Lipids play a crucial role in the intensive aquaculture industry, not only because it provides essential fatty acids but also due to its high energy density. It can impart a ‘protein-sparing effect’, thereby reducing dietary protein requirement, resulting in feed cost reduction(Reference Wang, Zhang and Gladstone1). Studies have demonstrated that increasing dietary lipid level could improve protein efficiency ratio and reduce N discharge in different fish species(Reference Ai, Mai and Li2–Reference Cho and Bureau5). Thus, the use of energy-dense high-lipid (HL) feeds is prevalent in aquaculture, especially in the farming of marine fish species. However, ingesting excessive lipid generally leads to fatty liver, which is detrimental to liver function. Since the liver is the central organ involved in carbohydrate, lipid and protein metabolism, its impairment eventually causes poor health condition and induces other diseases (e.g. inflammatory reaction and oxidative stress)(Reference Cao, Liu and Zheng6). Excessive lipid accumulation also poses severe health problems and reduces harvest yields, which is directly responsible for economic losses(Reference Yang, Yu and Luo7).
Several studies reported that some feed additives could reduce liver lipid deposition and alleviate the adverse effects caused by HL diets, such as bile acids(Reference Liao, Sun and Zhang8,Reference Ding, Xu and Liu9) , l-carnitine(Reference Jin, Pan and Cheng10), fenofibrate(Reference Ning, Liu and Wang11), choline, betaine(Reference Adjoumani, Abasubong and Phiri12) and xylooligosaccharides(Reference Abasubong, Li and Zhang13). Berberine (BBR) (18,5,6-dyhydro-9,10-dimethoxybenzo(g)-1,3-benzodioxolo(5,6-a) quinolizinium) is an isoquinoline alkaloid isolated from Coptis chinensis (Chinese goldthread), Hydrastis canadensis (goldenseal), Berberis vulgaris (barberry) and other herbs(Reference Wang and Zidichouski14) or manufactured by chemical synthesis. It has been commonly used in Chinese medicine for its anti-inflammatory, antibacterial, antioxidant and antiapoptotic features(Reference Zhou, Rahimnejad and Lu15). BBR is also used as an effective medicine in treating fatty liver, obesity, hypercholesterolaemia, hypertension, type 2 diabetes and gastrointestinal infection(Reference Brusq, Ancellin and Grondin16–Reference Feng, Shou and Zhao18). Generally, BBR presents few side effects and little toxicity(Reference Pang, Zhao and Zhou19,Reference Wang, Yi and Ghanam20) . In aquaculture, BBR was reported to be an effective immunostimulant for the prevention and treatment of some bacterial diseases(Reference Xu, Chen and Chen21–Reference Doan, Hoseinifar and Jaturasitha23). Recently, studies on blunt snout bream (Megalobrama amblycephala)(Reference Zhou, Rahimnejad and Lu15,Reference Chen, Liu and Zhou24) and zebrafish (Danio rerio)(Reference Chen, Zheng and Zhang25) reported the prominent lipid-lowering effect of BBR, but as far as we know, the effects of dietary BBR in marine fish have not been investigated.
Black sea bream (Acanthopagrus schlegelii) is a high-value commercially farmed tropical marine fish with high tolerance to environmental changes, desirable growth rate and good meat quality(Reference Wang, Zhang and Gladstone1,Reference Shao, Ma and Xu26) . Wang et al. (Reference Wang, Zhang and Gladstone1) reported that black sea bream is prone to store lipid in their liver. Previous studies in black sea bream reported that l-carnitine(Reference Ma, Xu and Shao27), choline(Reference Jin, Pan and Tocher28) and bile acids(Reference Jin, Pan and Cheng10) supplementation could improve immune and antioxidative abilities and reduce the histopathological changes caused by HL diets. The present study aimed to investigate whether BBR supplementation in HL diet could effectively reduce lipid accumulation in the liver of black sea bream. Other studies have reported that supplementation of BBR in normal lipid level diets could improve the growth performance of fish(Reference Xu, Chen and Chen21,Reference Doan, Hoseinifar and Jaturasitha23,Reference Chen, Liu and Zhou24) . However, BBR shows poor solubility and the absorption rate partly relies on gut microbiota to convert it into its absorbable form(Reference Feng, Shou and Zhao18). A study in rats observed that the oral bioavailability of BBR was less than 1 %(Reference Imenshahidi and Hosseinzadeh29), which might be due to the poor aqueous dissolution and solubility(Reference Kumar, Ekavali and Chopra30). Besides, the absorption of BBR after oral administration has a limit, and low dietary BBR level presents rapid intestinal absorption(Reference Kheir, Wang and Hua31). Zhou et al. (Reference Zhou, Rahimnejad and Lu15) reported that an HL diet with 50 mg/kg BBR improved growth performance and attenuated the hepatic abnormalities in blunt snout bream, whereas 100 mg/kg BBR reduced feed palatability and feed intake (FI), thus 50 mg/kg BBR was recommended in fish feed. Supplementation of 50 mg/kg and 100 mg/kg BBR in an HL diet showed similar effects on hepatocytes health in blunt snout bream(Reference Lu, Wang and Zhang32). Therefore, in the present study, we investigated the supplementation of 50 mg/kg BBR in normal and HL diets on growth performance, liver function and lipid metabolism of black sea bream fingerlings.
Experimental methods
Animal ethics
All the experimental processes followed the Guidance of the Care and Use of Laboratory Animals in China. The Committee on the Ethics of Animal Experiments of Zhejiang University approved the present study.
Diets preparation
The control (Con) and HL diets were formulated as indicated in Table 1, and they were designed as isonitrogenous (42 % crude protein) practical diets. The Con diet contained 11·19 % lipid and the HL diet contained 20·26 % lipid. Fishmeal, wheat gluten meal, soya protein concentrate and fermented soyabean meal were used as the major protein ingredients, while maize oil and fish oil served as the main lipid sources. These main protein and lipid ingredients were provided by the Minghui Feed Company. BBR (purity, HPLC ≥ 98 %) was purchased from the Spring and Autumn Biotechnology Company. BBR was supplemented to Con and HL diets at 50 mg/kg and named as ConB and HLB diets, respectively. After thorough grinding, all the ingredients were mixed and homogenised. Crystalline methionine, arginine and lysine were coated with carrageenan and carboxymethyl cellulose and added to the diet mix. The mixture was subsequently extruded through a feed machine (Modle HKJ-218; HUARUI) as described by Wang et al. (Reference Wang, Zhang and Gladstone1). The experimental diets were subsequently cooked by steam for 10 min and placed on a clean floor for drying by an electric fan in an air-conditioned room. The diets were stored at −20°C until use, and representative samples were taken for proximate composition analysis.
Table 1. Feed formulation and proximate composition of the experimental diets
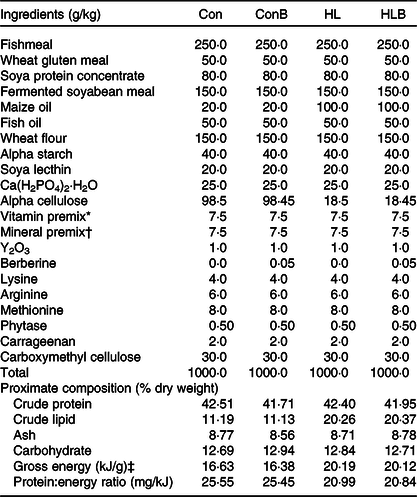
Con, control; ConB, control diet with berberine supplementation; HL, high lipid; HLB, high-lipid diet with berberine supplementation.
