Dietary recommendations of the World Strategy for populations( 1 ) recommend the consumption of whole grain (WG). The beneficial effects of WG on health are owing to fibre, micronutrients and phytochemicals present in the outer layer of the grain and germ. Epidemiological studies suggest that the consumption of WG reduces mortality from CVD, which is probably related to its antioxidant (AO) properties( Reference Awika and Rooney 2 ), as WG is an important source of AO( Reference Miller, Rigelhof and Marquart 3 ). There has been an increased interest in finding natural AO from plants to scavenge free radicals to be effective in the treatment of oxidative damage. Polyphenols from WG can protect against reactive O2 species-mediated damage by elevating cellular AO defence, showing increased superoxide dismutase (Cu/Zn SOD), glutathione peroxidase (GPx) and catalase (CAT) levels( Reference Lee, Han and Song 4 ). Expression of these AO enzymes is mediated by the nuclear transcription factor (erythroid-derived 2)-like 2, a key factor regulating genes that encode AO and detoxifying enzymes. WG also have both fermentable and non-fermentable fibre. Consumption of different types and amounts of dietary fibre can influence the production of bacterial enzymes with toxicological significance, such as mucinase, β-glucosidase and β-glucuronidase( Reference Goñi, Jiménez-Escrig and Gudiel 5 , Reference Kosmala, Zduńczyk and Kołodziejczyk 6 ). Dietary fibre with polyphenols not absorbed in the small intestine reaches the colon and can be fermented, modifying the production of certain microbial metabolites( Reference Manach, Scalbert and Morand 7 ) and SCFA, which can significantly increase the biomass, reduce the luminal pH, change the composition of flora and modify the gastrointestinal epithelial kinetic pattern( Reference Kaczmarczyk, Miller and Freund 8 ).
Sorghum is a good source of phytochemical compounds and fibre that have been associated with AO, anti-inflammatory and antiproliferative capacities( Reference Van Hung 9 ). The AO activities of whole-grain sorghum are higher than those reported in other cereals, such as rice, wheat and oat( Reference Soong, Tan and Leong 10 ), which suggests that sorghum is a good source of natural AO. Sorghum grains can contain substantial levels of a wide range of phenolic compounds, mostly flavonoids, and only some varieties with pigmented testa have condensed tannins( Reference Dykes and Rooney 11 ). Thus, different varieties of sorghum can be roughly divided into two categories: tannin-free sorghums, such as white or red sorghum, and tannin sorghums, such as brown sorghum. Phenolic acids are mainly found in pericarp, testa, aleurone layer and endosperm( Reference Hahn, Rooney and Earp 12 ). Free phenolics are found in the outer layers (testa, pericarp and aleurone), whereas bound ones are associated with the cell wall( Reference Hahn, Rooney and Earp 12 – Reference Yu and Tuinstra 14 ). Phenolic compounds could be negative in terms of reducing starch, protein and mineral digestibility( Reference Stefoska-Needham, Beck and Johnson 15 ). However, recently, phenolic compounds have gained increased interest because of their AO activity. The most abundant phenols in sorghum are caffeic acid, coumaric, ferulic and sinapic( Reference Hahn, Faubion and Rooney 13 , Reference Verbruggen, Beldman and Voragen 16 ). The most common anthocyanins in sorghums are the 3-deoxyanthocyanidins, which include orange luteolinidin and yellow apigeninidin( Reference Hahn, Faubion and Rooney 13 , Reference Verbruggen, Beldman and Voragen 16 ). On the other hand, sorghum has been known to be a slowly digestible cereal. Bran from black and brown sorghum is known to alter rat faecal SCFA concentrations, which suggests possible changes in the intestinal microbiota and epithelial barrier integrity( Reference Turner, Taddeo and McDonough 17 , Reference Lemlioglu-Austin, Turner and McDonough 18 ). Furthermore, secondary sorghum metabolites from polyphenols are reported to modify differentially luminal bacterial populations and induce systemic effects in vitro and in animal pathological models( Reference Rastmanesh 19 ). Thus, because of its low starch digestibility, high polyphenolic levels and AO capacity, WG sorghum has been recognised for the development of health functional foods for chronic disease risk protection.
