Provitamin A carotenoids are the major source of vitamin A (VA) in the human diet for most people worldwide(1). Three common carotenoids (α-carotene (AC), β-carotene (BC) and β-cryptoxanthin (CX)) are precursors of VA(1–Reference Olson, Shils, Olson and Shike3). Of the three, BC is the most widely distributed in edible plant sources(4) and usually attains the highest concentrations in human blood(Reference Ford and Giles5).
BC is converted to VA by simple mechanisms involving either BC 15,15′-mono-oxygenase (CMO1) for central cleavage or BC 9′,10′-mono-oxygenase (CMO2) for eccentric cleavage(Reference Hessel, Eichinger and Isken6, Reference Kiefer, Hessel and Lampert7). Theoretically, one molecule of BC can be cleaved by CMO1 into two molecules of VA. However, BC-rich foods do not contribute as much VA to the body as these reaction mechanisms would lead one to expect. The VA activity of carotenoids is denoted by retinol activity equivalents (RAE). Currently, the international estimate of VA activity is that 12 mg of BC from food are required to produce 1 mg of retinol, for an RAE ratio of 12:1(1, Reference Matsuda-Inoguchi, Date and Sakurai8). The reasons for this relatively poor RAE conversion ratio are multifactorial(1, Reference Tanumihardjo9). However, a major reason appears to be poor absorption of BC from its most important food sources(Reference de Pee, West and Permaesih10, Reference Burri, Nguyen and Neidlinger11). Dietary sources of BC include orange and bright green vegetables such as carrot, sweet potato, pumpkin, broccoli and collard greens, as well as other foods like red palm oil and vitamin supplements(4). The amount of VA formed from BC depends on the source of BC. BC in orange fruits and vegetables appears to be better absorbed and utilised, or more bioavailable, than BC in leafy green vegetables(1, Reference de Pee, West and Permaesih10). Therefore, orange fruits and vegetables are better sources of VA than leafy green vegetables with comparable BC concentrations.
Current estimates of RAE for AC- and CX-rich foods are half that of BC, so that 24 mg of AC or CX from food are expected to produce 1 mg of retinol, for an RAE ratio of 24:1(1, Reference Matsuda-Inoguchi, Date and Sakurai8). RAE ratios for AC and CX do not appear to be estimated empirically with CX- or AC-rich foods, or to be based on carotenoid cleavage reaction kinetics(Reference John, Kishor and Subbarayan2, Reference Kim and Oh12–Reference Davis, Jing and Howe14). Instead, it appears that RAE for AC and CX were set at half the RAE for BC because one molecule of BC can be cleaved to form two molecules of retinal, whereas one molecule of AC or CX on cleavage can only yield one molecule of retinol(1, Reference Tanumihardjo9). However, the RAE of BC-rich foods seem to depend more on how well BC is absorbed from food than on the reaction mechanisms that form VA(1, Reference Matsuda-Inoguchi, Date and Sakurai8). Therefore, we hypothesised that the RAE of AC- and CX-rich foods would also depend primarily on how well these carotenoids were absorbed from food and retained in the body. Thus, comparing apparent bioavailability of AC, BC and CX from the diet would be useful for delineating the relative importance of major provitamin A carotenoids. We further hypothesised that a good indicator of how well the carotenoids are absorbed and retained in the body would be their concentrations in the blood.
AC is found in a limited number of orange vegetables such as carrots and pumpkin(4). Good food sources of AC also tend to be good food sources of BC(4). CX, on the other hand, is found primarily in orange fruits such as mandarins, oranges, peaches and chillies that are not good sources of BC(4, Reference Chadrika, Jansz and Wickranasinghe15). Since the most important food sources of CX differ from those of AC and BC, the absorption of CX from the diet may also differ. Herein we describe our investigations comparing dietary intakes of BC, CX and AC to their serum concentrations to determine the relative bioavailability of these carotenoids from the diet. Three studies were conducted in our own laboratory. In addition, we identified and analysed data from other recent (post 2000) human nutrition studies that reported both dietary intakes and concentrations of AC, BC and CX in the blood.