* Vitamin premix (mg/kg): α-tocopherol, 80; retinyl acetate, 40; cholecalciferol, 0·1; menadione, 15; niacin, 165; riboflavin, 22; pyridoxine HC1, 40; thiamin mononitrate, 45; D-Ca pantothenate, 102, folic acid, 10; vitamin B12, 0·9; inositol, 450; ascorbic acid, 150; Na menadione bisulphate, 15; thiamin, 5; choline chloride, 320 and p-aminobenzoic acid, 50.
† Mineral premix (mg/kg): Na2SiO3, 0·4; CaCO3, 544·9; NaH2PO4·H2O, 200; KH2PO4, 200; MgSO4·7H2O, 10; MnSO4·H2O, 4; CuCl2·2H2O, 2; ZnSO4·7H2O, 12; FeSO4·7H2O, 12; NaCl, 12; KI, 0·1; CoCl2·6H2O, 0·1; Na2MoO4·2H2O, 0·5; AlCl3·6H2O, 1; and KF, 1.
‡ Gross energy = (crude protein × 23·6 + crude lipid × 39·5 + carbohydrate × 17·2)/1000.
Fish and feeding trial
Black sea bream fingerlings (mean initial weight 1·47 (sd 0·03) g) were purchased from a local fish farm. The feeding trial was conducted at the Marine Fisheries Research Institute of Zhejiang Province in Zhoushan, China. Before the feeding trial, all fish were kept in a cement tank (4·5 m × 2·5 m × 1·2 m) for a 14-d acclimatisation period. Then 420 healthy fish were selected and randomly assigned to twelve tanks (400 litres water volume) with thirty-five fish in each tank. Each diet was fed to triplicate groups of fish. Aerated and filtered sea water was provided to each tank at a rate of 2 litres/min during the feeding trial. The water quality parameters were constant throughout the experiment with temperature of 27 (sd 1)°C, pH 8·2 (sd 0·1), dissolved oxygen >5·0 mg/l and salinity 28 (sd 2) g/l. The fish were fed three times (07.00, 12.00 and 16.00 hours) daily to apparent satiation for 8 weeks.
Sample collection
At the end of the feeding trial, all fish were anesthetised (MS-222, 60 mg/l) 24 h after the last feeding. The fish in each tank was counted and weighed, and body length was measured. Blood from twelve fish was obtained from the caudal vein using 1-ml syringe, and pooled blood samples were centrifuged at 10 000 g after a 2-h settlement at 4°C. The fish were subsequently dissected on ice to excise the intraperitoneal fat, liver and dorsal muscle samples. Viscera, intraperitoneal fat and liver were weighed and recorded. Five fish from each tank were taken for whole-body and muscle proximate composition. Triplicate of liver and muscle samples from each treatment for gene expression measurements and all the other samples for further analyses were stored at −80°C. The liver from three fish per treatment was cut into small pieces and fixed in 10 % formalin and 2·5 % glutaraldehyde for histological observation.
Histological observation
The liver samples were stained by Oil Red O following the processes introduced by Volatiana et al. (Reference Volatiana, Wang and Gray33). The slide microphotography was observed using an Olympus CX21 microscope. The transmission electron microscope (TEM) samples were fixed using 1 % OsO4 for 2 h and desiccated in a graded mixture of alcohol and isoamyl acetate (1:1, v/v) for 30 min. The samples were subsequently kept in isoamyl acetate for 8 h, then washed and dried by liquid CO2 (Hitachi Model HCP-C critical-point dryer). Gold-palladium was used to coat the desiccated samples (Hitachi Model E-1010 ion sputter), and the samples were viewed in Hitachi H7650 TEM. Morphometry of lipid accumulation, liver cell and nuclear diameters were carried out using Image-Pro Plus 6.0.
Samples analysis
The diets, whole fish and dorsal muscle proximate compositions were determined following the methods of the Association of Official Analytical Chemists(34). Moisture was analysed by drying sample at 105°C for 24 h, crude lipid was determined by diethyl ether extraction, crude protein was estimated by determining N content following the Kjeldahl N method, and ash content was determined by heating sample at 550°C for 8 h. The liver supernatant was obtained following the method of Wang et al. (Reference Wang, Xiao and Hua35). The fatty acid synthase (FAS), lipoprotein lipase (LPL), hormone-sensitive lipase (HSL), lipase (LPS), aspartate aminotransferase (AST), alanine aminotransferase (ALT) activities and total cholesterol, TAG, LDL-cholesterol and HDL-cholesterol contents were determined using the diagnostic reagent kits for fish (Nanjing Jiancheng Bioengineering Institute, China) following the manufacturer’s instructions.
Quantitative RT-PCR
The PCR primers of sterol regulatory element-binding protein-1 (srebp-1), acetyl-CoA carboxylase α (accα), fatty acid synthase (fas), glucose 6-phosphate dehydrogenase (g6pd), 6-phosphogluconate dehydrogenase (6pgd), lipoprotein lipase (lpl) pparα, pparγ, carnitine palmitoyltransferase 1a (cpt1a) and hormone-sensitive lipase (hsl) were designed by Primer premier 5.0 (Table 2). The housekeeping gene β-actin was used as the reference gene. The mRNA expression levels of the genes in liver and muscle tissues were assayed by quantitative RT-PCR using the SYBR Premix Ex Taq™ II Kit (Takara, Japan). Triplicate samples of each sample were used for quantitative RT-PCR amplification. The relative mRNA expression levels of target genes were calculated by the 2−ΔΔCt method described by Livak and Schmittgen(Reference Livak and Schmittgen36). The PCR process was performed as follows: 94°C for 30 s, 52°C for 30 s and 72°C for 90 s, followed by 72°C for 10 min. The PCR products were measured by visualised UV transillumination and 1·5 % (w/v) agarose gel electrophoresis.
Table 2. Primers of real-time quantitative PCR for lipid metabolism-related genes and β-actin of black sea bream
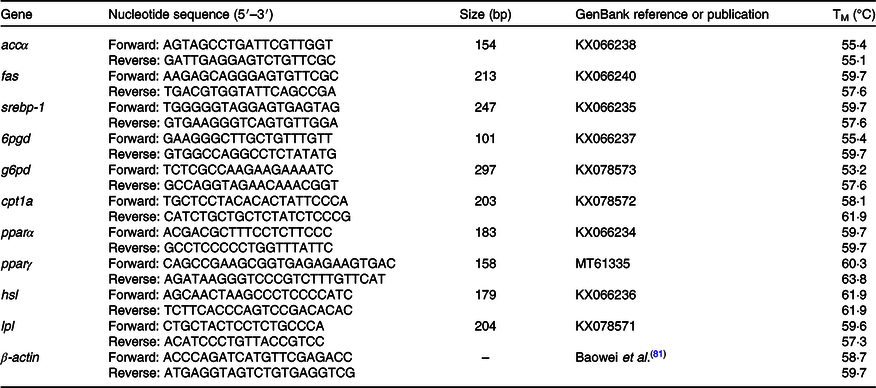
accα, Acetyl-CoA carboxylase α; fas, fatty acid synthase; srebp-1, sterol regulatory element-binding protein-1; 6pgd, 6-phosphogluconate dehydrogenase; g6pd, glucose 6-phosphate dehydrogenase; cpt1a, carnitine palmitoyltransferase 1a; hsl, hormone-sensitive lipase; lpl, lipoprotein lipase.
