The impact of dietary n-3 PUFA on human health has been increasingly examined in recent years. This unique class of fatty acids comprises the essential parent precursor fatty acid α-linolenic acid and principal metabolites include DHA and EPA. α-Linolenic acid cannot be manufactured de novo and must be supplied via dietary sources and is primarily found in seed oils such as flax and rapeseed. The more biologically relevant longer-chain fatty acids DHA and EPA can be synthesised from α-linolenic acid; however, this process is limited in humans(Reference Burdge and Calder1). A better dietary source of these fatty acids is therefore preformed DHA and EPA, which are abundant in oily fish. DHA is heavily concentrated in the mammalian brain and comprises 10–20 % of the fatty acids found in this organ(Reference McNamara and Carlson2). DHA maintains the structural integrity of neuronal cell membranes and has an impact on numerous signalling pathways and neurotransmitter systems(Reference Horrocks and Farooqui3, Reference Innis4). EPA, although not a major component of the brain, could potentially influence brain function via the action of its many eicosanoid derivatives; regulators of key immune, endocrine and cardiovascular functions(Reference Piomelli5, Reference Calder6). A large body of research now exists describing the effects of dietary n-3 PUFA on behaviour; however, very little attention has been given to evaluating their impact on brain function in physiological terms, with only a limited number of preclinical and clinical trials to date addressing this issue(Reference Tsukada, Kakiuchi and Fukumoto7–Reference McNamara, Able and Jandacek9).
A viable approach to exploring the effects of dietary n-3 PUFA on brain function is by measuring cerebral haemodynamics using functional near IR spectroscopy (NIRS), a well-validated and non-invasive optical imaging technique. NIRS is based on the intrinsic optical absorption properties of oxygenated Hb (oxy-Hb) and deoxygenated Hb (deoxy-Hb), and can be used as a measure of activation of neural tissue or simply as a measure of local cerebral blood flow (CBF) in the top layers of the cortex. Total-Hb (the sum of oxy- and deoxy-Hb) is closely related to cerebral blood volume, from which changes in regional CBF (rCBF) can be inferred(Reference Steinbrink, Villringer and Kempf10). Total-Hb also correlates well with rCBF changes as measured using the labelled water technique(Reference Villringer and Dirnagl11), although studies using rat brain models suggest that oxy-Hb is a better measure of rCBF than total-Hb(Reference Hoshi, Kobayashi and Tamura12). NIRS has been successfully used to image activation in the frontal cortex (Fallgatter & Strik(Reference Fallgatter and Strik13, Reference Fallgatter and Strik14)) and other areas of the brain including the temporal, visual and parietal cortices (Schecklmann et al. (Reference Schecklmann, Ehlis and Plichta15); Jasdzewski et al. (Reference Jasdzewski, Strangman and Wagner16)). Only a small number of trials published to date have utilised NIRS in pharmacological interventions (Kanamaru et al. (Reference Kanamaru, Kikukawa and Miyamoto17); Bonoczk et al. (Reference Bonoczk, Panczel and Nagy18)); data from our own laboratory have recently demonstrated increased rCBF following administration of a vasodilator (resveratrol(Reference Kennedy, Wightman and Reay19)) and decreased rCBF following a vasoconstrictor (caffeine(Reference Kennedy and Haskell20)), supporting the application of NIRS in this area.
To date, research has primarly focused on the effects of DHA on brain function, given the high proportion of this fatty acid found in this organ. However, it is also possible that EPA could also have an impact on the cerebrovascular parameters, given that this fatty acid is readily incorporated into endothelial cell membranes elsewhere in the cardiovascular system(Reference Hashimoto, Hossain and Yamasaki21) and has been shown to modulate local CBF in aged, stroke-prone rats(Reference Katayama, Katsumata and Muramatsu22). Therefore, the objective of the present pilot trial was to investigate any treatment-related effects of 12 weeks of supplementation with DHA- and EPA-rich fish oil (FO) in healthy volunteers on the cerebral haemodynamic response to cognitive tasks in the prefrontal cortex.
