in SMM of legs over 3 years of follow-up than those in the lower two betaine tertiles (all P < 0·05). A significant dose-dependent reduction in loss of leg and limb SMM and ASMI was observed with the increase of dietary betaine intake (all P trend < 0·05). However, there were neither significant differences in relative change in SMM of arms across tertiles of dietary betaine intake (all P > 0·05) nor significant linear association between loss of arm SMM and dietary betaine intake (P trend = 0·116).
Table 4. Relative change (%) in skeletal muscle mass (SMM) and appendicular skeletal mass index (ASMI)* † over 3 years estimated by tertiles (T) of energy-adjusted dietary betaine intake
(Adjusted mean values with their standard errors)
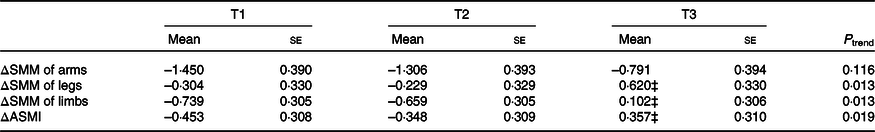
* Relative change (Δ) in SMM or ASMI = ((SMM or ASMI in GNHS 2014–2017) – (SMM or ASMI in GNHS 2011–2013))/(SMM or ASMI in GNHS 2011–2013) × 100 %.
† Adjusted for age, sex, energy intake, baseline SMM or ASMI, waist circumference, height, physical activity level, protein intake, choline intake and use of multivitamins.
‡ The values in T3 were significantly different from the values in T1 and T2.
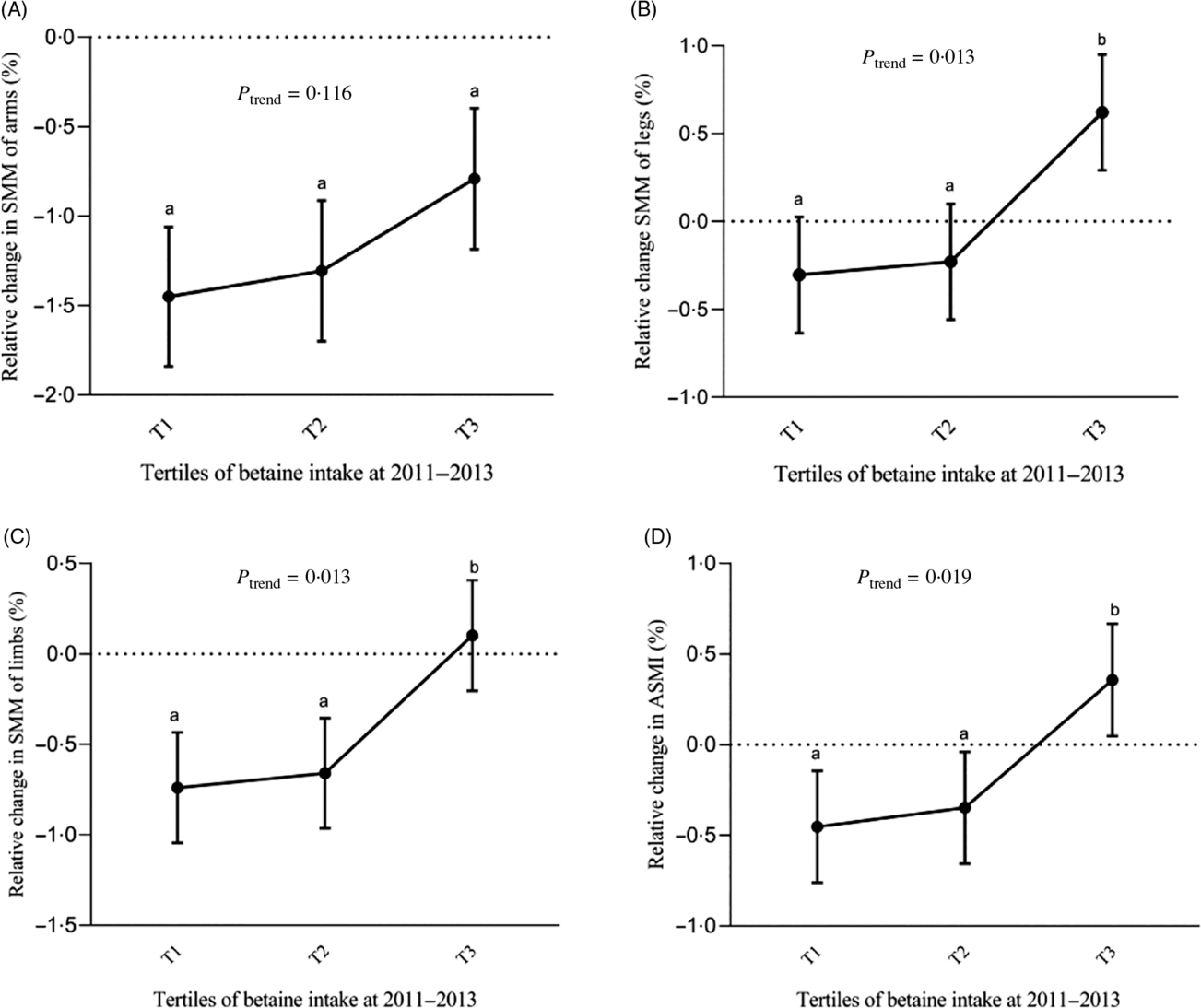
Fig. 2. Adjusted relative change in skeletal muscle mass (SMM) of arms (A), legs (B), limbs (C) and appendicular skeletal muscle index (ASMI) (D) over 3 years by tertiles (T) of energy-adjusted dietary betaine intake in middle-aged (age < 60 years) adults from the Guangzhou Nutrition and Health Study. Adjusted for sex, age, energy intake, baseline SMM or ASMI, height, waist circumference, physical activity level, protein intake, choline intake, marital status and use of multivitamins. Tests for a linear trend across tertiles of dietary betaine intake were conducted by using the median value in each tertile as a continuous variable in the linear regression models. a,b Least-squares means with unlike letters are significantly different (P < 0·05; t test). Median energy-adjusted dietary betaine intake by tertile (from T1 to T3) was 145·8, 241·4 and 361·3 mg/d.
Discussion