Statistical analysis
All the results are presented as mean values and standard deviations. Statistical analyses were conducted by IBM SPSS Statistics 20.0 (SPSS). Kolmogorov–Smirnov and Levene’s tests were applied to determine normality and homogeneity of all data, respectively. Two-way ANOVA was applied to evaluate the main effects of BBR supplementation, different basal diet and their interactions on each parameter. One-way ANOVA by Duncan’s multiple range test was used to determine the significant differences between different treatments, and P < 0·05 was considered as significant.
Results
Growth performance, morphological and feed utilisation parameters
The effects of BBR supplementation in normal and HL diets on fish growth performance, morphological and feed utilisation parameters are presented in Table 3. Growth performance was significantly affected by dietary lipid level (P < 0·05) but not by BBR supplementation. Significantly lower body length and specific growth rate were found in fish fed the HL diets compared with feeding the Con diets. Besides, fish fed the HLB diet obtained significantly higher viscerosomatic index (VSI) value than that of the other groups. Hepatosomatic index values were significantly increased by HL diets compared with the Con diets. Fish fed the ConB diet had significantly lower FI than that of fish fed the Con diet. Significantly reduced feed efficiency ratio was found in fish fed the HL diets. Besides, a significant interaction between dietary BBR supplementation and dietary lipid level was observed with intraperitoneal fat ratio, VSI and FI. The survival, condition factor and protein efficiency ratio showed non-significant differences in all groups (P > 0·05).
Table 3. Effects of berberine supplementation in normal and high-lipid diets on growth performance, morphological and feed utilisation parameters of black sea bream*
(Mean values and standard deviations; n 3)
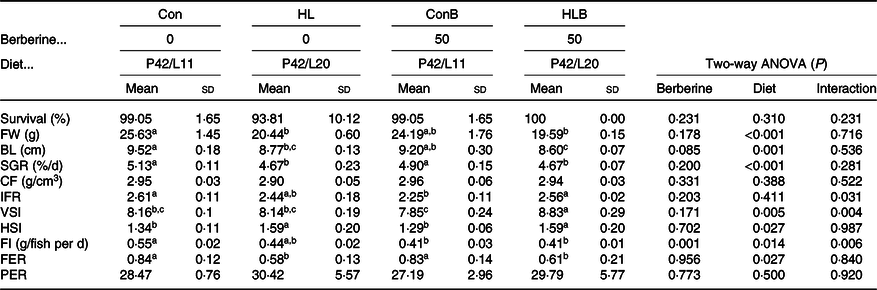
Con, control; HL, high lipid; ConB, control diet with berberine supplementation; HLB, high-lipid diet with berberine supplementation; FW, final weight; BL, body length; SGR, specific growth rate; CF, condition factor; IFR, intraperitoneal fat ratio; VSI, viscerosomatic index; HSI, hepatosomatic index; FI, feed intake; FER, feed efficiency ratio; PER, protein efficiency ratio.
a,b,c Mean values in a row with unlike superscript letters were significantly different (P < 0·05).
* Survival = 100 × (final fish number/initial fish number); SGR = 100 × (ln final body weight − ln initial body weight)/d; CF = final body weight/final body length3 × 100; IFR = 100 × (intraperitoneal fat weight/body weight); VSI = 100 × viscera weight/whole body weight; HSI = 100 × (liver weight/body weight); FI = dry diet feed/final fish number/d; FER = wet weight gain/dry diet fed; PER = 100 × weight gain/protein intake.
Whole-body and muscle proximate compositions
The effects of BBR supplementation in normal and HL diets on whole-body and muscle proximate compositions are presented in Table 4. Lipid contents of whole body and muscle were significantly affected by BBR supplementation and dietary lipid level. BBR supplementation in the Con and HL diets did not affect the whole-body and muscle lipid contents significantly. A significant interaction between BBR supplementation and diet was found in whole-body ash content. While whole-body and muscle moisture, protein, and muscle ash contents were not significantly affected by different treatments.
Table 4. Effects of berberine supplementation in normal and high-lipid diets on whole-body and muscle proximate compositions of black sea bream (wet weight %)
(Mean values and standard deviations; n 3)
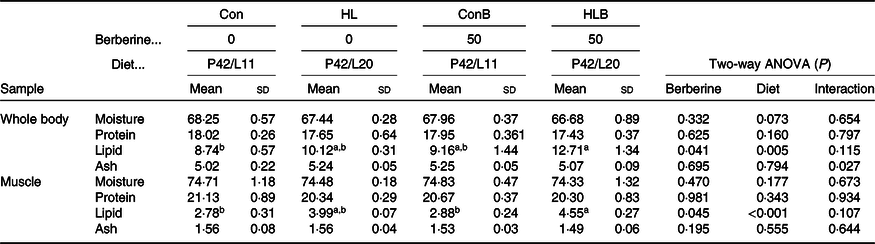
Con, control; HL, high lipid; ConB, control diet with berberine supplementation; HLB, high-lipid diet with berberine supplementation.
a,b Mean values in a row with unlike superscript letters were significantly different (P < 0·05).
Serum biochemical parameters
As shown in Table 5, the fish fed the HL diet had significantly higher serum LDL-cholesterol and TAG contents compared with other groups. Serum LDL-cholesterol and TAG contents were affected by BBR supplementation and dietary lipid level significantly. A significant interaction between BBR supplementation and diet lipid level was observed in serum LDL-cholesterol content. Besides, serum ALT was significantly influenced by BBR supplementation. Fish fed the HLB diet resulted in significantly lower serum ALT activity compared with that of the HL group, whereas the total cholesterol and HDL-cholesterol contents, as well as the AST activity showed no statistical differences among different groups.
Table 5. Effects of berberine supplementation in normal and high-lipid diets on serum biochemical parameters of black sea bream
(Mean values and standard deviations; n 3)
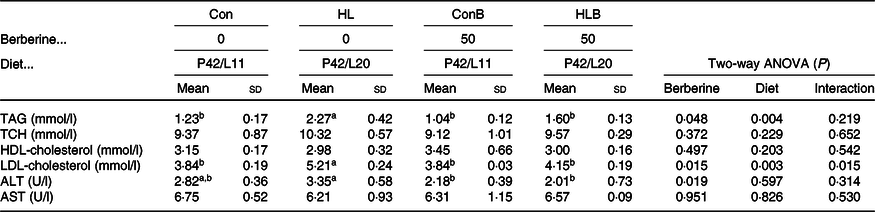
Con, control; HL, high lipid; ConB, control diet with berberine supplementation; HLB, high-lipid diet with berberine supplementation; TCH, total cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
a,b Mean values in a row with unlike superscript letters were significantly different (P < 0·05).