Experimental methods
Participants
A total of twenty-two healthy adults took part in the study (nine male; mean age 21·96 years; mean BMI 23·04 kg/m2). All participants declared that they were in good health, non-smokers, free from prescription medication and social drugs, free from n-3 supplements and native English speakers. The participants also confirmed that they did not consume more than one portion of oily fish per week. At the time of testing, the participants were enrolled in a larger intervention trial assessing the cognitive and mood effects of 12 weeks of dietary supplementation with FO and were invited to take part in an extra testing session once they had completed their 12-week cognitive assessment(Reference Jackson, Deary and Reay23).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving participants were approved by the Northumbria University School of Life Sciences Ethics Committee. Written informed consent was obtained from all the participants.
Treatments
Before the start of the behavioural intervention study, a restricted randomisation list matching treatments to participant code numbers was generated by the computer. The participants were therefore randomly allocated to one of three treatment groups, DHA-rich FO, EPA-rich FO and placebo. The 1 g daily dose was provided by 2 × 500 mg capsules. Each active 500 mg capsule contained 497·5 mg of deodorised FO+2·5 mg mixed tocopherols. The total daily dose of n-3 PUFA provided by 2 × 500 mg capsules for the DHA-rich FO was 450 mg DHA+90 mg EPA (DHA 5:1 EPA), and for the EPA-rich FO, this was 300 mg EPA+200 mg DHA (EPA 3:2 DHA). The total daily dose for the placebo treatment was 1 g olive oil, again provided by 2 × 500 mg capsules. Treatment oils were purchased from EPAX AS (Aalesund, Norway) and encapsulated by Cardinal Health UK. All capsules used a brown bovine gelatine casing, and all treatments were packaged, labelled and randomised on site by a disinterested third party.
Near IR spectroscopy
The guiding principle of NIRS is that the chromophores oxy-Hb and deoxy-Hb absorb light at different wavelengths. Therefore, by measuring the amount of light absorbance at a specific wavelength, the concentrations of oxy-Hb and deoxy-Hb can be calculated(Reference Fallgatter and Strik13). The third outcome produced by NIRS measurements – total Hb – is simply the sum of oxy-Hb and deoxy-Hb. In the present study, relative changes in the absorption of near IR light were measured using a 12-channel continuous-wave Oxymon system (Artinis Medical Systems B.V., Zetten, The Netherlands), described previously by Kennedy et al. (Reference Kennedy, Wightman and Reay19). In this system, two nominal wavelengths of light (approximately 765 and 855 nm) are introduced through the skull via a laser emitter and measured, following transit through the upper surface of the cortex, by an optode placed at a preset distance from the light source (40 mm in this case). At this distance, the NIR signal is sensitive to haemodynamic changes within the top 2–3 mm of the cortex(Reference Chance, Leigh and Miyake24). Given the experimental aims a simple two-channel configuration was used in this instance, with data collected from the areas of the frontal cortex corresponding to the International 10-20 system Fp1 and Fp2 EEG positions. NIRS data were time stamped by the researcher at the start and end of each task so that only NIRS data collected during the tasks were analysed. Recorded data were stored as a. TXT file and analysed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA).
Cognitive tasks
A total of four tasks were administered using the Computerised Mental Performance Assessment System, a software platform for the presentation of classic and bespoke computerised cognitive tasks. For accurate measurements, the NIRS optodes are required to make good contact with skin; therefore, the measures of haemodynamic response in the frontal cortex are most effective, given that the forehead is naturally free from hair. To this end, the tasks were selected on the basis that execution of the same or a similar task has previously been shown to activate the prefrontal cortex in brain imaging studies (Stroop task(Reference Vanderhasselt, De Raedt and Baeken25); peg-and-ball task, 3-back task(Reference Fitzgerald, Srithiran and Benitez26); Wisconsin card sort task(Reference Toone, Okocha and Sivakumar27)).
