In the UK, breast cancer is the most commonly diagnosed cancer among women accounting for almost one-third of all female cancers. Endometrial and ovarian cancers are the next most frequently diagnosed hormone-related cancers among British women( 1 ). These cancers are all age dependent and commonly diagnosed postmenopausally( 2 ). The mechanisms involved in the pathogenesis of these cancers are not completely elucidated. Reproductive and hormonal risk factors such as an early age at menarche, late age at menopause, lack of oral contraceptive use, lack of tubal ligation, postmenopausal hormone therapy, nulliparity, all contribute to the lifetime oestrogen exposure( Reference Kelsey, Gammon and John3 , Reference McPherson, Sellers and Potter4 ) as well as a family history have been consistently associated with these reproductive cancers( Reference Hunn and Rodriguez5 ). Moreover, smoking has also been associated with an increased risk of breast and ovarian cancers while it reduces the risk of endometrial cancer( Reference Cramer6 , Reference Jones, Schoemaker and Wright7 ). In addition, evidence from observational studies has indicated that obesity-related metabolic disorders such as diabetes and the metabolic syndrome can be linked to the aetiology of these cancers( Reference Gallagher and LeRoith8 ). These metabolic disorders are partly outcomes of poor dietary quality( Reference de Oliveira, McLellan and Vaz de Arruda Silveira9 ).
In addition to being one of the triggering factors in the development of obesity, diet also potentially influences the endogenous hormonal milieu, thereby increasing the risk of these hormone-related cancers( Reference Henderson and Feigelson10 ). As demonstrated in previous studies, dietary changes have been linked to changes in menstrual cycle length, circulating sex hormone-binding globulin levels and also oestradiol levels( Reference Thomas, Davey and Key11 – Reference Boyd, Lockwood and Greenberg14 ). Even though studies have shown that diet may be related to the risk of breast, endometrial and ovarian cancer, the specific dietary components involved in the aetiology of these cancers remain unclear. For instance, according to the recent World Cancer Research Fund/American Institute for Cancer Research report( 15 ), there was strong evidence that alcohol consumption increases both the risk of pre- and postmenopausal breast cancers. In addition, there was suggestive evidence demonstrating that a high consumption of non-starchy vegetables, foods sources of carotenoids, dairy products and Ca-rich diets were associated with a decreased risk of breast cancer. On the other hand, the link between other foods and risk of breast cancer remains limited and inconclusive. Likewise, the relationship between diet and endometrial as well as ovarian cancer was sparse and conflicting. Therefore, using data from the UK Women’s Cohort Study (UKWCS), this study aims to investigate the associations between food intake and the risk of breast, endometrial and ovarian cancer.
The aetiology of these cancers also differs by whether the cancer is pre- or postmenopausal. While evidence suggests a link between endogenous oestrogens and risk of these cancers among postmenopausal women, there is only weak evidence supporting this relationship among premenopausal women( Reference Samavat and Kurzer16 , Reference Allen, Key and Dossus17 ). In addition, the menstrual cycle variations in circulating sex hormone levels make deciphering the aetiology behind premenopausal breast, endometrial and ovarian cancer risk a challenge( Reference Lukanova and Kaaks18 ). This study thus also seeks to look into the relationship between diet and risk of the hormone-dependent cancers by menopausal status.
Methods
Study design, study population and ethical approval
At baseline, the UKWCS involved 35 372 women across England, Wales and Scotland who responded to a postal questionnaire between 1995 and 1998. The recruitment process has been detailed elsewhere( Reference Cade, Burley and Alwan19 ). Recruited women were aged between 35 and 69 years. Dietary data, lifestyle as well as health-related data were collected at baseline. Approximately 4 years later, further diet, lifestyle and health-related data were collected between the years 1999 and 2002 (40·1 % response), which formed the follow-up cohort. Reproductive history including menopausal status was also collected at study baseline and follow-up. At its initiation in 1993, ethical approval was obtained from 174 local research ethics committees (Research Ethics Committee reference number: 15/YH/0027).