Processing and cooking conditions do influence sorghum nutrient bioavailability. Extrusion is a high-temperature−short-time process used widely in the production of a variety of foods such as snack, ready-to-eat cereals, textured vegetable protein, confectioneries and pet foods. It has been shown that extrusion of WG sorghum can lead to enhanced mineral bioavailability and protein digestibility( Reference Llopart, Drago and De Greef 20 ). Results of recent studies indicated that the extrusion of sorghum improves the bioavailability of catechins, total phenolics and cinnamic acid( Reference Gu, House and Rooney 21 ), which positively affected in vitro AO and anti-inflammatory capacity( Reference Salazar López, Loarca-Piña and Campos-Vega 22 ). Thus, extruded sorghum could be considered as a viable commercial source of AO and be incorporated as a functional food ingredient. Currently, direct proof of health-enhancing effects of sorghum is lacking as most studies have been carried out on the grains or grain extracts, and not extruded grain itself, and also little research works have been performed in vivo. Currently, little information about the effects of consumption of extruded sorghum on the lipid profile, the AO activity and its impact on the gastrointestinal tract (GIT) is available.
The aim of this study was to evaluate the effect of consumption of white and red whole-grain sorghum precooked by extrusion on systemic, hepatic and colonic oxidative status using a growing Wistar rat model. In addition, its impact on modification in the crypt population of the colonic mucosa has been evaluated.
Methods
Raw materials and ingredients
Two commercial sorghum (Sorghum spp) hybrids, white and red, were evaluated. They were free of condensed tannins according to discolouring chlorine test. Grains were ground in a roll mill (Buhler MiagBUA AG) according to a milling diagram, which allows obtaining whole-grain grits with adequate particle size for extrusion (between 1920 and 420 µm), with <1 % fine fraction (below 420 µm).
Extrusion was carried out using a 20 DN Brabender single-screw extruder at the following conditions: 4:1 screw compression ratio, 3×20 mm (diameter–length) die and 150 rpm screw speed, 182°C temperature and 14 g water/100 g grits moisture.
Animals and diets
In all, twenty-four male Wistar rats (43·0 (sem 4·5) g) were obtained from the Animal Service Laboratory, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires. Throughout the experiment, animals were allowed free access to deionised water and food, and were housed in individual stainless steel cages in a temperature (21±1°C)- and humidity (60±10 %)-controlled room with a 12 h light–12 h dark cycle. They were fed the following diets (n 8/group) during a 60-d period (Table 1):
-
(1) control group (C): in this group, rats were fed a semi-synthetic diet prepared according to the American Institute of Nutrition Diet (AIN 93) having 5 g of cellulose/100 g diet;
-
(2) extruded white sorghum group (EWS): in this group, rats were fed the AIN 93 containing 5 g of fibre per 100 g of diet from extruded whole-grain white sorghum; and
-
(3) extruded red sorghum group (ERS): in this group, rats were fed the AIN 93 containing 5 g of fibre per 100 g of diet from extruded whole-grain red sorghum.
Table 1 Composition of control (C), extruded white sorghum (EWS) and extruded red sorghum (ERS) diets
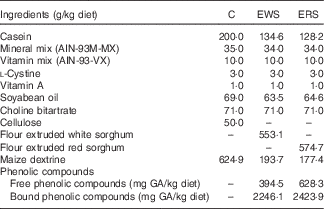
GA, gallic acid.
The analysis of the diets confirmed that they were isoenergetic and supplied a similar amount of macronutrients. The combination of nutrients used in this animal study could reasonably be expected to be achieved in the human population.
This study was carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Committee of Health Guide for the Care and Use of Laboratory Animals of the Facultad de Bioquímica y Farmacia, Universidad de Buenos Aires.
Sampling procedures
Body weights (BW) were recorded once a week and food intakes every 3 d throughout the experience. Total intakes were calculated. Feed efficiency of diets was determined as the relationship between weight gained by the animal and food consumed – that is weight gain/food intake.