The tasks were presented via a laptop computer in the same order as listed below. Progress through the battery of tasks was controlled by the participant, with brief instructions given onscreen before the start of each task. A 60 s rest period between each task was incorporated into the battery commencing as soon as the last response of the previous task had been made and ended with the presentation of the onscreen instructions for the next task. The cognitive data were not analysed.
The tasks were completed in the following order: Stroop task (5 min); peg-and-ball task (2·5 min); 3-back task (4 min); Wisconsin card sort task (3 min). Full descriptions of the Stroop, peg-and-ball and Wisconsin card sort tasks can be found in Kennedy et al. (Reference Kennedy, Veasey and Watson28). For the 3-back task, a continuous string of letters (upper and lower case; inter-stimulus interval of 2·5 s) was presented: seventy-eight letters in total with thirty-six target pairs. For each stimulus, the participants were instructed to indicate using a yes/no key press whether this was the same letter that appeared three letters previously.
Procedure
As part of a larger randomised, intervention trial investigating the effects of two types of FO on cognitive function and mood, the participants had been taking a daily 1 g supplement of either EPA-rich FO, DHA-rich FO or olive oil placebo for 12 weeks. Towards the end of the 12-week period, the participants were invited via email to take part in an extra cognitive testing session with concurrent NIRS data collection. In all cases, this assessment either took place on the same day as the final behavioural testing session or the following day (average time on treatment 88·59 (sem 3·9) d). Therefore, the final treatment dose was consumed 1 or 2 d before the NIRS testing session. Testing took place in the afternoon, and the participants were instructed to refrain from consuming food and drink except water for a minimum of 2 h before testing.
On arrival, the participants were seated in front of the laptop computer used for presenting the cognitive tasks. They were fitted with the NIRS headband and were instructed to sit quietly with their eyes closed until they were given further instruction by the researcher to start the cognitive tasks. Recording of NIRS data was initiated following 30 s of eyes-closed relaxation, and the participant was instructed to begin the tasks after a further 2 min of eyes-closed relaxation. This 2 min period was utilised as the NIRS resting baseline measurement. The participants completed each task with NIRS data collected throughout.
Treatment compliance
The results for compliance as measured by capsule count were similar in all three treatment conditions (89 % DHA-rich FO, 87 % EPA-rich FO and 88 % placebo). As part of the behavioural intervention trial, the participants were required to give two venous blood samples, once before supplementation and again at the week 12 time point. The samples were collected and analysed as described by Jackson et al. (Reference Jackson, Deary and Reay23); available data for the participants who completed the NIRS assessment are presented in Table 1.
Table 1 Serum concentrations of DHA and EPA (% total fatty acid methyl esters) of samples collected at baseline and week 12
(Mean values with their standard errors)
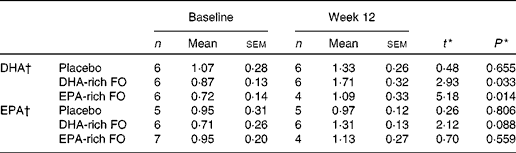
FO, fish oil.
* t and P values are obtained from paired sample t tests (baseline and week 12).
† Serum fatty acid analytical methods are described in full in Jackson et al. (Reference Jackson, Deary and Reay23).
Statistical methods
Changes in concentration (compared with resting baseline) of oxy-Hb and deoxy-Hb were averaged across the two channels (hemispheres) before analysis. Data collected during each task were averaged over the 2·5–5 min of task performance. The resultant data therefore represent the change in the concentration of each of the chromophores, plus their sum–total Hb–during each complete task period with respect to the pre-testing 2 min baseline measurement. As the duration of each task was substantially longer than the potential physiological oscillations that can cause a drift in shorter periods of the NIRS recording (e.g. heartbeat and respiration(Reference Hoshi29)), no adjustment was made to the data for this phenomenon.
