Bioelectrical impedance analysis is a portable, easy-to-perform, non-invasive bedside method, which allows an assessment of body composition in both ambulatory and hospitalised patients. Since the calculation of body composition is limited in several clinical conditions, the consideration of raw bioelectrical impedance analysis parameters has gained increasing attention. Moreover, phase angle (PhA) is an excellent predictor of morbidity and mortality in HIV/AIDS, pancreatic, colorectal, breast and lung cancer, liver cirrhosis, dialysis, pulmonary disease, amyotrophic lateral sclerosis, geriatric and surgical patients(Reference Ott, Fischer and Polat1–Reference Barbosa-Silva and Barros16). PhA is calculated from resistance (R, pure opposition of a biological conductor to alternating electric current) and reactance (X c, capacitive resistance of cell membranes).
PhA primarily reflects the electrical integrity of vital cell membranes(Reference Ott, Fischer and Polat1) and indicates the distribution of water between intracellular and extracellular space(Reference Schwenk, Beisenherz and Romer2). Theoretically, changes in reactance associated with variability in cell size, membrane permeability or intracellular composition may contribute to the variation among individuals in PhA at a fixed frequency(Reference Baumgartner, Chumlea and Roche17). Higher PhA suggests large quantities of intact cell membranes(Reference Selberg and Selberg9), while lower PhA suggests cell death or decreased cell integrity. PhA is decreased in patients with significant weight loss(Reference Norman, Stobäus and Zocher8, Reference Gupta, Lis and Dahlk18–Reference Oliveira, Kubrusly and Mota20) and is considered a marker of clinically relevant malnutrition characterised by both increased extracellular fluid and decreased body cell mass(Reference Selberg and Selberg9).
In healthy subjects, age, sex and BMI are the major determinants of PhA(Reference Barbosa-Silva, Barros and Wang21–Reference Bosy-Westphal, Danielzik and Dorhofer24). Ageing is associated with decline in tissue mass, which results in decreasing PhA. Males have higher PhA values due to higher muscle mass. Higher BMI is associated with more cells, which increases PhA until a BMI of 30 kg/m2. Interestingly, at BMI >40 kg/m2, an inverse relationship with PhA is observed. This has been attributed to increased tissue hydration or a pathological fluid overload(Reference Bosy-Westphal, Danielzik and Dorhofer24).
PhA is useful in clinical practice, since it allows identification of patients at risk of impaired nutritional status and decreased survival. However, what contributes to a low PhA in disease is not yet completely understood. We therefore investigated the determinants of PhA in a retrospective sample of hospitalised patients and, moreover, studied the risk factors for patients' individual deviation from age-, sex- and BMI-stratified reference values (standardised PhA, SPhA)(Reference Bosy-Westphal, Danielzik and Dorhofer24).
Patients and methods
We pooled data from 777 patients with full data sets from prospective cross-sectional studies(Reference Norman, Stobäus and Zocher8, Reference Norman, Schutz and Kemps25–Reference Valentini, Wirth and Schweizer27) and previously unpublished results with the same method (N Stobäus, unpublished results). Patients admitted to the Charité University Hospital, Berlin, Germany aged >18 years were originally consecutively recruited. The study protocols were approved by the Ethics Committee of the Charité, and all studies were conducted according to the guidelines laid down in the Declaration of Helsinki. All subjects signed informed consent forms. Age, sex, weight and height as well as clinical variables were recorded.
Bioelectrical impedance analysis
Bioelectrical impedance analysis was performed at 50 kHz and 0·8 mA by experienced observers according to a standardised protocol described elsewhere(Reference Kyle, Bosaeus and De Lorenzo28), using a Nutrigard M (Data Input, Darmstadt, Germany). R and X c (Ω) were measured and PhA was calculated (![]() ). The percentage of patients with PhA below the fifth percentile of age-, sex- and BMI-stratified reference values was determined(Reference Bosy-Westphal, Danielzik and Dorhofer24). PhA was also standardised according to the reference values:
). The percentage of patients with PhA below the fifth percentile of age-, sex- and BMI-stratified reference values was determined(Reference Bosy-Westphal, Danielzik and Dorhofer24). PhA was also standardised according to the reference values: ![]() , where sd is standard deviation of PhA, where mean and sd are from age-, sex- and BMI-stratified reference values.
, where sd is standard deviation of PhA, where mean and sd are from age-, sex- and BMI-stratified reference values.
Assessment of nutritional status
Weight and height were measured to calculate BMI: weight (kg)/height (m2). Nutritional status was assessed by subjective global assessment (SGA)(Reference Detsky, McLaughlin and Baker29). Patients were classified as well nourished (SGA A), moderately malnourished (SGA B) or severely malnourished (SGA C).
Inflammation
C-reactive protein (CRP) was determined according to standard methods (mg/l).
Statistical analysis
Statistical analysis was performed with PASW Statistics 18 (SPSS, Inc., Chicago, IL, USA). Data are presented as means and standard deviations. Comparison between groups was made using Student's t test and χ2 test. The relationship between PhA and its determinants was described by Pearson's correlation. Regression analysis was performed with the general linear model allowing adjustment for continuous and categorical variables, in order to assess the impact of age, sex, BMI, nutritional status defined by SGA, disease (malignant/benign) and inflammation on PhA. The estimates of the effect size are expressed as percentages of η2. A P value < 0·05 was considered significant.
Results
A total of 777 patients (367 men) were included in the pooled analysis. Mean age was 53·6 (sd 16·7) years and women were significantly older (P = 0·006). According to the BMI, 10 % were underweight while 37·8 % were overweight or obese; 54·8 % were considered moderately or severely malnourished by SGA. Patients with tumours (34 %) were older (63·3 (sd 12·6) v. 48·5 (sd 16·3) years, P < 0·001) and had a higher BMI than patients with benign disease (24·7 (sd 4·7) v. 23·8 (sd 4·9) kg/m2, P = 0·01). Mean length of hospital stay was 13·1 (sd 11·5) d.