Experimental methods
Chemicals and supplies
Retinyl palmitate, retinol, retinyl acetate, AC and BC standards were purchased from Sigma Aldrich Chemical Company (St Louis, MO, USA). CX standard was purchased from Chromadex (Irvine, CA, USA). Purities of BC and CX standards were checked by HPLC with diode array detection and were found to be 95 and 94 %, respectively. All other chemicals and solvents were obtained from Sigma, Fisher Scientific (Pittsburgh, PA, USA) or J.T. Baker (Phillipsburg, NJ, USA). All the chemicals were reagent or HPLC grade.
Human studies
We conducted three human nutrition studies that derived dietary intake estimates and serum concentrations of AC, BC and CX between 1996 and 2006(Reference Burri, Nguyen and Neidlinger11, Reference Dixon, Burri and Neidlinger16, Reference Burri and Neidlinger17). All the studies were conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board of the University of California Davis. Written consent was obtained from all the subjects. The main purpose of these studies was to test methods for improving dietary analysis(Reference Burri, Nguyen and Neidlinger11, Reference Dixon, Burri and Neidlinger16) or to investigate antioxidant status in people with disabilities(Reference Burri and Neidlinger17). The study plans and demographic characteristics of the volunteers for these studies were published elsewhere(Reference Burri, Nguyen and Neidlinger11, Reference Dixon, Burri and Neidlinger16, Reference Burri and Neidlinger17). All the participants were healthy adult volunteers, whose age ranged from 18 to 69 years. Subjects in these studies could consume a daily multivitamin supplement containing up to 2000 IU (1·2 mg) BC, but could not consume carotenoid supplements in higher dosages or megavitamins. In the case of Dixon et al. (Reference Dixon, Burri and Neidlinger16), dietary information was re-analysed in 2001, after more carotenoid information was added to the United States Department of Agriculture database. The carotenoid data herein are presented for the first time.
Dietary intake estimates
Dietary intakes were estimated for 6 months before the study by the Block FFQ (Block Dietary Systems, Berkeley, CA, USA; HHHQ, Scantron version 98.2, Scantron Corporation, Santa Ana, CA, USA)(Reference Coates, Eley and Block18). Food frequency estimates were collected on the same day as the blood draw. Food records (3 d) were collected on two non-consecutive week and one weekend day, and were analysed with Nutrition Data System for Research (NDS-R) software 4.02 (http://www.ncc.umn.edu/products/ndsr.html)(Reference Burri, Nguyen and Neidlinger11). These dietary intake estimates cover different amounts of time. Food records from all but three subjects were collected within 3 weeks after the blood draw and covered a 1-week reporting period. The FFQ covers 6 months of usual intake before the blood draw. Thus, the 3 d record reflects short-term intake, while the food frequency reflects long-term intake.
HPLC
Carotenoids from human plasma or serum were extracted as described previously(Reference Burri, Nguyen and Neidlinger11, Reference Dixon, Burri and Neidlinger16, Reference Burri and Neidlinger17). Following an overnight fast, 15–70 ml of blood (depending on the study) were collected from an antecubital vein. Plasma was harvested within 3 h of collection and stored at − 80°C until use. Plasma samples (500 μl) were mixed with 1 ml of 95 % aqueous ethanol containing 5 mg/l butylated hydroxytoluene, vortexed for 60 s and then mixed with an equal volume of hexane. The hexane layer was removed, and the extraction was repeated. The combined hexane layers were dried under a stream of nitrogen. Finally, the sample was re-suspended in 100 μl solvent buffer.
HPLC analysis was performed with an Agilent 1100 gradient chromatograph (Waldbronn, Germany). Data analyses were carried out using a HP Chemstation for LC 3D revision A.08.03 (847) for Agilent Technologies (Hewlett-Packard, Waldbronn, Germany).