Dietary assessment
A detailed validated( Reference Taylor, Burley and Greenwood20 ) 217-food item FFQ was used to assess the dietary intake of the participants over a period of 12 months. Daily intake of each food item (g/d) was determined using the frequency categories to estimate the portion size. Using a standard portion size, these were then converted into weights. According to the recent World Cancer Research Fund report, one of the identified critical areas of research included better characterisation of diet( 15 ) and their cancer prevention recommendations( 21 ) suggests consumption of a fibre-rich diet, limiting consumption of foods high in fat, starches or sugars as well as limiting consumption of red and processed meat. Therefore, in this study, the individual food items were collapsed into sixty-four food groups based on their fibre and fat contents, the type of meat or according to their culinary uses. Details on grouping of the foods have been described previously( Reference Dunneram, Greenwood and Burley22 ). The standard portion sizes were estimated by calculating the average portion size of the individual food items within the food group as per the Food Standards Agency( 23 ).
Case definition
Incident cases of invasive breast carcinomas, endometrial and ovarian cancers were identified through linkage to the National Health Service Central Register( Reference Cade, Taylor and Burley24 ). The International Classification of Diseases 9 and 10 were used to code incident cancer cases. Participants were followed from study entry till diagnosis of the breast cancer (International Classification of Diseases (ICD)-9 code 174 or ICD-10 code C50), endometrial cancer (ICD-9 code 182 or ICD-10 code C54.1 or C54.9), ovarian cancer (ICD-9 code 183 or ICD-10 code C56), date of death or until the censor date (1 April 2016) whichever came first.
Statistical analysis
Descriptive statistics were used to describe lifestyle characteristics of participants for breast, endometrial and ovarian cancer separately as well as for women without any incident case of a malignant cancer. Cox proportional hazards regression was used to provide hazard ratios (HR) and 99 % CI to account for potential multiple testing of breast, endometrial and ovarian cancers in relation to diet. For ease of interpretation, the HR were presented per standard portion size of the food group per d. The proportional hazards assumption was tested graphically as well as using the Cox–Snell residuals for all terms in the model. Time in the study was used as the time variable calculated from the date of questionnaire receipt until either death or censor date.
Risk factors for cancer previously identified in the literature were considered to build a directed acyclic graph. A parsimonious age-adjusted model was firstly used to estimate the association between each individual food groups and risk of the cancers in separate models (model 1). According to the minimal sufficiency set of adjustments, the final models for risk of breast and ovarian cancer were adjusted for age (years), physical activity (h/d)( Reference Clague and Bernstein25 ), ethanol intake (g/d)( Reference Bagnardi, Rota and Botteri26 ), smoking status (never, current or former smoker)( Reference Stockwell and Lyman27 ), cumulative duration of breast-feeding (weeks)( Reference Wang, Li and Shi28 – Reference Su, Pasalich and Lee30 ), menopausal status (pre- or post-menopausal),( 2 ) and socio-economic status (professional/managerial, intermediate or routine and manual)( Reference Liu, Deapen and Bernstein31 ) (model 2). For risk of endometrial cancer, history of diabetes( Reference Friberg, Mantzoros and Wolk32 ) and hypertension( Reference Aune, Sen and Vatten33 ) were also included in model 2. Participants with incomplete data on these variables were excluded.
Subgroup analyses by pre-menopausal cancer and postmenopausal cancer were also performed. A premenopausal cancer was defined as an incident case diagnosed before the last menstrual period, while a postmenopausal cancer case was one diagnosed either at or after the last menstrual period. For premenopausal cancer, cases contributed to person-time from age at baseline until the diagnosis of the event. If the participant did not have a premenopausal cancer, the age until last menstrual period was considered as the time variable instead. Women who were already postmenopausal at study entry were excluded from the model (adjusted for model 2). For postmenopausal cancer, cases contributed to person-time from age at last menstrual period until the diagnosis of the event. Women who were incident cases of premenopausal cancer and those who were still premenopausal at censor date were excluded from the model (adjusted for model 2).