At the end of the experience, rats were anaesthetised with an intraperitoneal injection of 0·1 mg/100 g BW of ketamine hydrochloride, with 0·1 mg/100 g BW of acepromazine maleate. An abdominal incision was made; blood was withdrawn from the abdominal aorta and centrifuged at 3000–3500 rpm for 20 min at 4°C. Serum samples were stored at −80°C and examined within the following 3 d. The liver was excised, weighed, divided in small portions and stored at −80°C for analysis. The caecum was removed and weighed (Mettler Toledo). Caecal content was collected and weighed. After measuring its pH with a pH-metre (IQ Scientific Instruments), it was diluted 1/4 with PBS buffer (0·01 mol/l, pH 7·3), vortexed and centrifuged at 3000 g for 20 min. Caecal enzymes were analysed in these samples. The activities of selected bacterial enzymes (β-glucosidase and β-glucuronidase) released into the caecal environment were measured according to the rates of p- and o-nitrophenol release from their nitrophenylglucosides( Reference Djouzi and Andlueux 23 ). Protease inhibitor (P8340; Sigma) was added to an aliquot of this supernatant for further analysis of secretory IgA (sIgA) using kit. All samples were stored in a freezer at −80°C for later analysis. The colon section was dissected. Sections (2 cm) of colon (proximal and distal) were routinely processed and paraffin embedded. Sections were cut, stained with haematoxylin–eosin (H&E) and processed for immunohistochemical staining.
Analytical methods
TAG and cholesterol
Pieces of 250 mg of liver were extracted with chloroform–methanol (2:1) mixture( Reference Folch, Lees and Sloane Stanley 24 ). Aliquots were evaporated and cholesterol and TAG were analysed using enzymatic methods (commercial kits: ColestatEnzimático and TAG Color GPO/PAP ascorbic acid; Wiener Lab, respectively). Serum cholesterol and TAG were determined using the kits mentioned before.
Proteins and polyphenols
Protein content was determined according to the method of Lowry et al.( Reference Lowry, Rosebrough and Farr 25 ) using bovine serum albumin as standard (A7906; Sigma Aldrich).
A double extraction was used to extract free (FPC) and bound phenolic compounds (BPC) from sorghum diets( Reference Qiu, Liu and Beta 26 ). Then, phenolics were determined by the Folin–Ciocalteu( Reference Singleton, Orthofer and Lamuela-Raventos 27 ) method using gallic acid (GA) as standard. Total phenolic compounds (TPC) was calculated as the sum of FPC and BPC.
Thiobarbituric acid reactive substances assay
The assay proposed by Buege & Aust( Reference Buege and Aust 28 ) with some modifications was performed to measure thiobarbituric acid (TBA) reactive substances in serum and liver as estimation of lipid peroxidation. In brief, 0·5 g of liver was homogenised with 5 ml of 30 mmol/l phosphate buffer (pH 7·4) using a homogeniser (PRO250-Homogenizer; PRO Scientific). An aliquot of liver homogenate was combined with BHT in ethanol (0·4 g/l) and TCA (200 g/l) and the mixture was centrifuged for 10 min at 5000 g . The supernatant was combined in a 1:1 ratio with TBA (7 g/l) and the mixture was heated at 100°C for 1 h. The absorbance was measured at 535 nm in the spectrophotometer (Genesys 5; Milton Roy). The results were expressed as malondialdehyde equivalent (MDA eq) (nmol/100 g protein) using MDA molar extinction coefficient (1·56×105 litres/mol per cm). The same method was used for serum and the results were expressed as MDA eq (nmol/g protein).
Catalase activity
Hepatic CAT was analysed according to Aebi( Reference Aebi 29 ). In brief, 0·5 g of liver was homogenised with 5 ml of 50 mmol/l phosphate buffer (pH 7–7·4) and centrifuged for 5 min at 8000 g . Aliquots of the supernatant were diluted 1/400 with phosphate buffer, added with 30 mmol/l H2O2 solution and shaken vigorously. The decrease in absorbance at 240 nm during 1 min was measured using the spectrophotometer. CAT activity was calculated using the slope of the absorbance v. time (s) curve in a logarithmic scale (log 10). Results were expressed as μmol/s per g protein using H2O2 molar extinction coefficient (0·0394 litres/mmol per cm).
Glutathione reductase and glutathione peroxidase
Glutathione reductase (GR) activity was analysed according to Horn( Reference Horn 30 ). A piece of liver was mixed with 30 mmol/l phosphate buffer (pH 7·4) with 1 mmol/l EDTA and 4 mmol/l dithiothreitol, homogenised on ice and centrifuged for 5 min at 10 000 g (Z160M; Hermle). An aliquot of liver homogenate was added with pH 7·6 phosphate buffer (100 mmol/l KH2PO4, 3·4 mmol/l EDTA), 30 mmol/l GSSG and 0·8 mmol/l NADPH. The reduction of GSSG to GSH by GR activity was measured every 30 s during 5 min in a plate reader at 340 nm (UVM 340; ASYS Hitech). Results were expressed as nmol NADPH/per min per mg protein using the slope of the Absorbance v. time (s) curve, NADPH molar extinction coefficient (3·732 nmol/ml), 0·6 cm length of multi-well plate, 190 µl final reaction volume and protein content of liver homogenate.