Activities of hepatic metabolic enzymes
The effects of BBR supplementation in normal and HL diets on hepatic metabolic enzyme activities are shown in Table 6. Hepatic LPL and LPS activities were significantly affected by BBR supplementation, while ALT activity was significantly affected by dietary lipid level. BBR supplementation in the HL diet reduced LPS activity significantly. In addition, hepatic FAS, HSL and AST activities were not significantly influenced by different treatments.
Table 6. Effects of berberine supplementation in normal and high-lipid diets on hepatic metabolic enzymes activities of black sea bream
(Mean values and standard deviations; n 3)
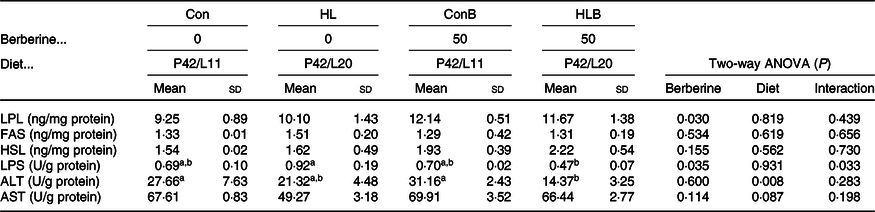
Con, control; HL, high lipid; ConB, control diet with berberine supplementation; HLB, high-lipid diet with berberine supplementation; LPL, lipoprotein lipase; FAS, fatty acid synthase; HSL, hormone sensitive lipase; LPS, lipase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
a,b Mean values in a row with unlike superscript letters were significantly different (P < 0·05).
Liver lipid deposition and ultrastructure
Oil Red O-stained liver pictures are shown in Fig. 1. Fish fed the HL diet had significantly higher hepatic lipid accumulation compared with the other groups. BBR supplementation in Con and HL diets significantly reduced liver lipid accumulation as indicated by the dramatic decrease in the relative areas of the liver that was stained red.
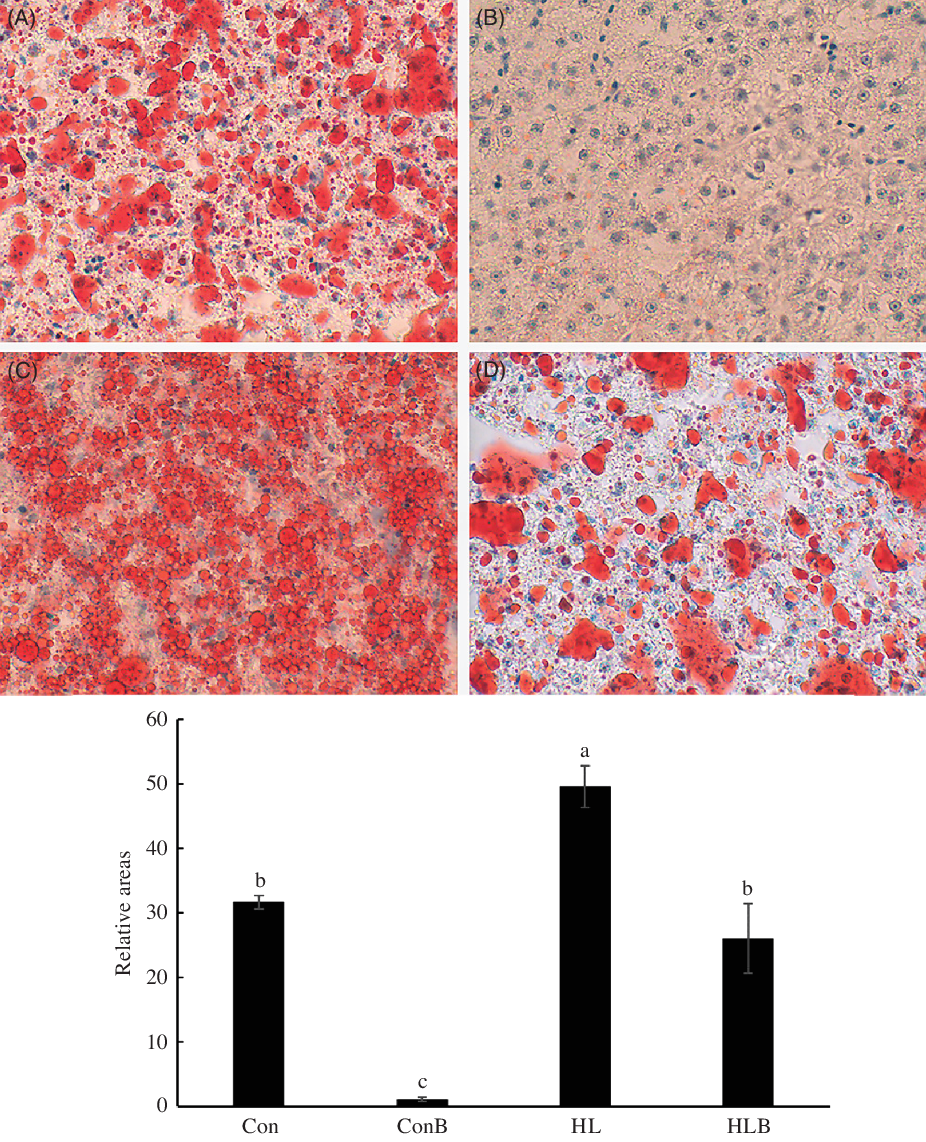
Fig. 1. Oli Red O-stained liver (400×) of black sea bream fed control (A), control + berberine (BBR) (B), high-lipid (C) and high-lipid + BBR (D) diets. Relative areas stained by Oil Red O were determined by Image-Pro Plus 6.0. a,b,c Mean values with unlike letters were significantly different (P < 0·05). Con, control; ConB, control diet with berberine supplementation; HL, high lipid; HLB, high-lipid diet with berberine supplementation.
The liver ultrastructure pictures of fish fed the different diets are presented in Fig. 2. Fish fed the Con or ConB diets exhibited normal ultrastructure with round and clear nucleus. Fewer lipid droplets were visible in the liver of ConB group compared with the Con group. However, fish fed the HL diet showed hypertrophied liver cells with significantly larger cell diameter than that of the other treatments. Large lipid droplets were also observed in the cytoplasm along with nuclear translocation to the cell periphery in the liver of the HL group. In contrast, fish fed the HLB diet showed alleviated hepatic lipid accumulation with fewer droplets surrounding the centred nucleus compared with the HL group.