The primary analysis of the averaged NIRS data was conducted by one-way ANOVA for each task with a priori planned comparisons of the data from each task being made between placebo and each treatment group (DHA-rich FO, EPA-rich FO) using t tests calculated with the mean squares error of the overall ANOVA(Reference Keppel30). To protect against an increased type I error rate, only those planned comparisons associated with a significant main effect of treatment on the ANOVA are reported.
Results
Oxygenated Hb
There was a significant main effect of treatment on oxy-Hb, while participants completed the Stroop task (F(2,19) = 3·72, P = 0·043). Reference to the planned comparisons showed a significant increase in the concentration of oxy-Hb following the DHA-rich FO during this task compared with placebo (P = 0·013). The trends for an effect of treatment were observed for the peg-and-ball and the 3-back tasks (F(2,19) = 3·35, P = 0·057; F(2,19) = 2·69, P = 0·093, respectively) (Fig. 1).

Fig. 1 Concentration changes in oxygenated (Δoxy-Hb) and total Hb (ΔTotal-Hb) by treatment group (placebo (□), DHA-rich fish oil (FO; ![]() ), EPA-rich FO (
), EPA-rich FO (![]() )) during the cognitive task periods. Data are averaged across both hemispheres (left/right). Significance is compared with placebo (t tests using the mean squares error from the omnibus ANOVA). Values were significantly different: * P < 0·05; ** P < 0·01; t, 0·05 < P < 1. Stroop, Stroop task; P-B, peg-and-ball task; 3-back, 3-back task; WCST, Wisconsin card sort task.
)) during the cognitive task periods. Data are averaged across both hemispheres (left/right). Significance is compared with placebo (t tests using the mean squares error from the omnibus ANOVA). Values were significantly different: * P < 0·05; ** P < 0·01; t, 0·05 < P < 1. Stroop, Stroop task; P-B, peg-and-ball task; 3-back, 3-back task; WCST, Wisconsin card sort task.
Deoxygenated Hb
The ANOVA revealed no treatment-related effects on the concentrations of deoxy-Hb during any of the cognitive tasks.
Total Hb
The ANOVA showed that there was a significant effect of treatment on total-Hb during the Stroop, peg-and-ball and 3-back tasks (F(2,19) = 4·93, P = 0·019; F(2,19) = 4·21, P = 0·031; F(2,19) = 3·57, P = 0·048, respectively). Reference to the planned comparisons showed a significant increase in total-Hb in the DHA-rich FO treatment condition compared with placebo during all three of these tasks (Stroop (P < 0·006); peg-and-ball task (P < 0·009); 3-back task (P < 0·016) (Fig. 1).
Planned comparisons did not reveal any significant difference in task-related concentration changes in any of the chromophores in the EPA-rich FO treatment condition, in comparison with placebo. Averaged data for each treatment group during the task periods are shown in Table 2.
Table 2 Average change in concentration (μmol/l, calculated from a 2 min pre-testing baseline) of oxygenated Hb (oxy-Hb), deoxygenated Hb (deoxy-Hb) and total Hb for each of the treatment groups (placebo, DHA-rich fish oil (FO), EPA-rich FO) during the cognitive tasks
(Mean values with their standard errors)
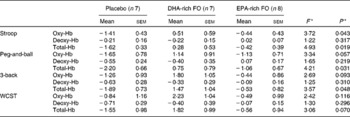
* F and P values are obtained from the primary ANOVA.
Discussion
The present results reveal that supplementation with DHA-rich FO was associated with a significant increase in rCBF in the prefrontal cortex in response to cognitive task performance, in comparison with placebo, as indexed by changes in the concentration of oxy-Hb and total-Hb. Given that a similar pattern of results was not observed in participants who were administered EPA-rich FO, which also contained DHA, it would appear that the rCBF response to the tasks is only modulated following supplementation with DHA at a dose higher than 200 mg/d.