Age at natural menopause was further explored as an effect modifier for the foods that were significantly associated with the risk of the cancers. Previous studies have also demonstrated an increased risk of these cancers with a later age at natural menopause due to longer exposure to oestrogen( Reference Persson34 ). Age at last period was self-reported at both baseline and phase 2. This variable was grouped as having a menopause either between 40 and 49 years (n 10 505) or 50 and 65 years (n 6295). To include only postmenopausal women with a natural menopause, those who had a hysterectomy or bilateral oophorectomy as well as those who reported current or ever use of hormone replacement therapy (HRT) before their last period were excluded from the analyses. In addition, women who had their last period before the age of 40 years were also excluded as this could be due to other treatments or surgical procedures that could not be ascertained in this study. All statistical analyses were conducted using Stata version 15 statistical software.
Sensitivity analysis was also conducted using model 2, further adjusting for both family history of any cancer and family history of breast cancer in the first-degree relatives to estimate the association between food groups and the risk of breast cancer. To estimate the association of the risk of endometrial cancer, family history of endometrial cancer was included in the model, and for the risk of ovarian cancer, a family history of ovarian cancer and breast cancer was adjusted for in addition to model 2. Sensitivity analyses also involved adjusting for total energy intake (kJ/d) to account for under- and over-reporters (model 3). Adjustments were also made for current HRT use( Reference Simin, Tamimi and Lagergren35 , Reference Beral36 ), use of oral contraceptive pills and parity( Reference Salazar-Martinez, Lazcano-Ponce and Lira-Lira37 , Reference Tsilidis, Allen and Key38 ) (model 4) in addition to model 3 as these are known risk factors for breast, endometrial and ovarian cancers.
Results
Baseline characteristics according to cancer type
Of the 35 372 women at baseline, 695 women who were not flagged on the National Health Services (NHS) digital, 2340 women reporting history of any previous malignant cancer at baseline (except for non-melanoma of the skin) and women who were diagnosed with breast (n 68), endometrial (n 7) and ovarian (n 12) cancer within 1 year of baseline were excluded. After the exclusions, 32 228 women were eligible for the breast cancer analysis, 32 289 for the endometrial cancer analysis and 32 284 for the ovarian cancer analysis.
Baseline characteristics of the participants according to cancer type are summarised in Table 1. After approximately 18 years of follow-up, there were 1822 incident cases of breast cancer, 294 and 285 incident cases of endometrial and ovarian cancer, respectively. Women with endometrial and ovarian cancer were on average overweight at baseline with a BMI of 27·3 and 25·1 kg/m2, respectively, while women with breast cancer were borderline overweight (24·8 kg/m2) and women without any cancer had a normal weight (24·4 kg/m2). Women with endometrial cancer were less likely to be current smokers and had lower ethanol intake in comparison to those with breast and ovarian cancer as well as those without any cancer. A majority of women with incident breast cancer were current users of HRT at baseline (58·3 %). Women without any cancer had an earlier natural menopause (mean=47·5 years) as compared with women with breast, endometrial and ovarian cancer. Around 42–46 % of women with breast, endometrial and ovarian cancer had a family history of any cancer at baseline as compared with 38·4 % for the non-cancer cases. Total energy intake and fibre intake was quite similar between the cancer cases and non-cancer cases.