Liver GPx activity was measured according to Paglia & Valentine( Reference Paglia and Valentine 31 ) with some modifications. An aliquot of the same liver homogenate obtained for GR was diluted (1/100) and mixed with phosphate buffer, pH 7·0 (0·0828 mol/l KH2PO4, 0·0083 mol/l EDTA), 0·0028 mol/l NADPH, GR (451 U/ml), 0·075 mol/l NaN3 and 0·05 mol/l GSH. The mixture was equilibrated during 5 min at 20°C and the reaction begins with the addition of 0·0007 mol/l H2O2. The absorbance was measured every 30 s during 5 min at 340 nm. The activity was calculated using the slope of absorbance v. time (s) curve, NADPH molar extinction coefficient (4·354 µmol/l), 0·7-cm length of multi-well plate, 200 µl final reaction volume and protein content of liver homogenate. Results were expressed as nmol NADPH/min per mg protein.
GSSG and GSH
Liver GSSG and GSH contents were determined by the fluorometric method of Hissin & Hilf( Reference Hissin and Hilf 32 ) with modifications. A portion of liver was mixed with phosphate buffer, pH 8 (0·1 mol/l Na2HPO4, 0·005 mol/l EDTA), and an aliquot of 25 g/100 ml HPO3 solution, homogenised on ice and centrifuged at 10 000 g for 10 min. For GSSG assay, an aliquot of the supernatant was mixed with 0·04 ml of N-ethylmaleimide and incubated during 20 min at room temperature. To this mixture, 0·1 mol/l NaOH was added, shaken and an aliquot was used for reacting with 1 mg/ml O-phthaldialdehyde. After 15 min, the fluorescence at 420 nm was determined (excitation at 350 nm) using a fluorescence spectrophotometer (F2000; Hitachi). For GSH assay, an aliquot of the supernatant, phosphate buffer and 1 mg/ml O-phthaldialdehyde were incubated during 15 min at room temperature, and fluorescence was determined as mentioned above. GSSG and GSH contents were expressed as µg/g of liver.
Assessment of apoptosis
Apoptotic cells in colonic sections were detected using terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) procedure( Reference Gavrieli, Sherman and Ben-Sasso 33 ). In brief, colonic sections after deparaffinisation and rehydration were permeabilised with proteinase K (20 μg/ml) for 15 min at 37°C. Thereafter, the sections were quenched of endogenous peroxidase activity using 3 % hydrogen peroxide for 10 min. After thorough washing with PBS, the sections were incubated with equilibration buffer for 10 min and then terminal deoxynucleotidyl transferase reaction mixture was added to the sections, except for the negative control, and incubated at 37°C for 1 h. The reaction was stopped by immersing the sections in saline-sodium citrate buffer for 15 min. The incorporated biotinylated nucleotides were detected by streptavidin-horseradish peroxidase (HRP) (1:500) for 30 min at room temperature, and after repeated washings sections were incubated with diaminobenzidine (DAB) until colour development (5–10 min). The sections were then mounted after dehydration and counterstained with methyl green. The TUNEL labelling index (LI) (%) was calculated as the number of apoptotic cells×100/total number of cells per crypt column height. For the quantification of the TUNEL LI, at least fifty perpendicular well-oriented crypts were examined and counted for each animal at 400× magnification. These studies were performed using a Leica DM LB2 microscope and a digital Leica DFC 320 camera (Leica).