Human plasma samples were run by a reversed-phase binary gradient method(Reference Burri, Dopler-Nelson and Neidlinger19). Solvent A contained acetonitrile–tetrahydrofuran–methanol–ammonium sulphate (85:5:5:5, by vol.), and solvent B contained acetonitrile–tetrahydrofuran–methanol–ammonium sulphate (55:35:5:5, by vol.). HPLC was run at 1·0 ml/min on a Prodigy 5 micron C18 250 × 4·6 mm column (Phenomenex, Torrance, CA, USA). Total run time was 48 min. The run was 0–10·0 min, 5 % solvent B; 10·0–29·0 min increasing linearly to 95 % solvent B; 29·0–35·9 min, maintaining 95 % solvent B; 35·9–36·0 min, abruptly decreasing to 60 % solvent B; maintaining 60 % solvent B from 36·0 to 44·9 min; then abruptly decreasing to 5 % solvent B at 45 min. The column re-equilibrated with 5 % solvent B from 45·0 to 48·0 min. Retinyl acetate (measured at 325 nm) was used as the internal standard. This method separates α-cryptoxanthin and CX, and all trans-BC and cis-BC. The BC and CX data reported are, respectively, for BC and CX alone, not in combination with their isomers (α-cryptoxanthin and cis-BC, respectively).
Literature review and analysis of studies
We searched reference databases Medline, Food Science and Technology Abstracts and Agricola between October 2008 and April 2010 to identify studies that reported dietary intakes and concentrations of AC, BC and CX in the blood. Literature searches were limited to human observational studies published subsequent to 2000, after more carotenoid concentrations had been added to the United States Department of Agriculture Nutrient Database for Standard Reference. Key words used for carotenoids were ‘caroten*’, ‘cryptoxanthin’, ‘RN = 7235-40-7’, ‘RN = 7488-99-5’ and ‘RN = 472-70-8’. Key words for dietary intake were ‘dietary intake’, ‘diet’, ‘food frequency’ or ‘food record’. Key words for blood were ‘blood’, ‘serum’ or ‘plasma’. Keywords for human were ‘human*’ or ‘subject*’. The articles retrieved were hand searched to retrieve other relevant articles. A study was included if it met all of the following criteria: it was a human study published in English as a full-length study in a peer-reviewed journal; it reported mean or median carotenoid concentrations in the blood and total dietary intake estimates (as opposed to the intake of major foods or food groups) for AC, BC and CX. We found twelve studies that met these criteria.
Mathematical analysis
All dietary intake estimates were converted into μmol carotenoid/d. All the blood (serum or plasma) concentrations were converted into μmol/l. Then, carotenoid concentrations in the blood were divided by dietary intakes of that carotenoid to estimate the amount of carotenoid absorbed and circulated (bioavailable carotenoid (bio)) in the blood (e.g. ACblood/ACdiet = ACbio). Since BC is the major carotenoid source of VA, we used it as our standard of comparison. Comparisons of bioavailability are given as the ratios of ACbio/BCbio and CXbio/BCbio. Mean ratios of ACbio/BCbio and CXbio/BCbio were calculated by averaging the ACbio/BCbio and CXbio/BCbio ratios derived for each subpopulation.
Descriptive statistics are presented as means with their standard errors and 99 % CI. Results from the studies were reviewed for their suitability for meta-analysis(Reference Norman20). However, all of these studies are observational, most are small, and the subject selection criteria and analysis methods diverse. Furthermore, none of the studies in this report was a planned comparison of the bioavailability of AC, BC and CX from the diet. We concluded that formal meta-analysis of the data was premature. We were able to examine the relationships between carotenoid concentrations in the blood and diet using the Student's t test, ANOVA and linear regression. All the statistical comparisons were two-sided. Statistics were calculated with Sigmaplot (Systat Software, Inc., San Jose, CA, USA).
Results
Dietary intakes
Dietary intakes of carotenoids estimated by food record (mean or median when noted) are reported in Table 1. Results for dietary intake estimates calculated from FFQ are shown in Table 2 (for our laboratory data) and Table 3 (for literature data). Data are listed by the subpopulations studied in the orginal reports (for example, men and women; normal weight, overweight and obese).