Mean PhA was 4·91 ± 1·17°, ranging from 1·62 to 8·51°, and was significantly higher in male patients; 41 % of whom exhibited PhA beneath the fifth percentile of age-, sex- and BMI-stratified reference values. SPhA ranged from − 7·2 to 2·5 sd, 56·5 % of patients had PhA below − 1 sd of the population average. PhA correlated inversely with age (men: r − 0·479, P < 0·001; women: r − 0·503, P < 0·001), while only a weak positive correlation existed between PhA and BMI, which was significant in women (r 0·116, P = 0·019).
PhA and SPhA were significantly decreased in moderate and severe malnutrition. Patients with benign disease had higher PhA than patients with tumours (5·14 ± 1·14° v. 4·48 ± 1·09°, P < 0·001). CRP correlated inversely with PhA (r − 0·248, P < 0·001).
Determinants
Age, sex, BMI, nutritional status (SGA), inflammation and malignant v. benign disease were investigated as possible determinants of PhA and SPhA in a general linear model regression analysis (Table 1). Next to age, malnutrition emerged as a major determinant of PhA. Moreover, sex, CRP and BMI significantly influenced PhA, whereas diagnosis showed no effect in this model. When investigating SPhA, the only significant determinants were malnutrition and CRP.
Table 1 Results from the general linear model regression analysis determinants of phase angle and standardised phase angle
(β Coefficients and percentages)
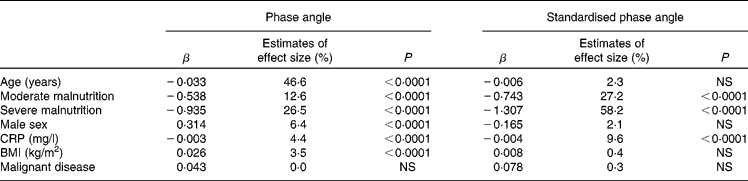
CRP, C-reactive protein.
Interestingly, after stratifying the patients according to CRP (no inflammation, < 5 mg/l; low to moderate, 5–50 mg/l; high, >50 mg/l), the association between CRP and SPhA was statistically significant only in patients with low-to-moderate inflammation, whereas SGA exerted a significant impact in all CRP categories (data not shown).
Discussion
We pooled data from cross-sectional studies in order to investigate the determinants of PhA in disease. Age, malnutrition, sex, inflammation and BMI were identified as significant predictors of PhA. When PhA was standardised according to age-, sex- and BMI-stratified reference values, only moderate and severe malnutrition, as well as inflammation, were significant risk factors.
Age, sex(Reference Barbosa-Silva, Barros and Wang21, Reference Piccoli, Pillon and Dumler30) and BMI(Reference Dittmar22) are the strongest predictors in healthy populations. Few studies have, however, investigated the risk factors for decreased PhA in disease. In haemodialysis, creatinine and log-soluble leptin receptor:leptin ratio(Reference Beberashvili, Sinuani and Azar31) as well as albumin, mid-arm muscle circumference, SGA and normalised protein catabolic rate(Reference Maggiore, Nigrelli and Ciccarelli32) were significant independent predictors of PhA. Moreover, IL-6 predicted lower PhA as well as greater loss of PhA over time in haemodialysis patients(Reference Johansen, Kaysen and Young33).
In the present analysis, age exerted the largest impact on PhA, explaining 46·6 % of its variability. Ageing is associated with loss of muscle mass (decline in X c) and decrease in total body water (increase in R), which results in decreased PhA. This inverse relationship was also found in healthy(Reference Barbosa-Silva, Barros and Wang21–Reference Bosy-Westphal, Danielzik and Dorhofer24) and ill populations(Reference Chertow, Lazarus and Lew34). PhA was reduced in malnutrition, which is in accordance with previous findings(Reference Norman, Stobäus and Zocher8, Reference Gupta, Lis and Dahlk18, Reference Norman, Smoliner and Valentini19). The impact of CRP is expected, as several studies have shown an association between PhA and inflammation(Reference Bosy-Westphal, Danielzik and Dorhofer24, Reference Johansen, Kaysen and Young33, Reference Avram, Fein and Rafiq35–Reference Demirci, Demirci and Ozdogan37).
However, only malnutrition and inflammation predicted SPhA. Transforming PhA into a Z-score allows assessment of individual deviations from age-, sex- and BMI-specific population average, which clearly enhances its validity. SPhA has been shown to be the strongest predictor of 6-month mortality in tumour patients(Reference Norman, Stobäus and Zocher8), and of increased post-operative complications(Reference Barbosa-Silva and Barros16).
The present results are limited since they are a retrospective analysis of prospectively collected data. This precluded the assessment of potentially interesting parameters such as physical activity, probably a contributing factor as suggested by earlier findings in healthy individuals(Reference Dittmar22, Reference Marra, Caldara and Montagnese38). However, to what extent a disease-specific impact might override the influence of physical activity in patients is not known. Future studies should include the assessment of physical activity when studying PhA in disease.
In conclusion, together with the known biological determinants, malnutrition and inflammation had a strong impact on PhA in disease. However, only malnutrition and inflammation predicted SPhA. As a result, SPhA, the individual deviation from population average, is therefore considered a more suitable indicator of nutritional and inflammatory status.
Acknowledgements
K. N. and N. S. designed the study and performed the analysis. L. V., M. P. and J. D. S. contributed the data and significant advice. N. S. and K. N. wrote the manuscript. The authors have no conflicts of interest. The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.