Previous cross-sectional studies have showed that higher betaine intakes or higher serum betaine levels were associated with greater lean body mass in middle-aged and older adults. However, few longitudinal studies have investigated whether higher betaine intake is beneficial for preventing age-related loss of SMM. To our knowledge, this is the first prospective cohort study examining the association between dietary betaine intake and changes in SMM among community-dwelling middle-aged adults. In the GNHS cohort, a higher dietary betaine intake was associated with less loss in SMM of legs and limbs and ASMI over 3 years of follow-up in middle-aged adults. Of note, middle-aged adults in the highest tertile of betaine intake even experienced an increase in SMM of legs and limbs and ASMI during the 3-year follow-up.
There is strong biological plausibility for the association between betaine and changes in SMM. First, homocysteine thiolactone can inhibit insulin/insulin-like growth factor-1 mediated mRNA expression and enzyme activity involved in protein synthesis. Betaine can reduce the production of homocysteine thiolactone through the remethylation of homocysteine to methionine, thereby enhancing protein synthesis in skeletal muscle(Reference Najib and Sanchez-Margalet13). Second, betaine can promote muscle fibre differentiation and increases myotube size by activating the insulin-like growth factor-1 pathway(Reference Senesi, Luzi and Montesano32). Third, accumulation of betaine in cells during metabolism leads to an increase in sarcoplasmic osmolality via the osmoregulated betaine/GABA transporter-1, resulting in cellular swelling. Cellular swelling can promote protein synthesis and suppresses proteolysis through enhancing amino acid uptake or inducing a series of cascades via activating mitogen-activated protein kinase(Reference Courtenay, Capp and Anderson33). Fourth, betaine as a methyl donor provides its labile methyl groups for the synthesis of carnitine and creatine(Reference Eklund, Bauer and Wamatu34), which have been used to increase muscle mass in community-dwelling adults older than 55 years(Reference Evans, Guthrie and Pezzullo35,Reference Rawson, Clarkson and Price36) .