Table 1 Mean dietary intakes estimated from food records v. mean concentrations of α-carotene (AC), β-carotene (BC) and β-cryptoxanthin (CX) in the blood
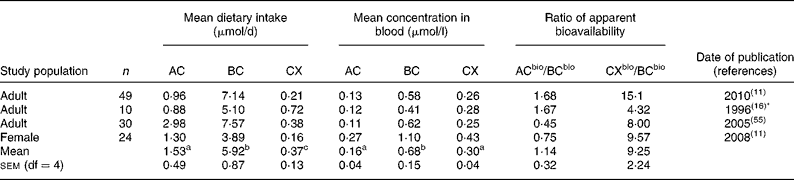
bio, Bioavailable.
a,b,c Superscript letters indicate significant differences between mean dietary intakes or between mean carotenoid concentrations in the blood (P < 0·05).
* Dietary information re-analysed 2001.
Table 2 Mean dietary intakes estimated from FFQ v. mean concentrations of α-carotene (AC), β-carotene (BC) and β-cryptoxanthin (CX) in the blood
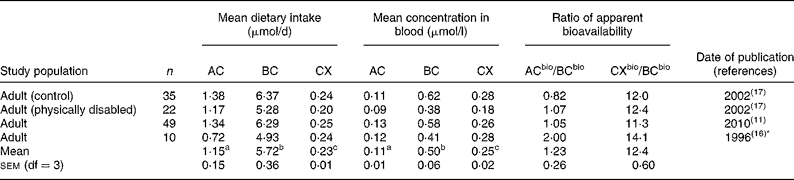
bio, Bioavailable.
a,b,c Superscript letters indicate significant differences between mean dietary intakes or between mean carotenoid concentrations in the blood (P < 0·05).
* Dietary information re-analysed 2001.
Table 3 Mean dietary intakes estimated from FFQ v. mean concentrations of α-carotene (AC), β-carotene (BC) and β-cryptoxanthin (CX) in the blood (from literature)

bio, Bioavailable.
a,b,c Superscripts letters indicate significant differences between mean dietary intakes or between mean carotenoid concentrations in the blood, P < 0·05.
* Converted from mg/d per 1000 kcal.
† Medians, converted from mg/d.
One subpopulation found in our literature search (females with osteoporosis)(Reference Yang, Zhang and Penniston21) was an outlier, as determined by its z score. This population had very high dietary intakes of VA and BC, and considerably had higher ACbio and BCbio values than any other subpopulation. Osteoporosis is linked to high VA intakes(Reference Penniston and Tanumihardjo22), and these high intakes or the medications that this population takes appear to have influenced their carotenoid absorption or metabolism. If this subpopulation was included in our calculations, none of the present results would differ significantly from those we report.
Mean dietary carotenoid intakes estimated by 3 d food records ranged from 0·88 to 2·98, 3·89 to 7·57 and 0·16 to 0·72 μmol/d for AC, BC and CX, respectively (Table 1). These were generally somewhat lower than mean dietary intakes estimated by food frequency, which ranged from 0·27 to 2·31, 3·12 to 9·80 and 0·18 to 0·89 μmol/d for AC, BC and CX, respectively (Tables 2 and 3). A comparison of mean dietary intakes (Tables 1–3) for the subpopulations shows that intakes of BC were always greater than the intakes of AC (P = 0·00 001), which in turn were usually greater than the intakes of CX (P = 0·00 001).
Mean dietary intakes of AC and BC in these subpopulations were positively correlated with each other (r 2 0·48, P = 0·0001). Mean dietary intakes of CX were not correlated with either AC or BC (r 2 0·01 and 0·12, respectively). Because of the small number of reports for each subpopulation, no conclusions could be drawn on differences in subpopulations, though men usually had higher mean carotenoid intakes than women did (P = 0·01).