Immunohistochemical staining
Before immunostaining, the sections were deparaffinised, rehydrated and then treated in 3 % hydrogen peroxide in methanol to inhibit peroxidase activity. They were then boiled in a microwave oven in 0·01 m sodium citrate buffer (pH 6·0) for 20 min. To avoid background staining, blocking serum was derived from the same species in which the secondary antibody had been raised. After that, the sections were incubated with the following primary antibodies overnight at 4°C: mouse monoclonal anti-proliferating cell nuclear antigen (PCNA; PC-10) (1:50; Lab Vision Corporation and BionovaCientífica SL), rabbit polyclonal anti-cyclo-oxygenase 2 (COX-2) (1:200), rabbit polyclonal anti-inducible nitric oxide synthase (iNOS), rabbit polyclonal anti-Cu, Zn SOD (1:200), rabbit polyclonal anti-Mn SOD (1/200), anti-GPx-1 (1:200), anti-GR (1:200), anti-CAT (1:200) and anti-Nrf-2 (1/50) (Santa Cruz Biotechnology). After washing with PBS, the sections were covered for 30 min at room temperature with biotinylated goat anti-mouse or goat anti-rabbit (1:400) as secondary antibodies (Santa Cruz Biotechnology). Immunochemical staining was performed for 30 min using streptavidin–biotin-conjugated HRP (Sigma Aldrich) and visualised by incubation with 3, 3'-DAB (Sigma Aldrich) for 10 min at room temperature. The sections were counterstained with Harris’s haematoxylin, dehydrated and mounted. Brown colour indicates specific protein immunostaining and light blue colour indicates nuclear haematoxylin staining. Positive and negative controls were used during the optimisation of the methods.
For quantification of the PCNA LI %, at least 20 perpendicular well-oriented crypts were examined in each animal under light microscopy at 400× magnification. LI was calculated as the number of positive nuclei×100/total number of cells per crypt column height.
The staining intensity of epithelial COX-2, iNOS, SOD, CAT, GR and GPx expression was evaluated according to a semi-quantitative immuno-histochemical scoring system as follows: score 0–4: 0, none; 1, equivocal; 2, low; 3, moderate; 4 and above, intense. The number of nuclear Nrf-2-positive cells was quantified by a percentage score with grading between 0 and 4: 0, no nuclear staining; 1, 1–10 %; 2, 10–30 %; 3, 30–60 %; and 4, 60–100 % positive cells. An additional evaluation was performed in which the stained cells were attributed either to the basal, the middle or the luminal crypt compartments.
The crypt length was measured from H&E slices and was determined as a distance (μm) between the basal side of the lamina epithelialis at the bottom of the crypt and the apical side of the lamina epithelialis at the top of the crypt. Only crypts with an open longitudinal crypt axis were analysed.
Data analysis
Data were presented as the arithmetic means with their standard errors for each treatment group (n 8). One-way ANOVA was performed and the statistical differences among samples were determined using least significant difference test. Significance was accepted at P<0·05.
The data obtained of the semi-quantitative immunohistochemical staining were comparatively analysed using the Kruskal–Wallis test. Multiple comparisons were made through the decomposition of the interaction by applying Tukey’s test. The level of significance was set to 5 % (P<0·05) on all tests. Results were expressed as means with their standard errors. All statistical analyses were performed with SPSS 17.1 for Windows (SPSS).
Results and discussion
Effects of sorghum diets on food intake and weight gain
Animals fed EWS and ERS diets had lower total intakes than C diet (C: 1115·62b (sem 16·62), EWS: 931·35a (sem 31·42) and ERS: 985·71a (sem 23·31) g/60 d (P=0·0030)), probably because of the effect on satiety of WG fibre, resulting in less body weight gain (BWG) along 60 d (310·69b (sem 4·78), 279·49a (sem 7·30) and 279·70a (sem 6·17) g/60 d for C, EWS and ERS, respectively (P=0·0557)). However, efficiency did not differ among diets (0·26a (sem 0·01), 0·29a (sem 0·02) and 0·28a (sem 0·01) BWG/g diet for C, EWS and ERS, respectively (P=0·2247)). Similar results were observed in the previous study using the same model of growing rats fed extruded WG maize( Reference Albarracín, Weisstaub and Zuleta 34 ). Sorghum WG has been proposed as a promising novel ingredient in foods targeting satiety as an adjunct for weight control( Reference Stefoska-Needham, Beck and Johnson 15 ).