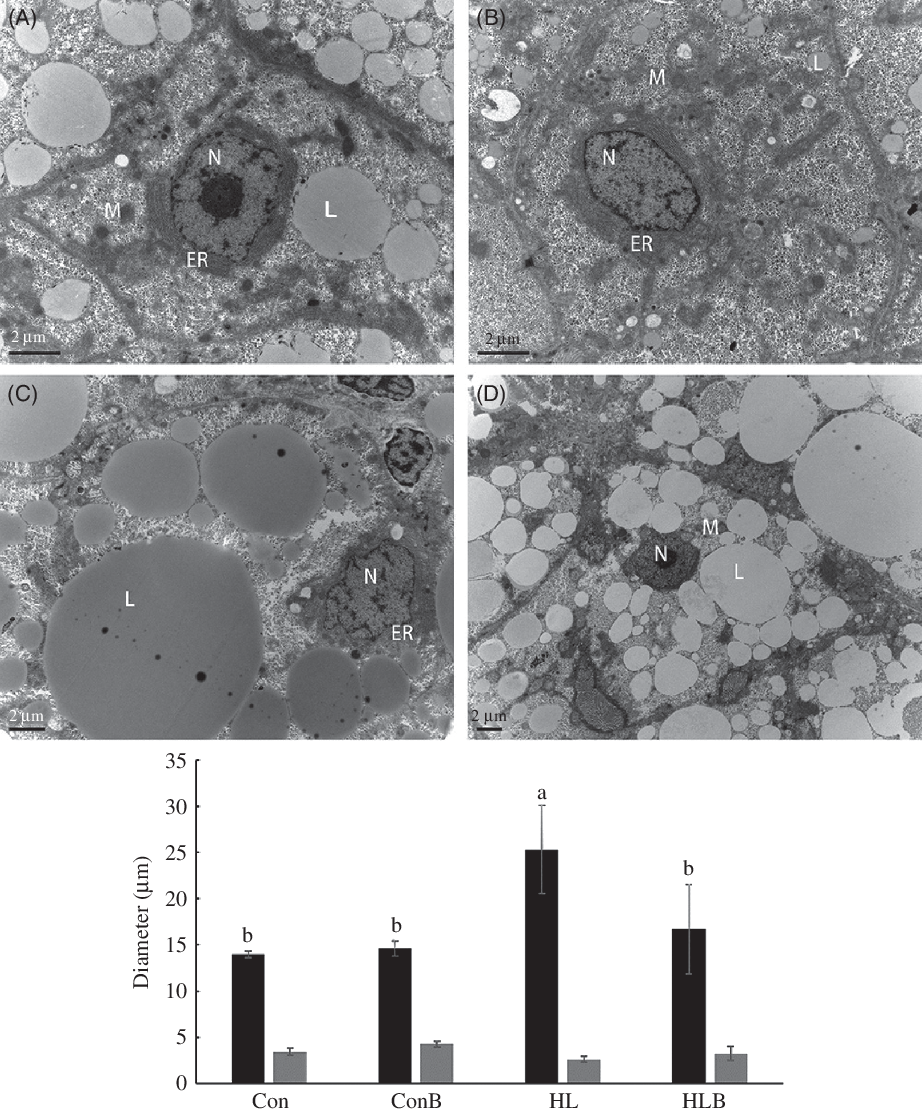
Fig. 2. Transmission electron microscope images of juvenile black sea bream liver fed different diets for 8 weeks. (A) Con; (B) Con + berberine (BBR); (C) HL; (D) HL + BBR. a,b Mean values with unlike letters were significantly different (P < 0·05). ![]() , Hepatocyte diameter;
, Hepatocyte diameter; ![]() , Nucleus diameter. N, nucleus; M, mitochondria; ER, endoplasmic reticulum; L, lipid droplet; Con, control; ConB, control diet with berberine supplementation; HL, high lipid; HLB, high-lipid diet with berberine supplementation.
, Nucleus diameter. N, nucleus; M, mitochondria; ER, endoplasmic reticulum; L, lipid droplet; Con, control; ConB, control diet with berberine supplementation; HL, high lipid; HLB, high-lipid diet with berberine supplementation.
Gene expression
As shown in Fig. 3 and Table 7, BBR supplementation in the HL diet significantly down-regulated hepatic expression levels of accα, srebp-1, 6pgd, g6pd and pparγ. In contrast, hepatic lpl, hsl and cpt1a expression levels were significantly up-regulated by BBR supplementation in the HL diet. However, the hepatic mRNA levels of these genes in the Con and ConB groups showed non-significant differences except for a down-regulation of pparγ in CoB group. According to the two-way analysis, BBR supplementation affected the expression levels of accα, 6pgd, pparγ, hsl and lpl in liver significantly. And dietary lipid level affected the expression levels of fas, 6pgd, pparγ, cpt1a, pparα, hsl and lpl in liver significantly. Significant interactions between BBR supplementation and dietary lipid level were observed for the hepatic srebp-1, 6pgd, g6pd, cpt1a and lpl expression levels.
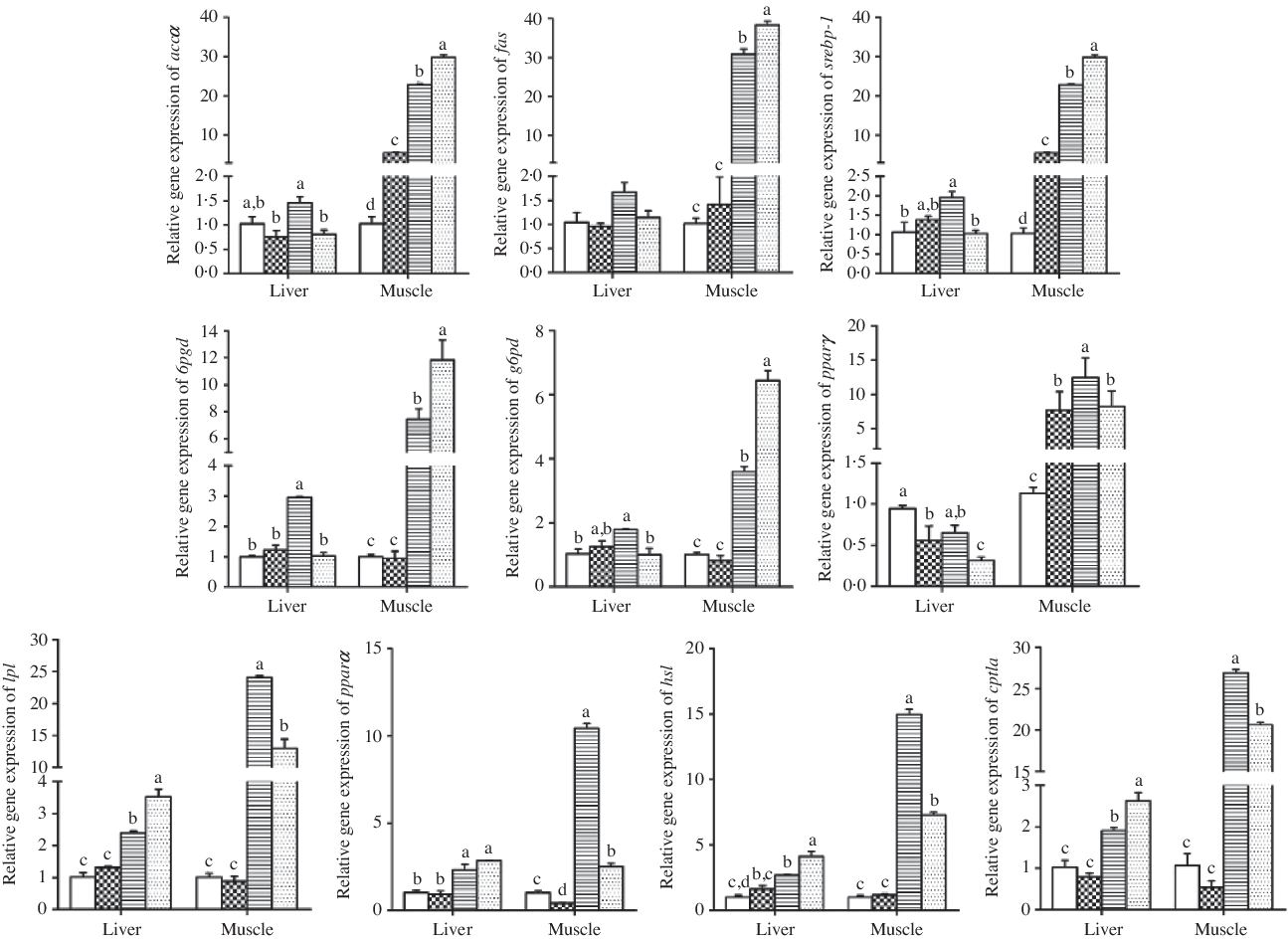
Fig. 3. Results of quantitative real-time PCR analysis carried out for acetyl-CoA carboxylase α (accα), fatty acid synthase (fas), sterol regulatory element-binding protein-1 (srebp-1), 6-phosphogluconate dehydrogenase (6pgd), glucose 6-phosphate dehydrogenase (g6pd), pparγ, lipoprotein lipase (lpl), pparα, hormone-sensitive lipase (hsl), carnitine palmitoyltransferase 1a (cpt1a) in the liver and muscle of black sea bream fed different diets for 8 weeks. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05). ![]() , Control;
, Control; ![]() , control diet with berberine supplementation;
, control diet with berberine supplementation; ![]() , high lipid;
, high lipid; ![]() , high-lipid diet with berberine supplementation.