Carotenoid concentrations in the blood
Mean carotenoid concentrations in the blood are also shown in Tables 1–3. Mean concentrations of AC, BC and CX in the blood ranged from 0·06 to 0·27, 0·20 to 1·10 and 0·09 to 0·43 μmol/l, respectively. Mean concentrations of BC were always greater than mean concentrations of CX (P < 0·00 001) or AC (P < 0·00 001) measured in the same study population. Mean concentrations of CX were greater than concentrations of AC in all but three of twenty-eight subpopulations (P < 0·00 001). Mean carotenoid concentrations in the blood in these studies (Tables 1–3) were well within the reported US ranges of 0·02–0·47, 0·04–2·26 and 0·03–0·70 μmol/l for AC, BC and CX, respectively(1, Reference Ford and Giles5).
Mean carotenoid concentrations in the blood were correlated with each other, so that a subpopulation that had high concentrations of BC also tended to have high concentrations of AC and CX. The correlations between AC and BC, AC and CX, and BC and CX were r 2 0·41, P < 0·0002; r 2 0·39, P = 0·0012 and r 2 0·57, P = 0·00 001, respectively.
Comparing carotenoid bioavailability
Tables 1–3 compare the impact of dietary intakes on concentrations of carotenoids in the blood. The ratios of ACbio/BCbio vary from study to study. However, ratios are quite consistent, being>1 in all but five of the twenty-eight comparisons (Fig. 1). The mean ratio of ACbio/BCbio was 1·53 (99 % CI 1·23, 1·83). Thus, eating comparable amounts of AC- and BC-rich foods resulted in 53 % higher AC concentrations in the blood.
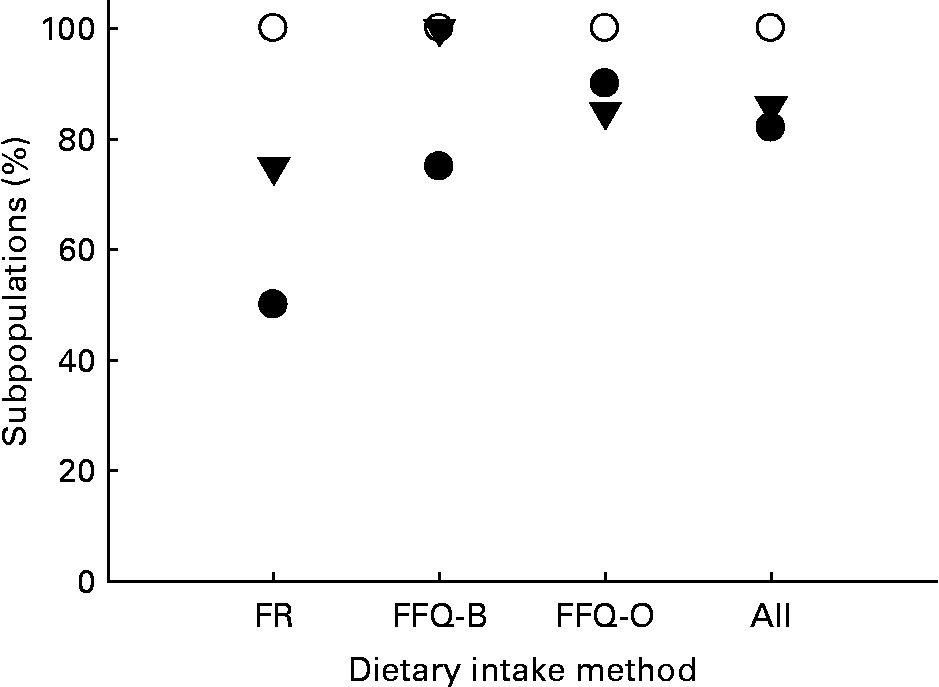
Fig. 1 Percentage of subpopulations with ratios of α-carotene (AC)bioavailable (bio)/β-carotene (BC)bio or β-cryptoxanthin (CX)bio/BCbio >1, grouped by the methods used to estimate dietary intake. Dietary intake methods: FR, food record; FFQ-B, FFQ measured in Burri's laboratory; FFQ-O, FFQ measured in other laboratories; All, combined data, for either dietary methods. ●, ACbio/BCbio>1; ○, CXbio/BCbio>1; ▾, CXbio/BCbio>5.