The pattern of concentration changes in oxy-Hb and total-Hb in the EPA-rich FO group did not differ from placebo, suggesting that the addition of dietary EPA at a dose of 300 mg/d for 12 weeks has no impact on cerebral haemodynamic response to cognitive tasks in this population. However, referring to the limited serum fatty acid data indicates that serum concentrations of EPA in this treatment group did not significantly increase following the 12-week intervention, despite a similarly high level of compliance with the treatment regimen being followed as in those administered the DHA-rich FO. Therefore, the impact of EPA supplementation on cerebrovascular response to cognitive tasks may require further exploration, and future investigations at doses higher than the present study would be advised. That being said, Sublette et al. (Reference Sublette, Milak and Hibbeln31) found significant correlations between cerebral metabolic rates of glucose (tempoparietal and frontal cortices, anterior cingulate) and peripheral concentrations of DHA and arachidonic acid, but not of EPA, suggesting instead that EPA may simply not have an impact on cortical activity, possibly due to, as these authors also predict, the very small amount of EPA found in the brain (approximately 1 % total fatty acids(Reference McNamara and Carlson2)).
The pattern of increased oxy-Hb and total-Hb in the DHA-rich FO treatment group, compared with placebo, is in agreement with recent data published by McNamara et al. (Reference McNamara, Able and Jandacek9). Using fMRI, these authors revealed that compared with placebo, DHA supplementation for 8 weeks resulted in increased activation of the dorsolateral prefrontal cortex during a sustained attention task in thirty-three schoolchildren (male, 8–10 years). It is worth noting that the NIRS parameters generated in the present study have been shown to closely correspond to the fMRI blood oxygen level-dependent signal(Reference Huppert, Hoge and Diamond32), suggesting consistency of findings across these two paradigms. Interestingly, the authors also report null effects of the treatment on cognitive performance measures, despite the observed increase in cortical activity. Although the sample size in the present study was too small to permit an analysis of the behavioural data, performance measures analysed from the larger intervention trial described by Jackson et al. (Reference Jackson, Deary and Reay23) from which the current sample was drawn revealed no effect of either active treatment (DHA-rich FO and EPA-rich FO) on cognitive performance overall. The results from both studies suggest that the administration of DHA may improve the overall function and health of the brain, in terms of increased CBF, but that this is simply not manifested in a strong pattern of observable behavioural measures in young, healthy populations. A possibility is that improved function in terms of both haemodynamic response and behaviour may only be apparent in groups characterised by either natural or disease-related cerebrovascular insufficiency. Of course, it may also be the case that the length of the intervention in both our own study and the study described by McNamara et al. (Reference McNamara, Able and Jandacek9) was too short for any potential behavioural effects to be observed. However, longer intervention trials, in children at least, have reported similar null findings (Kirby et al. (Reference Kirby, Woodward and Jackson33); Osendarp et al. (Reference Osendarp, Baghurst and Bryan34)).
Overall, the results of the present pilot study provide evidence of modulation of CBF parameters in healthy young adults following dietary supplementation with DHA-rich FO, and at a dose (1 g/d) that would be achievable by dietary intake of oily fish alone. Previously, studies that have aimed to evaluate the effect of n-3 PUFA supplementation on behavioural outcomes in healthy individuals have provided mixed results. However, the data from the present study suggest that an n-3 PUFA dietary supplement may indeed have an impact on cerebrovascular function. Given these interesting findings, further studies using NIRS and other imaging techniques to assess the causal relationship between n-3 PUFA intake and brain function are warranted.
Acknowledgements
The present study was completed as part of the PhD degree of P. A. J., which was funded by Ginsana SA, Switzerland, who also provided participant payments. Ginsana SA did not have any other input into the study design or analysis of the data. P. A. J. collected the data, and all authors contributed to the design, analysis and manuscript preparation. The authors declare no conflicts of interest.