, high-lipid diet with berberine supplementation.
Table 7. Two-way ANOVA (P) analysis of gene expression of fish fed the experimental diets
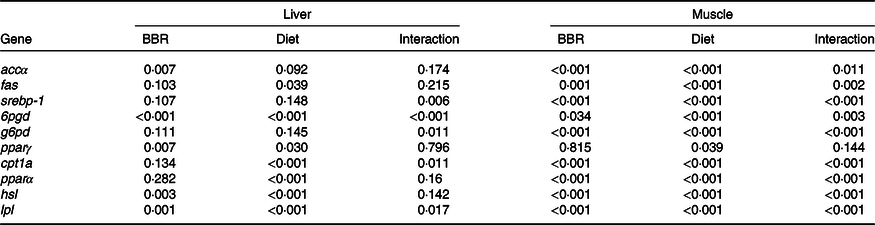
BBR, berberine; accα, acetyl-CoA carboxylase α; fas, fatty acid synthase; srebp-1, sterol regulatory element-binding protein-1; 6pgd, 6-phosphogluconate dehydrogenase; g6pd, glucose 6-phosphate dehydrogenase; cpt1a, carnitine palmitoyltransferase 1a; hsl, hormone-sensitive lipase; lpl, lipoprotein lipase.
In the muscle, fish fed the BBR-supplemented diets had significantly higher expression levels of accα (4·5-fold increase in ConB group) and srebp-1 (8·2-fold increase in ConB group), but significantly lower pparα expression level in muscle. Pparγ expression level was significantly up-regulated in fish fed the ConB diet compared with the Con group, whereas an opposite trend was found in the HL and HLB groups. BBR supplementation in the HL diet up-regulated the expression levels of fas, 6pgd and g6pd but down-regulated lpl, hsl and cpt1a expression. Based on the two-way analysis, all these genes (except for pparγ) were affected by both dietary lipid level and BBR supplementation significantly, and significant interactions between BBR and dietary lipid level were observed in all these gene expression levels (except for pparγ).
Discussion
In the present study, an HL diet reduced growth performance, which could be due to reduced FI observed in fish fed the HL and HLB diets. Feed consumption is directly influenced by dietary energy level(Reference Wang, Zhang and Gladstone1), and HL diets had been reported to decrease FI and growth performance in giant croaker (Nibea japonica)(Reference Han, Li and Wang37), grass carp (Ctenopharyngodon idella)(Reference Du, Liu and Tian38), blunt snout bream(Reference Zhou, Rahimnejad and Lu15) and black sea bream(Reference Wang, Zhang and Gladstone1). Furthermore, it was also observed in the present study that BBR supplementation in Con diet caused significant depression of FI, but BBR supplementation in both the Con and HL diets did not affect growth performance significantly. BBR tastes bitter and influences the palatability of diet and was reported to inhibit the FI in blunt snout bream at dietary inclusion level of 100 mg/kg(Reference Zhou, Rahimnejad and Lu15). However, several studies demonstrated that some Chinese herbs have hepatoprotective effects, which are beneficial for growth(Reference Zhou, Li and Wang39). Xu et al. (Reference Xu, Chen and Chen21) observed that supplementation of 50 mg/kg BBR in an HL diet could improve the growth performance of blunt snout bream, as BBR attenuated the adverse effects (hepatocyte apoptosis, suppressed immune function and increased oxidative stress) caused by high dietary lipid. Nevertheless, some other studies in blunt snout bream obtained contradictory results about BBR supplementation and growth performance. Zhou et al. (Reference Zhou, Rahimnejad and Lu15) observed that supplementation of 50 mg/kg BBR in the HL (15 % crude lipid) diet significantly improved FI and growth performance, while 100 mg/kg BBR decreased both parameters. Moreover, 50 mg/kg BBR improved the growth performance of fish when supplemented to the normal diet (4·7 % crude lipid) rather than the HL diet (10·1 % crude lipid)(Reference Chen, Liu and Zhou24). Xu et al. (Reference Xu, Chen and Chen21) reported that fish fed the normal diet (5·0 % crude lipid) with 50 mg/kg BBR at a 2-week interval mode obtained remarkably better growth performance than other feeding modes (no-supplement, 4-week intervals and continuous feeding). Supplementation of 1, 3, 6 and 9 g/kg BBR in normal diet improved the growth performance of Nile tilapia (Oreochromis niloticus), which might be attributed to the promotion of beneficial microflora regulated by BBR(Reference Doan, Hoseinifar and Jaturasitha23). However, zebrafish larvae pretreated with high-cholesterol diet exposed to high dosage of BBR (5 and 25 μm) for 10 d caused a decrease in weight gain(Reference Chen, Zheng and Zhang25). Therefore, the relationship between growth performance and BBR supplementation may vary with fish species, feeding mode, basal diet used, dietary BBR concentration and lipid level. In addition, chronic administration of BBR led to the reduction of weight gain in rats(Reference Lee, Kim and Kim40) and humans(Reference Hu, Ehli and Kittelsrud41), which might be because BBR could improve energy expenditure(Reference Kim, Lee and Cha42).