Even more remarkably, the ratios of CXbio/BCbio were always>1, and in all but four out of the twenty-eight comparisons, the ratio of CXbio/BCbio was>5 (Fig. 1). The mean ratio of CXbio/BCbio was 8·25 (99 % CI 6·35, 10·15). Thus, eating comparable amounts of CX- and BC-rich foods resulted in 725 % greater CX concentrations in the blood. In a single study of adipose tissue(Reference Chung, Ferreira and Epstein23), the ratio of ACbio/BCbio was 1·32, and the ratio of CXbio/BCbio was 7·0, similar to the mean ratios obtained for blood.
We observed few consistent differences in the ratios of ACbio/BCbio or CXbio/BCbio between population subgroups, probably because of the small number of studies available for our analysis, and the variability created by differences in the test methods and populations of these studies.
Discussion
Mean dietary intakes and concentrations of AC, CX and BC in the blood varied widely from study to study. Despite this variability, several relationships between carotenoid concentrations in the subpopulations could be observed. Mean dietary intakes and concentrations of BC in the blood were always greater than those of AC or CX in the studies reported herein, which appears to be typical worldwide(1, Reference Olson, Shils, Olson and Shike3, Reference Ford and Giles5, Reference Matsuda-Inoguchi, Date and Sakurai8). Mean dietary intakes of AC and BC in the subpopulations that we studied were positively correlated with each other, which was not surprising since these carotenoids are often found in the same foods(4). Mean dietary intakes of CX were not correlated with either AC or BC, which again was expected since the most important food sources of CX are different than the most important food sources of AC and BC(4). Mean concentrations of all carotenoids in the blood correlated positively with each other. Therefore, the subpopulations that had high concentrations of BC in their blood usually had high concentrations of AC and CX as well. Few studies have compared AC, BC and CX concentrations in the blood of regionally or culturally distinct populations, but these studies also suggest that populations that have higher BC concentrations also tend to have higher AC and CX concentrations(Reference Stimpson and Urrutia-Rojas24, Reference Al-Delaimy, van Kappel and Ferrari25).
ACbio/BCbio and CXbio/BCbio ratios varied between studies. This is not surprising, since these studies were conducted in different populations by different laboratories, using different dietary and HPLC analysis methods. Despite this, these results show that the ratio of ACbio/BCbio may be>1, while the ratio of CXbio/BCbio appears to be>5 (Fig. 1). Overall, the results from our laboratory studies and the literature suggest that eating comparable amounts of AC- and BC-rich foods in mixed diets would result in about 53 % greater AC concentrations in the blood (Tables 1–3; 99 % CI 23, 83 %, P = 0·00 004). Results obtained for adipose tissue(Reference Chung, Ferreira and Epstein23) were similar, with a 32 % greater AC concentration. The results for CX are even more striking. These results suggest that comparable dietary intakes of BC and CX from mixed Western diets would result in 7-fold (99 % CI 5·3, 9·1, P = 0·00 001) greater concentrations of CX in the blood. Again, results for adipose tissue are similar, since the mean ratio of CXbio/BCbio in adipose was 7·0(Reference Chung, Ferreira and Epstein23). Thus, the apparent bioavailability of CX from the diet is greater than BC.