Table 1 Baseline characteristics from the UK Women’s Cohort Study according to cancer type (Mean values and standard deviations; numbers and percentages)

Diet and risk of breast, endometrial and ovarian cancer
For the association between food intake and risk of breast cancer, in both the age-adjusted model and fully adjusted model, a standard portion of 83 g of tomato consumption was associated with a significant risk reduction (HR 0·87, 99 % CI 0·75, 0·999). In the fully adjusted model, a standard portion of both processed meat and total meat intake was associated with higher risk of breast cancer, 36 and 17 %, respectively (HR 1·36, 99 % CI 1·02, 1·81; HR 1·17, 99 % CI 1·00, 1·36) (Table 2). According to the subgroup analysis by pre- and postmenopausal breast cancer, consumption of tomatoes reduced the risk of postmenopausal breast cancer but not premenopausal breast cancer. Consumption of processed meat and total meat were both associated with a significant higher risk of postmenopausal breast cancer only. In addition, intake of 15 g of biscuits per d was associated with a 17 % higher risk of premenopausal breast cancer (Table 3).
Table 2 Breast, endometrial and ovarian cancer by food groups (Hazard ratios (HR) and 99 % confidence intervals)
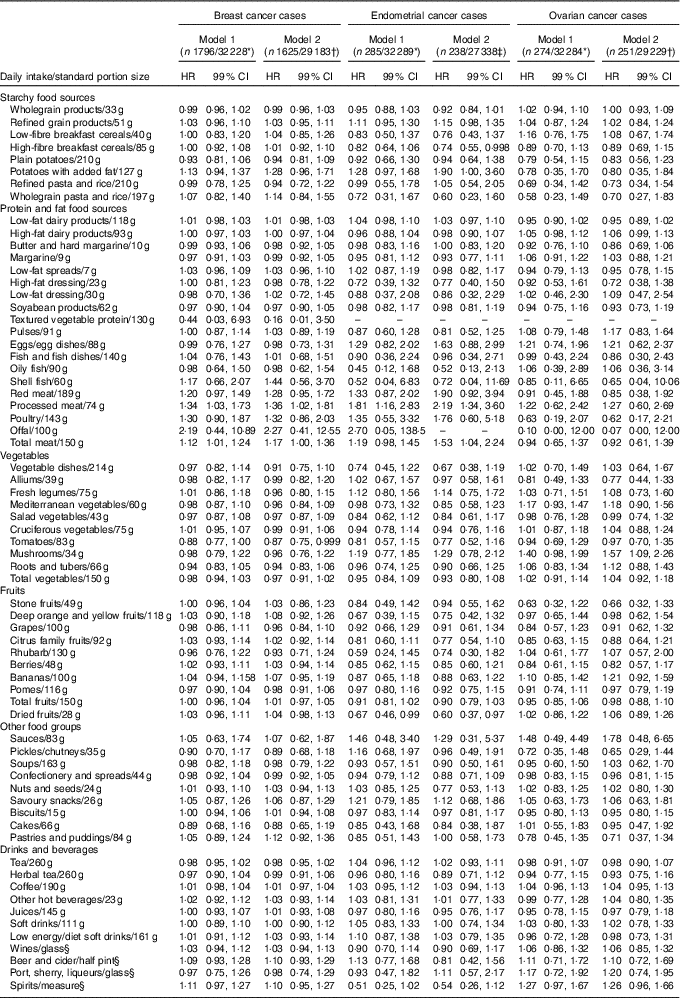
* Model 1: adjusted for age.
† Model 2: adjusted for age, ethanol intake, duration of breast-feeding, physical activity, smoking, social class and menopausal status.
‡ Model 2 (endometrial cancer): adjusted for age, ethanol intake, duration of breast-feeding, physical activity, smoking, social class, menopausal status, history of diabetes and history of hypertension.
§ Not adjusted for ethanol intake.
Table 3 Associations between various food groups and risk of breast, endometrial and ovarian cancer by incidence of premenopausal and postmenopausal cancer cases (Hazard ratios (HR) and 99 % confidence intervals)

* Fully adjusted for age, ethanol intake, duration of breast-feeding, physical activity, smoking, social class and menopausal status.
† Fully adjusted for age, ethanol intake, duration of breast-feeding, physical activity, smoking, social class, menopausal status, history of diabetes and history of hypertension.
‡ Not adjusted for ethanol intake.