HL diets causing excessive lipid deposition in liver have been widely reported in different fish species(Reference Zhou, Rahimnejad and Lu15,Reference Lu, Wang and Zhang32,Reference Wang, Li and Hou43) . HL diets have been reported to result in cellular swelling, nuclear translocation, lipid droplet accumulation(Reference Cao, Liu and Zheng6,Reference Kang-Le, Wei-Na and Wen-Bin44) and hypertrophied liver cells with larger cell diameter(Reference Lu, Wang and Zhang32,Reference Ragab, Abd Elghaffar and El-Metwally45) , which were also observed in the present study. These pathologies might also inhibit lipoprotein secretion and fatty acid oxidation, which then result in a vicious cycle(Reference Du, Clouet and Huang46). However, these abnormalities can be mitigated by BBR supplementation. The mitigative effect of BBR against the impact of HL diets was reported in blunt snout bream(Reference Zhou, Rahimnejad and Lu15,Reference Lu, Wang and Zhang32) and obese rats(Reference Ragab, Abd Elghaffar and El-Metwally45). BBR attenuated high-cholesterol diet -induced hepatic steatosis, liver lesion and mitochondrial damage in zebrafish(Reference Chen, Zheng and Zhang25). In mammals, BBR alleviated fatty liver through promoting AMP-dependent protein kinase pathway and improved fatty acid oxidation while suppressing the genes involved in lipogenesis(Reference Kim, Lee and Cha42). BBR could also recover mitochondrial function and reduce steatosis caused by high-fat diet(Reference Teodoro, Duarte and Gomes47,Reference Liang and Wang48) . In the present study, based on histological observation, BBR supplementation in the normal diet resulted in little lipid accumulation in the liver, whereas considerable hepatic lipid deposition was still observed in the fish fed the HLB diet. This means that the lipid-lowering effect of BBR may vary with dietary lipid and BBR levels.
Cpt1a is a rate-limiting enzyme of fatty acid oxidation which is located in the outer membrane of mitochondria and manages uptake of long-chain fatty acids into mitochondria(Reference Lu, Zhang and Wang49,Reference McGarry and Brown50) . Pparα can bind the promoter region of cpt1a, and it also regulates some enzymes involved in fatty acid β-oxidation(Reference Lu, Zhang and Wang49,Reference Zheng, Luo and Zhuo51) . Hsl is the key rate-limiting enzyme for TAG mobilisation(Reference Lafontan and Langin52). Lpl hydrolyses plasma TAG to release fatty acid for oxidation or storage(Reference Goldberg53) and highly expressed in tissues that have a high demand for lipid storage or oxidation(Reference Zheng, Luo and Zhu54). It is well known that the imbalance of lipid consumption and oxidation is the main reason for lipid accumulation(Reference Lu, Xu and Wang55). In the present study, BBR supplementation in the HL diet up-regulated the hepatic expressions of lpl, cpt1a and hsl, indicating that BBR could improve lipolysis and fatty acid β-oxidation in liver. Consistent with the present study, enhanced fatty acid oxidation by BBR supplementation was also reported in blunt snout bream(Reference Zhou, Rahimnejad and Lu15,Reference Lu, Zhang and Wang49,Reference Lu, Xu and Wang55) , obese rats(Reference Kim, Lee and Cha42) and in vitro (Reference Brusq, Ancellin and Grondin16,Reference Sun, Chen and Liu56) .
Accα is a committed enzyme that regulates long-chain fatty acid biosynthesis by producing malonyl-CoA in cytosols. In mammals, accα is predominantly expressed in lipogenic tissues, such as liver and adipose(Reference Zheng, Luo and Zhu54). Srebp-1 is a transcription factor responsible for regulating cholesterol and fatty acid/lipid biosynthesis pathways(Reference Zheng, Luo and Zhu54,Reference Minghetti, Leaver and Tocher57) . Fas is involved in de novo fatty acid synthesis(Reference Jin, Pan and Tocher28). G6pd and 6pgd participate in NADPH production and are important genes for fatty acids biosynthesis(Reference Chen, Luo and Liu58). Pparγ regulates lipogenesis and promotes lipid storage by binding to the promoter region of adipogenic genes(Reference Zheng, Luo and Zhuo51,Reference Oku and Umino59) , and it is a major metabolic transcription regulator particularly for hepatic lipogenesis(Reference Gavrilova, Haluzik and Matsusue60). In the present study, BBR supplementation in the HL diet significantly reduced the expression levels of accα, srebp-1, 6pgd, g6pd and pparγ in the liver. Notably, numerous studies have demonstrated that BBR can reduce lipogenesis by down-regulating pparγ expression in different tissues(Reference Ning, Liu and Wang11,Reference Imenshahidi and Hosseinzadeh29,Reference Ragab, Abd Elghaffar and El-Metwally45,Reference Zhang, Xiao and Feng61,Reference Zhou and Zhou62) . Hence, the lipid-lowering effect of BBR was mainly attributed to the fact that it can mitigate lipid accumulation in liver through down-regulating lipogenesis and up-regulating lipolysis pathways, similar to mechanisms reported in mammals(Reference Wang and Zidichouski14,Reference Kim, Lee and Cha42,Reference Zhang, Xiao and Feng61) .
In line with the gene expression results, higher hepatic LPS activity was observed in the HL group compared with the Con group, while BBR supplementation significantly reduced the activity. Hepatic LPS hydrolyses TAG-rich LDL to form denser and smaller LDL particles; thus its activity is positively related to hypertriacylglycerolaemia(Reference Carr and Brunzell63). Accordingly, BBR supplementation had been reported to reduce LPS activity in rats fed the high-fat/sucrose diet(Reference Ragab, Abd Elghaffar and El-Metwally45). However, the activities of FAS and HSL did not correspond with the gene expression results and showed no significant differences among different groups. This might be because the mRNA level was influenced by time and mRNA stability(Reference Zheng, Luo and Zhuo51,Reference Rigault, Le Borgne and Tazir64) . Similarly, the inconsistent relationship between enzyme activity and mRNA level had been reported in other fish species(Reference Zheng, Luo and Zhuo51,Reference José Ibáñez, Peinado-Onsurbe and Sánchez65) .
Conversely, the expression levels of accα, srebp-1, 6pgd, g6pd, lpl, cpt1a and hsl in the muscle showed the opposite trends with those in the liver of the HL and HLB groups. BBR supplementation in the HL diet up-regulated the expression of fas but down-regulated pparα expression in the muscle. This means BBR could inhibit lipolysis and promote lipogenesis in muscle, which is in line with the increased crude lipid contents in muscle and whole body of the fish fed the BBR-supplemented diets. In contrast to the present study, BBR reduced malonyl-CoA level and up-regulated fatty acid oxidation genes in muscle of rats(Reference Kim, Lee and Cha42). One plausible explanation for the present result is that the dietary protein level (42 %, DM) was not high enough and black sea bream used both lipid and carbohydrate as energy resources as reported by Wang et al. (Reference Wang, Zhang and Gladstone1), and BBR supplementation might improve the utilisation of carbohydrate. In the present study, the HL diets decreased hepatic ALT activity. It is well known that hepatic ALT and AST activities are related to protein deposition and energy provision, and their activities were negatively associated with dietary lipid and carbohydrate contents(Reference Wang, Hu and Sun66), which means black sea bream might use carbohydrate and lipid for energy to compensate for protein catabolism(Reference Wang, Zhang and Gladstone1). Besides, up-regulated expression levels of hepatic pparα, hsl and cpt1a were found in fish fed the HL diet, indicating improved fatty acid oxidation in HL treatments. Some studies proved that BBR has a hypoglycemic effect through enhancing insulin sensitivity and secretion and stimulating glucose uptake and transport(Reference Ko, Choi and Park67–Reference Zhou, Yang and Wang69). This assumption is supported by the up-regulated pparγ level in muscle of ConB group, which is positively related to insulin sensitisation(Reference Ahmadian, Suh and Hah70). Glucose can be oxidised for energy, thus inhibits lipid oxidation and improves lipid accumulation(Reference Hellerstein, Schwarz and Neese71). Besides, glucose also provides NADPH or carbon backbones for de novo lipogenesis(Reference Kamalam, Medale and Panserat72). However, supplementation of BBR in the Con and HL diets showed an opposite trend in regulating pparγ expression in muscle. The reason for this is unknown and deserves further investigation. Therefore, BBR supplementation might improve glucose utilisation of muscle, which was shown as improved lipogenesis and restricted lipolysis. Moreover, the lowest hepatic ALT activity was observed in the HLB group, which might be because BBR supplementation improved insulin sensitivity. A study in humans found that hepatic ALT activity is negatively related to insulin sensitivity(Reference Vozarova, Stefan and Lindsay73).