There are two methodological reasons that could account for the somewhat higher apparent bioavailability of AC from the diet. First, AC concentrations in diets may be poorly reported and thus underestimated. The difficulties in estimating dietary intakes are well known(Reference Block26, Reference Bingham27). AC concentrations might be more under-reported than BC concentrations because AC is found in smaller concentrations, in fewer foods. Secondly, the food composition data for AC- and BC-rich food may not reflect their current nutrient content well. The number of foods and plant varieties is immense and evolves with time. Furthermore, carotenoid concentrations in plants vary with growing conditions, handling, storage, cooking practices and plant variety, making nutrient intakes from dietary intakes difficult to estimate(4, Reference Chadrika, Jansz and Wickranasinghe15, Reference Maiani, Castón and Catasta28). If the carotenoid concentrations of an AC-rich food such as carrots increased with time because of the improvements in plant varieties, it is possible that the real AC concentrations in foods are higher than the concentrations reported by the United States Department of Agriculture database.
However, the difference in apparent bioavailability between AC- and BC-rich foods in the diet could be caused by physiological mechanisms. First, dietary intakes of AC are usually much lower than intakes of BC(1, Reference Olson, Shils, Olson and Shike3, Reference Ford and Giles5) (Tables 1–3). There is some evidence that lower concentrations of carotenoids are absorbed better than higher concentrations of carotenoids from food. If this were so, it could account for the difference in apparent bioavailability between AC- and BC-rich foods(Reference Diwadkar-Navsariwala, Novotny and Gustin29, Reference During, Hussain and Morel30). However, most reports have found little or no effect of carotenoid concentration on absorption(Reference Castenmiller and West31–Reference Chen, Oace and Wolf35). Overall, there is little evidence of a strong concentration effect on AC or BC absorption, except during high pharmacological intakes.
Secondly, AC might circulate longer in the blood than BC. This is likely because BC is preferentially cleaved to form VA by both carotenoid cleavage enzymes CMO1 and CMO2(Reference Hessel, Eichinger and Isken6, Reference Kiefer, Hessel and Lampert7). Data from carotenoid depletion studies support the possibility that AC circulates in the blood longer than BC. These results suggest that concentrations of all carotenoids decreased in a similar way by first-order kinetic processes. Differences in carotenoid circulation time (t 1/2) were not large(Reference Burri, Neidlinger and Clifford36); however, the t 1/2 of carotenoid disappearance from the blood was 45 d for AC, 37 for BC and 39 for CX. This difference in half-life probably accounts for the differences in apparent bioavailability that we observed between AC and BC.
However, AC may really be absorbed better than BC from mixed diets. Although BC is available in dietary supplements that are absorbed well(1, Reference Van Loo-Bouwman, West and van Breeman37), it is also found in a variety of foods including green leafy vegetables, which have poor biovailability(1, Reference de Pee, West and Permaesih10, Reference Maiani, Castón and Catasta28, Reference O'Connell, Ryan and O'Brien38). Thus, the overall absorption of AC from the diet might be greater than that of BC. Further research on the current concentration of carotenoids in foods, their absorption and circulation in the body and their interactions with CMO1 and CMO2 is necessary to determine whether the bioavailability of AC is better than that of BC from mixed diets.
The 7-fold difference between the apparent bioavailability of CX and BC in mixed diets is more difficult to explain as a product of methodological issues, even though artefacts associated with dietary assessment, and nutrient composition of foods could occur(Reference Castenmiller and West31–Reference Chen, Oace and Wolf35). As with AC and BC absorption, there is little evidence of a strong concentration effect on CX absorption(Reference Castenmiller and West31–Reference Chen, Oace and Wolf35). Finally, it is also unlikely that the small differences in circulation time(Reference Burri, Neidlinger and Clifford36) between CX and BC (37 and 39 d, respectively) could account for this large difference in apparent bioavailability.
Another explanation for the apparently high bioavailability of CX from the diet is that CX might be formed from BC. However, the metabolism of BC has been studied by radioisotope(Reference Burri and Clifford39, Reference Ho, de Moura and Kim40) and cell culture methods(Reference During and Harrison41), and these reports do not suggest that CX is a major metabolite of BC. Furthermore, the correlation between dietary intake estimates and serum concentrations of CX is no worse than that of other carotenoids(1, Reference Burri, Nguyen and Neidlinger11), as it might be if significant amounts of CX in the blood were actually being formed from BC. Thus, it appears that CX absorption from its major dietary sources must be high, compared with AC and BC.