Similarly, an increased risk of endometrial cancer was observed in the fully adjusted model with consumption of a standard portion of processed and total meat per d (HR 2·19, 99 % CI 1·34, 3·60; HR 1·53, 99 % CI 1·04, 2·24). Consumptions of 28 g of dried fruits per d and 85 g of high breakfast cereals were associated with a 40 and 26 % reduced risk of endometrial cancer, respectively (HR 0·60, 99 % CI 0·37, 0·97; HR 0·74, 99 % CI 0·55, 0·998) (Table 2). In the subgroup analysis, a standard portion of processed meat per d was associated with a higher risk of postmenopausal endometrial cancer. Consumption of dried fruits was associated with a significant reduced risk of only postmenopausal endometrial cancer (HR 0·55, 99 % CI 0·31, 0·98), while a higher intake of low-energy/-diet soft drinks was positively associated with the risk of postmenopausal endometrial cancer (HR 1·27; 99 % CI 1·00, 1·61). For ovarian cancer, 34 g of mushroom intake per d was associated with a significantly higher risk (HR 1·57, 99 % 1·09, 2·26). Furthermore, it was found that a higher mushroom intake was associated with an increased risk of postmenopausal ovarian cancer. A higher consumption of citrus fruits and total fruits was associated with an 87 and 37 % reduced risk of premenopausal ovarian cancer, respectively.
After further adjustment for family history of the respective cancers, similar results were obtained to those reported above. In addition, a significantly higher risk of breast and endometrial cancer was observed with frequent consumption of a standard portion of potatoes with added fat (i.e. chips/roast potatoes) (online Supplementary Table S1). The associations between diet and risk of breast, endometrial and ovarian cancer after further adjustments for total energy intake and current HRT use, oral contraceptive use and parity were also in agreement with the study’s main associations (online Supplementary Table S2). We also found that the risk of breast, endometrial and ovarian cancer significantly increased with an increase in age at natural menopause (online Supplementary Table S3). Subgroup analysis by age at natural menopause demonstrated that the diet of women with either an earlier or later age at natural menopause did not change the risk of the cancers (online Supplementary Table S4).
Discussion
In this prospective investigation of the consumption of food groups in relation to the risk of breast, endometrial and ovarian cancers, we consistently found that consumption of processed meat and total meat was associated with a significantly higher risk of breast and endometrial cancer. In addition, frequent consumption of a standard portion of tomatoes and dried fruits were associated with a reduced risk of breast and endometrial cancer, respectively. A higher consumption of mushrooms was found to be weakly associated with a higher risk of ovarian cancer. Subgroup analysis showed similar associations between these food items and cancer risk, when differentiating between a pre- and postmenopausal cancer as well as when further adjustments for family history of cancer, total energy intake, current HRT use, oral contraceptive use and parity were accounted for in the different models.
Previous studies have also reported an increased risk of breast and endometrial cancer with a higher consumption of processed meat and total meat. According to the recent UK Biobank cohort study( Reference Anderson, Darwis and Mackay39 ), a 6 % higher risk of breast cancer was reported in relation to processed meat consumption. Similar to our results, they also found only a significant increased risk of postmenopausal breast cancer. The European Prospective Investigation into Cancer and Nutrition (EPIC)( Reference Pala, Krogh and Berrino40 ) and NutriNet-Santé( Reference Fiolet, Srour and Sellem41 ) prospective cohort studies have also reported an increased risk of breast cancer associated with the consumption of processed meat. Our findings are further supported by a prospective randomised control trial conducted over a period of 8 years( Reference Pouchieu, Deschasaux and Hercberg42 ). Studies investigating the association between processed meat and the risk of endometrial cancer are limited and conflicting. While a case–control study( Reference Fabio, Carlo and Silvia43 ) including 274 participants with endometrial cancer found that intake of processed meats such as boiled ham, salami and sausages and canned meat was associated with an increased risk of endometrial cancer, findings from a cohort study, the National Institutes of Health – American Association of Retired Persons (NIH-AARP) Diet and Health Study( Reference Arem, Gunter and Cross44 ) including 1486 incident cases reported no evidence of an association. Another cancer multi-site study from the NIH-AARP Diet and Health Study also reported no association between processed meat consumption and risk of both breast and endometrial cancer( Reference Cross, Leitzmann and Gail45 ).