Consistently, fish fed the HL diet caused more lipid deposition in the liver, as shown in the higher hepatosomatic index value, which was in line with other studies(Reference Wang, Zhang and Gladstone1,Reference Adjoumani, Abasubong and Phiri12) . However, intraperitoneal fat ratio and VSI were not significantly affected by HL treatment, which means that black sea bream tends to accumulate lipid in the liver rather than intraperitoneally. Different fish species have different preferences for organs to store lipid, and HL diet increasing intraperitoneal fat ratio, hepatosomatic index and VSI values were reported in blunt snout bream(Reference Adjoumani, Abasubong and Phiri12) and common carp (Cyprinus carpio)(Reference Abasubong, Li and Zhang13). Interestingly, significant interactions between BBR supplementation and diet were found in VSI and intraperitoneal fat ratio. It seemed that dietary BBR supplementation in the HL diet could improve lipid deposition in intraperitoneal fat and viscera, while the opposite trend was found in that of Con and ConB groups. BBR had been reported to improve energy expenditure and accelerated the oxidation rate of fatty acids(Reference Kim, Lee and Cha42), thus reduced lipid accumulation in specific tissues when supplemented in the normal diet. On the other hand, BBR was observed to be distributed to all organs with various accumulation levels after oral administration, with the highest distribution in the liver, followed by kidney, muscle and the least accumulation was in fat(Reference Feng, Shou and Zhao18). Therefore, the diverse contents of BBR in different organs might have resulted in the variation of results. Besides, BBR might stimulate hepatic lipid transport to other tissues, such as mesenteric fat tissue and muscle, which is also supported by the increased lipid contents in the muscle of BBR-supplemented groups. In the present study, hepatic LPL activity was improved by BBR supplementation, which might indicate the increment of lipid importation for storage in the muscle(Reference Zheng, Luo and Zhuo51). Notably, BBR can regulate multiple pathways or molecules and reflects a comprehensive effect to treat energy-related metabolic disorders(Reference Yao, Kong and Jiang74). Hence, the tissue-specific manner of BBR supplementation in different diets might be correlated with a complicated mechanism, which deserves further study.
Feeding fish an HL diet usually causes excessive lipid accumulation and thus impairs health status(Reference Adjoumani, Abasubong and Phiri12). Increased plasma TAG, LDL and total cholesterol contents is a significant risk symbol of steatosis, liver dysfunction and regarded as a sign of poor health condition(Reference Kim and Spiegelman75,Reference Kikuchi, Furuta and Iwata76) . Besides, serum ALT and AST activities are common indicators for liver function and health(Reference Wang, Zhang and Gladstone1). In the present study, the HL diet induced higher serum TAG and LDL-cholesterol contents, as well as significantly higher serum ALT activity compared with that of the fish fed the Con diet. These evaluated serum parameters caused by the HL diet are in line with the histological result and some other studies in blunt snout bream(Reference Adjoumani, Abasubong and Phiri12,Reference Abasubong, Li and Zhang13) , large yellow croaker (Pseudosciaena crocea R.)(Reference Wang, Li and Hou43) and black sea bream(Reference Wang, Zhang and Gladstone1). Conversely, BBR supplementation in the HL diet reduced these serum parameters significantly, which indicated that impaired lipid metabolism and liver function resulting from the HL diet could be alleviated by BBR supplementation. Similarly, some studies reported that BBR supplementation reduced plasma TAG and total cholesterol contents in blunt snout bream(Reference Xu, Chen and Chen21), serum TAG and total cholesterol contents in grass carp(Reference Pan, Li and Xie77), serum TAG and LDL concentrations in rabbits fed with a high-fat diet(Reference Luo, He and Jin78), plasma total cholesterol and TAG contents, as well as ALT and AST activities in obese rats(Reference Kim, Lee and Cha42). Hepatic TAG, total cholesterol and glucose contents were reduced by BBR exposure in zebrafish larvae pretreated with high-cholesterol diet(Reference Chen, Zheng and Zhang25). Previous studies demonstrated that BBR could reduce plasma LDL by increasing LDL receptor gene expression through stabilising LDLR receptor mRNA(Reference Zhou, Rahimnejad and Lu15,Reference Kong, Wei and Abidi79) , inhibit cholesterol absorption of the intestine(Reference Wang, Yi and Ghanam20) and prevent hepatic cholesterol biosynthesis(Reference Wang and Zidichouski14). Moreover, BBR could regulate gut microflora population and structure, which was beneficial for fatty acid oxidation and reducing serum TAG(Reference Wang and Zidichouski14). In contrast, the administration of BBR showed a positive correlation with serum HDL-cholesterol concentration in rats(Reference Ragab, Abd Elghaffar and El-Metwally45,Reference Chueh and Lin80) , which was not observed in the present study.
Conclusion
In conclusion, the HL diet inhibited FI and reduced the growth performance of black sea bream. The high dietary lipid level also caused extensive lipid accumulation in liver and increased serum TAG, LDL-cholesterol contents and ALT activity. BBR supplementation in the HL diet reduced the expression levels of lipogenesis genes and increased lipolysis genes expression, thus alleviating hepatic lipid accumulation and improved the health condition. Besides, BBR supplementation increased lipid deposition in the muscle through the opposite mechanism of that in the liver. The lipid-lowering effect of BBR is affected by dietary BBR and lipid levels. Thus, BBR has the potential to work as a hepatoprotective feed additive to alleviate the harmful effects caused by HL diets in aquaculture.
Acknowledgements
We would like to thank the Bio-ultrastructure Analysis Lab of Analysis Center of Agrobiology and Environmental Sciences, Zhejiang University for the TEM analysis. We thank the Key Lab of Mari culture and Breeding of Zhejiang Province for providing the experimental rearing system and logistical support during the growth trial. We are grateful to the Animal Physiology Laboratory of Zhejiang University, Hangzhou, China for helping with the microscopic examination of samples.
This work was supported by the Zhejiang Provincial Bureau of Science and Technology (project no. 2015C02020).
L. W., J. Z. and Q. S. designed this study. L. W. carried out the rearing work and wrote the paper with contributions from the other authors.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.