Effect of sorghum diets on caecum
The consumption of sorghum diets reduced caecal pH compared with C diet containing cellulose, which resists fermentation (Table 2). In addition, animals fed extruded WG corn( Reference Albarracín, Weisstaub and Zuleta 34 ) or WG flours of different wheat varieties( Reference Adam, Levrat-Verny and López 35 ) or WG wheat bread( Reference Adam, Lopez and Leuillet 36 ) decreased caecal pH. The caecal fermentation of extruded sorghum may generate great quantities of SCFA and cause lowering of intraluminal colonic pH, which are considered beneficial for intestinal health, because they promote positive microbiota proliferation and decrease the growth of pathogenic bacteria species( Reference Topping and Clifton 37 – Reference Delcour, Aman and Courtin 39 ). Sorghum diets also supply polyphenols. Total polyphenol content of diets was 2640·6 mg GA/kg diet for EWS and 3052·2 mg GA/kg diet for ERS, respectively. It has also been proposed that a high concentration of phenols in caecum could exert local beneficial effects within the GIT( Reference Halliwell, Rafter and Jenner 40 ). However, only the consumption of ERS diet increased sIgA, which could imply a favourable effect on the intestinal barrier and its immune function, whereas EWS decreased sIgA with respect to C (Table 2). The dual effects of EWS (decreased) and ERS (increased) diets on IgA levels could be related to caecal pH values, SCFA concentrations and/or changes in the microbiota. In this work, the causal relation between an elevation or decrease of caecal IgA concentrations and lowered caecal pH values found in rats fed sorghum diets is still unclear. Further investigations are needed to elucidate the relationship between the effects of sorghum diets on the caecal IgA secretion. In addition, it was reported that diets containing 15 % sugar beet crude fibre significantly increased sIgA in rats( Reference Shiau and Chang 41 ).
Table 2 Caecal content (CC), CC PH, caecal polyphenols, reducing power, secretory IgA (sIgA), mucinase, β-glucosidase and β-glucuronidase activity (Mean values with their standard errors; n 8/group)
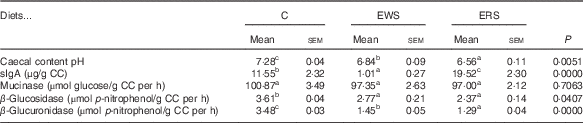
C, control lot; EWS, extruded white sorghum lot; ERS, extruded red sorghum lot.
a,b,c Mean values with unlike superscript letters were significantly different between samples analysed by the least significant difference test (P<0·05).
There were no differences in caecal mucinase activity among diets (Table 2). Shiau & Chang( Reference Shiau and Chang 41 ) reported that only 15 % guar gum diet reduced mucinase in rats with respect to diets free of fibre, or with 5 or 15 % pectin, carrageenan or cellulose. Rats fed WG sorghum diets decrease the activity of bacterial β-glucosidase relative to C (Table 2). In addition, Zdunczyk et al.( Reference Zdunczyk, Jankowski and Mikulski 42 ) reported a decreased β-glucosidase activity in turkeys fed ground WG wheat in relation to a diet without WG. Similarly, β-glucuronidase was significantly reduced with sorghum diets, even more with ERS (Table 2). This enzyme is characteristic of harmful bacteria species, and it has deconjugative properties that support the transformation of xenobiotics into more toxic substances. Shiau & Chang( Reference Shiau and Chang 41 ) reported lower activity of this enzyme in the case of rats fed 15 % pectin diets. The inclusion of extruded WG sorghum in the diet can be assumed to selectively modulate the composition of the microbiota and thus decrease bacterial enzymatic activity. β-Glucosidase and β-glucuronidase transform pre-carcinogens to carcinogens. Therefore, a decrease in the activity of these enzymes could play an important role in colon carcinogenesis prevention( Reference Rowland 43 , Reference Justil, Arroyo and Valencia 44 ). Our results indicate that the differential changes observed in caecal pH, sIgA, β-glucuronidase and β-glucosidase activities may depend on the nature of the sorghum consumed and different content of free and bound phenolics of each. In this regard, ERS exerted a stronger positive impact on caecal fermentation.