The underlying mechanisms for the pathogenesis of breast cancer are heterogeneous. High levels of nitrates, nitrites and amines, which are precursors of N-nitroso compounds, added in processed meat to enhance its colour and flavour have been consistently reported to be one of the causes of carcinogenicity( Reference Inoue-Choi, Sinha and Gierach46 ). In addition, cooking especially at high temperatures (e.g. frying, grilling or barbecuing) can lead to the formation of heterocyclic aromatic amines, which are also potent mutagens and carcinogens( Reference Zheng, Gustafson and Moore47 ). The N-nitro compounds, heterocyclic amines along with other compounds (haem Fe, saturated fat and oestradiol), present in meats can directly cause DNA damage and have been associated with mammary tumour development as demonstrated in both animal and human studies( Reference Inoue-Choi, Sinha and Gierach46 , Reference Nagao, Ushijima and Wakabayashi48 ). We also found that processed meat consumption was positively associated with postmenopausal breast cancer though not for premenopausal breast cancer. Disparities could be due to differing oestrogen metabolism pathways between the two groups. These results could suggest that processed meat influences breast cancer risk by interacting with oestrogen metabolism in scenarios where the levels of circulating oestrogens are lower( Reference Taylor, Burley and Greenwood20 ).
Endometrial cancer is a hormone-driven cancer, with approximately 80 % potentially arising due to either an excess of oestrogen or a lack of progesterone. In the normal endometrium, the proliferative effects of oestrogen are normally countered by progesterone but in the absence of progesterone, oestrogen can induce oncogenesis, an effect that is amplified in situations of excess oestrogen( Reference Carlson, Thiel and Yang49 ). In addition to being a source of N-nitroso compounds, processed meat is also rich in cholesterol, which can be converted into androgens and oestrogens through varying metabolic pathways( Reference Bremer and Miller50 ).
Our study further demonstrated that consumption of a standard portion of tomatoes per d was associated with a reduced risk of breast cancer. The protective association was mainly observed among women with postmenopausal breast cancer. Lycopene, a carotenoid widely available in tomatoes, has a very high antioxidant potential and can thus protect the DNA from damage. In a large pooled analysis which included more than 3000 breast cancer cases, Eliassen et al. ( Reference Eliassen, Hendrickson and Brinton51 ) also found an inverse association between lycopene and risk of breast cancer. The anti-proliferative effect of lycopene has also been demonstrated in mammary cancer cell lines by its inhibitory effect on insulin-like growth factor-I-stimulated cell multiplying( Reference Levy, Bosin and Feldman52 , Reference Karas, Amir and Fishman53 ). The observed inverse association could also be due to the high flavonol content of tomatoes which also confers enhanced antioxidant capacity.
Consumption of dried fruits and high-fibre breakfast cereals such as porridge, muesli and bran flakes were inversely associated with risk of endometrial cancer, in particularly among women who were incident cases of postmenopausal endometrial cancer. Dried fruits reportedly have a higher total phenolic content, flavonoids and total antioxidant capacity compared with fresh fruits, making dried fruits a potential candidate of a chemopreventive food( Reference Lutz, Hernández and Henríquez54 , Reference Capanoglu55 ). Previous studies have similarly reported an inverse association between wholegrain cereal consumption and endometrial cancer( Reference Goodman, Wilkens and Hankin56 , Reference Bidoli, Pelucchi and Zucchetto57 ). Dietary fibre has been found to interact with the metabolism of oestrogen, causing a reduced bioavailability of the hormone( Reference Sowers, Crawford and McConnell58 ). High-fibre cereals and dried fruits are also good sources of dietary lignans. Lignans, a type of phyto-oestrogens are plant compounds having structural similarity to 17-oestrodiol. They can lower endogenous oestrogen levels by potentially binding to oestrogen receptors( Reference Kuiper, Lemmen and Carlsson59 ), hence reducing the risk of endometrial cancer.