Although our study showed that a higher dietary betaine intake was significantly associated with a reduction in SMM loss over 3 years, betaine ingestion was not associated with SMM of arms, legs, limbs and ASMI at baseline. These findings were inconsistent with the findings from previous cross-sectional studies. Higher serum betaine levels have been observed to be associated with greater lean body mass in male adults who participated in the GNHS study and the CODING (Complex Disease in Newfoundland population: Environment and Genetics) study(Reference Huang, Zhu and Tan20,Reference Gao, Randell and Zhou21) . Humans obtain betaine either from foods that contain betaine or by de novo synthesis of betaine in the body. Serum betaine, as a biomarker of internal exposure of betaine, is a more accurate measurement of betaine status than dietary betaine intake. However, since serum betaine concentrations were not assessed in the GNHS 2011–2013, we could not explain whether the inconsistent results are due to the selection of different exposure markers (i.e. dietary or serum betaine) or not. In contrast, another cross-sectional study using data from the CODING study reported that higher dietary betaine intakes were significantly related to greater total percentage lean mass(Reference Gao, Wang and Randell37). Notably, dietary betaine intake is much lower in the CODING study than in our study (110·1 v. 259·0 mg/d). There may be a ceiling effect of betaine intake on muscle growth when betaine intake reaches a certain level. On the other hand, we assessed the association between dietary betaine intake expressed as mg/d and SMM of arms, legs and limbs, while the CODING study investigated the association between dietary betaine intake expressed as mg/kg per d and total body lean mass. Both the confounding effect of body weight and different muscle outcomes used may contribute to the inconsistent findings.
There are several strengths to the study. First, the present study was a prospective cohort study with a relatively large sample size. Second, the long follow-up (3 years) allowed us to observe the changes in SMM over time and to examine the association between dietary betaine and relative change in SMM. Third, detailed information on sociodemographic information and physical activity was collected, which was later used as covariates in the statistical model.
Some limitations must be acknowledged as well. First, we used self-reported FFQ to collect dietary information which raises the possibility of recall bias and misreporting of food consumption. However, photographs of generic foods and portion sizes were provided to help participants estimate their usual food consumption. Another defect is that we had little information on muscle function in this population. We could not infer the relationship between dietary betaine and muscle strength or performance. Third, although we have carefully adjusted for potential confounders including physical activity and energy and protein intake, residual confounding cannot completely be ruled out since the age-related loss of SMM might be influenced by a variety of genetic and environmental factors. Fourth, the lack of betaine biomarker precludes us from reaching a sound conclusion of betaine status and SMM loss. Besides, we had no information on changes in diets and physical activity during the follow-up, since dietary assessment and physical activity questionnaire were only performed in GNHS 2011–2013. And last, the observational nature of the present study precludes us from estimating a causal association between dietary betaine intake and changes in SMM.
Conclusion
Higher dietary betaine intake was associated with less loss of SMM in middle-aged adults, suggesting that increasing dietary betaine intake may help maintain or improve SMM during ageing. Further long-term prospective studies, especially randomised controlled trials, are needed to confirm the findings.
Acknowledgements
The authors thank the numerous individuals who participated in the present study.
The present study was jointly supported by the National Natural Science Foundation of China (no. 81773415) and the Key Project of Science and Technology Program of Guangzhou, China (no. 201704020035). The funding sources had no role in the design, analysis or writing of this article.
A.-P. F., H.-L. Z. and Y.-M. C. formulated the research question and designed the study; S. C., F. W., Y. L., X.-T. L. and D. Y. conducted the study; J.-A. L. and R.-H. Z. analysed the data; J.-A. L. wrote the paper; H.-L. Z. and A.-P. F. edited, revised and finally reviewed the manuscript critically for important intellectual content. All the authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520002433