Effect of sorghum diets on colon mucosae
Antioxidant status
The GIT is a major site for generation of pro-oxidants, whose production is primarily because of the presence of microbiota, food ingredients and interactions between immune cells. To investigate the effect of extruded sorghum diets on the colonic AO system, we examined the immunolocalisation of SOD (Mn SOD and Cu,Zn SOD), CAT, GPx and GR enzymes in proximal and distal mucosa by immunohistochemistry assay (Table 3 and online Supplementary material). The balance of the activity and expression of these enzymes may be critical in oxidative defence of colon. We demonstrated that both sorghum diets induced similar modulation on colonic AO system, showing the highest impact on distal mucosa. The expression of Cu,Zn SOD was significantly reduced in sorghum-fed rats compared with those of C rats (P<0·0001) (Table 3), although only EWS diet showed significant decreases in Mn SOD immunoreactivity score in both colonic areas (proximal: 92 %; distal 80 %; P<0·0001). In distal mucosa, CAT and GPx protein expression levels were significantly increased compared with C (CAT EWS: 93 %, ERS: 120 %; GPx EWS: 142 %, ERS: 42 %), resulting in a reduction in the SOD:CAT and SOD:GPx ratios, which may yield a system that can eliminate H2O2 in a higher rate than it is formed. The balance between the AO enzymes demonstrated a better state of AO protection in distal colon of sorghum-fed rats. This is in agreement with Dani et al.( Reference Dani, Oliboni and Pasquali 45 ) and López-Oliva( Reference López-Oliva, Agis-Torres and Goñi 46 ), who reported reduced SOD:CAT and SOD:GPx ratio in the liver of Wistar rats fed purple grape juice or colon of rats fed GADF, respectively, indicating an enhancement of the AO capacity.
Table 3 Copper zinc superoxide dismutase (SOD), manganese SOD, catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx), nuclear transcription factor (erythroid-derived 2)-like 2 (Nrf-2), cyclo-oxygenase 2 (COX-2), inducible nitric oxide synthase (iNOS), in proximal and distal colon of rats fed control (C), extruded white sorghum (EWS) and extruded red sorghum (ERS) (Mean values with their standard errors; n 8/group)

a,b,c Mean values with unlike superscript letters were significantly different between samples analysed by Tukey’s test (P<0·05).
High increases of GR expression compared with the C were found in all animals fed sorghum diets (proximal colon EWS: 475 %, ERS: 200 %; distal EWS: 573 %, ERS: 63 % (P<0·0001)). This suggests that sorghum diets might enhance the GSH reduction capacity from GSSG in colonic mucosa by inducing GR up-regulation.
These results demonstrated that a better AO protection state would be present in the colon after consumption of sorghum diets. The Nrf-2 AO response element signalling pathway is important to AO enzyme expression. To determine whether sorghum diets elicit their AO effects by regulating Nrf-2, we also investigated cytosolic and nuclear Nrf-2 immunolocalisation in proximal and distal colonic sections by immunocytochemistry analysis (Table 3). An intense positive Nrf-2 staining along the colonic axis of the crypts in the cytoplasm and nuclei of the epithelial cells affecting mainly the middle and luminal crypt compartments has been shown in slices from rats fed both sorghum diets. The number of Nrf-2-positive nuclei significantly increased with respect to C (P<0·0001) in the proximal colon for EWS-fed rats and distal colon for ERS-fed rats. However, C rats showed a weak and diffuse cytosolic Nrf-2 staining distributed on the top of crypt cells. These results confirm that sorghum diets enhance Nrf-2 nuclear translocation, which promotes the transcription of detoxifying or AO enzymes.
On the other hand, a similar COX-2 and iNOS immunostaining pattern was observed in all the groups (Kruskal–Wallis P>0·05) (Table 3). The immunolocalisation of COX-2 and iNOS enzymes showed very weak staining along the colonic axis of the crypts in the cytoplasm of the epithelial cells of the basal, the middle and the luminal crypt compartments. Similarly, Ritchie et al.( Reference Ritchie, Taddeo and Weeks 47 ) did not observe extensive inflammatory cell infiltration or fold changes in relative expression of COX-2, IL12b and IL6 in DSS-induced colitis in rats fed bran sorghum diets.