Contrary to a previous case–control study undertaken in Chinese women, which demonstrated an inverse association between white button mushrooms and risk of ovarian cancer( Reference Lee, Pasalich and Su60 ), our findings showed weak evidence of an increased risk in relation to the consumption of a standard portion of mushrooms per d. Furthermore according to a study among Korean women, high mushroom intake was reportedly associated with a lower risk of breast cancer among premenopausal women and a stronger association was reported among premenopausal women with oestrogen-receptor-positive and progesterone-receptor-positive tumours( Reference Shin, Kim and Lim61 ). However, in this study we do not have this level of detail in terms of types of mushroom consumption and breast cancer by hormone receptor type. This difference could also be attributed to the fact that Chinese cohorts most commonly consume fresh mushrooms, while in Europe the use of canned mushrooms is more widespread. In addition, in the UK, there is no other evidence suggesting that mushrooms can increase or decrease the risk of cancer( 62 ).
Strengths of this study include the prospective study design, a long follow-up time and large sample size. This is also the first study in the UK looking at multiple food groups in relation to the risk of breast, endometrial and ovarian cancers. We were also able to study the associations with specific types of meat, cereal products (wholegrain or refined) and dairy products (high fat or low fat). We adjusted for a wide range of confounders including sociodemographic and lifestyle using a consistent method (directed acyclic graph). However, as in any observational study, residual confounding is still possible. A limitation of our study was the inability to determine whether the associations varied according to the hormone receptor status of tumours, due to the lack of these data at present in this cohort. The UKWCS will soon be expanding to include additional details on the tumour types. Moreover, the use of an FFQ for dietary assessment could also be prone to low accuracy due to recall bias. However, the FFQ is a useful tool in providing a snapshot of the dietary habit over a longer period of time. Regression dilution might also be an issue, given participants’ diets may have changed over time, potentially introducing further measurement error. This study also does not take into account the use of pesticides which is also a potential carcinogen influencing cancer risk in women. Our sample was also more health conscious, given the high number of vegetarians in our sample population and more well-off participants than the general population. However, our study still included women from a range of different backgrounds, which implies that findings of this study may be extrapolated to other countries.
Primary prevention of cancer is important and a matter of consideration in public health. While factors such as parity, age at onset of natural menopause and family history are well established to have a link with the risk of breast, endometrial and ovarian cancer, they are non-modifiable risk factors. However, diet which has been shown to either increase or decrease the risk of carcinogenesis makes focus on diet an interesting opportunity in cancer prevention.
To summarise, this study suggests a link between specific foods: processed meat, total meat, tomatoes, dried fruits and wholegrain products and the risk of breast as well as endometrial cancer while a relationship between diet and risk of ovarian cancer is less evident. These findings support the call for further randomised controlled trials of dietary interventions to reduce the risk of these hormone-related cancers among pre- and postmenopausal women.
Acknowledgements
The authors thank the UKWCS steering group who initiated the study, collected data, managed and processed information of the cohort as well as the participants of the UKWCS.
Y. D. is in receipt of a scholarship from the Commonwealth Scholarships Commission, UK. J. E. C. acknowledges additional support from the Medical Research Council (MR/L02019X/1). The Commonwealth Scholarships Commission and the Medical Research Council had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
J. E. C. initiated and developed the cohort. Y. D. was primarily responsible for data analysis and writing the manuscript. All authors were involved in the study design, interpretation of findings, editing and approving the article.
J. E. C. is a director of the University of Leeds spin out company Dietary Assessment Ltd.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003665