Few studies have compared CX, BC and especially AC absorption from foods under controlled conditions. Studies in human subjects suggest that CX is very well absorbed(Reference Breithaupt, Weller and Wolters42), while studies in the preruminant calf model suggest that AC is absorbed similarly to BC(Reference Bierer, Merchen and Erdman43). Cell culture studies simulating carotenoid digestion are contradictory, suggesting that CX can be digested with similar(Reference Liu, Glahn and Liu44), greater(Reference O'Sullivan, Ryan and O'Brien45) or lesser(Reference Thakkar and Failla46) efficiency than BC, probably depending on the specific food chosen and on reaction conditions.
Other studies have investigated how bioefficacious CX or AC are in maintaining VA status, which probably depends mainly on how well the carotenoid is absorbed and retained, as well as on how well it functions as a substrate for the carotenoid cleavage enzymes CMO1 and CMO2. BC is clearly the preferred substrate for CMO1 and CMO2, but these enzymes also have significant activities with AC and CX(Reference Lindquist and Andersson47, Reference Kim, park and Oh48). There are few studies on the bioefficacy of AC, and most of the studies on the bioefficacy of CX were conducted with purified chemicals and used in inappropriate animal models such as rats, mice or chickens(Reference John, Kishor and Subbarayan2, Reference Deuel, Meserve and Johnston49, Reference Greenberg, Chatterjee and Calbert50). These studies estimated that CX was 56 and 22 % as bioactive as BC in the rat(Reference John, Kishor and Subbarayan2, Reference Deuel, Meserve and Johnston49), and 55 or 98 % as active in the chick(Reference Greenberg, Chatterjee and Calbert50, Reference Johnson, Swick and Baumann51). However, their results appear similar to the two reports that have compared the efficacy of VA formation by BC and CX in an accepted animal model of human carotenoid metabolism, the Mongolian gerbil, which estimated efficacies of over 90 %(Reference Davis, Jing and Howe14, Reference Mills, Tumuhimbise and Jamil52). A single study investigated the bioefficacy of AC in the Mongolian gerbil and found it to be half as effective as BC(Reference Tanumihardjo and Howe53).
A caveat about our research is that BCbio is probably increased by vitamin supplement intake, since many vitamin supplements contain BC in a highly bioavailable form. It is not possible for us to estimate the influence of vitamin supplement intakes on BCbio because many of the studies that we surveyed do not estimate vitamin supplement intake. Furthermore, the intake of vitamin supplements is restricted in many nutrition studies. For example, the subjects in our studies could consume no more than 1·2 mg BC/d as vitamin supplements, and only 22 % of our study population consumed any vitamin supplements.
Further studies are needed on the absorption of AC and CX from foods, and on their metabolism in the body. However, it appears that CX might have 7-fold greater bioavailability from its primary food sources than do AC or BC. Although the efficacy of CX in forming VA in humans is not established, animal model studies suggest that it is between 22 and 98 % as active as BC. These results, taken together, suggest that CX-rich foods may be a better source of VA than currently assumed. This is especially interesting, because the citrus fruits that are rich in CX are often consumed as whole fruit, instead of in mixed dishes with fat. The absorption of most carotenoids, including BC, is greatly improved by incorporating fat in the diet(Reference Mills, Tumuhimbise and Jamil52, Reference Brown, Ferruzzi and Nguyen54). Most VA deficiency occurs in populations who also have little fat in their diet(1, Reference Olson, Shils, Olson and Shike3), so if CX is absorbed well without fat, then CX-rich foods might be especially useful for dietary interventions to prevent VA deficiency.
Acknowledgements
This work was supported by the United States Department of Agriculture and the Paralyzed Veterans of America (Spinal Cord Research Grant 1803). There are no conflicts of interest. B. J. B. designed the research, analysed the data, wrote the paper and had primary responsibility for the final content. T. R. N. and J. S. T. C. conducted the research. All the authors read and approved the final manuscript.