The intestinal epithelial barrier integrity depends on a delicate balance between cellular proliferation and apoptosis. In the present work, the PCNA assay was used to quantify the colonocyte proliferation and the apoptotic process was assessed by TUNEL staining. In all animals, PCNA-labelled nuclei were typically located in the lower half of the crypts, which is the zone of proliferating cells, whereas the apoptotic bodies were typically located at the top of the crypts of the colonic epithelium (Fig. 1). A differential response to consumption of ERS or EWS has been found between proximal and distal colon. Microscopic examination of the colonic sections clearly showed a decreased level (23 %; P<0·0001) of PCNA-positive cells in proximal mucosa of rat fed ERS (PCNA LI: 35·69 (sem 1·68) v. 46·07 (sem 3·17) %) but did not significantly affect the TUNEL-positive cell (P>0·05). As a consequence, ERS diet induced a modification in the crypt population with shorter crypts as shown in H&E staining sections (Fig. 1, online Supplementary material). These results indicate that ERS elicits an antiproliferative effect but does not influence apoptosis in the proximal colonic mucosa. This decreased proliferation could enhance DNA repair. Positive effects of eating sorghum on cancer prevention have been documented( Reference Awika and Rooney 2 , Reference Turner, Diaz and Taddeo 48 ), with anthocyanins and ferulic acid being mainly involved in antiproliferative and AO effects. Thus, mainly the diet based in ERS may have protective effects against colorectal tumourigenesis in the proximal colon. These results could be associated with a reduction of oxidative environment of the proximal colonic mucosa through modulation of the AO system by ERS diet observed in this study. In this connection, several reports demonstrated that different plant polyphenolics could protect against pre-neoplastic conditions via induction of endogenous AO enzymes preventing oxidative stress( Reference Rodríguez-Ramiro, Ramos and López-Oliva 49 ). No further differences were detected for the distal colon in ERS-fed rats. This could be because of the fact that during passage through the intestinal tract SCFA and polyphenolic metabolites from fermentation are absorbed by colonic mucosa and a less local effect may be found in the distal colon. In addition, sequential metabolism in the colon means that regions of proximal and distal colon are probably exposed to distinct profiles of active compounds from the diet. Thus, rats fed a diet containing AO grape fibre, another type of fibre plus polyphenols, attenuated the mitochondrial apoptotic pathway, which was correlated with an increase in the AO capacity of the fibre( Reference López-Oliva, Pozuelo and Rotger 50 ), showing also an epithelial hypoplasia in the caecum and the distal colonic mucosa of Wistar rats( Reference López-Oliva, Agis-Torres and García-Palencia 51 ). On the other hand, consumption of EWS generated hyperproliferation in both proximal and distal colon, which could be associated with an increased risk of cancer, but also increased apoptosis of epithelial cells, indicating a faster renewal rate of the epithelium. Consequently, rats fed an EWS showed similar crypt depth as the C group (P>0·05), without histological modifications in the mucosal architecture (Fig. 1). As the majority of apoptotic cells were localised in the place where apoptosis normally takes place, the increase could implicate that EWS ensures the re-establishment of homoeostasis of the colonic mucosa by removal of damaged cells, inducing a balance between proliferation and apoptosis. Torres et al.( Reference Torres, Pizauro and Soares 52 ) observed significant changes in the small intestinal mucosa of broilers fed low-tannin sorghum diets, such as higher mitotic index and epithelial loss. The differential effect of sorghum fibres on colonocytes could be partially explained by taking into account the differences in fibre composition, particularly the fermentable components and their AO capacities. Ritchie et al.( Reference Ritchie, Sturino and Carroll 53 ) demonstrated that the presence of bioactive compounds such as 3-deoxyanthocianins and condensed tannins derived from fermentation of sorghum brans may alter the luminal environment and microbial populations. These diets also can alter faecal SCFA concentrations, as butyrate, which would mediate an increase in proliferation, differentiation and apoptosis in the crypt in normal colonic epithelium( Reference Davidson and McDonald 54 ).

Fig. 1 Effects of extruded sorghum diets on crypts depth, cell proliferation and apoptosis in the rat proximal and distal colonic mucosa. (A) Representative haematoxylin–eosin (H&E)-stained sections and the crypts depth measured as cells number per hemicrypt of the colon of rats fed with the control (C, ![]() ), extruded white sorghum (EWS,
), extruded white sorghum (EWS, ![]() ) or extruded red sorghum (ERS,
) or extruded red sorghum (ERS, ![]() ) diet (100×). (B) Representative photographs for immunohistochemical staining of proliferating cell nuclear antigen (PCNA) (
) diet (100×). (B) Representative photographs for immunohistochemical staining of proliferating cell nuclear antigen (PCNA) (![]() )-positive cells (400× magnification) and the PCNA labelling index (%) in colon tissues from rats fed the control, EWS or ERS diet. (C) Colonic epithelial apoptosis as revealed by terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay of control, EWS- and ERS-fed rats (light microscope, 400× magnification) and quantification of apoptotic cells by TUNEL labelling index (%). Values are means (n 8 rats/group), with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (at least P<0·05) between groups.
)-positive cells (400× magnification) and the PCNA labelling index (%) in colon tissues from rats fed the control, EWS or ERS diet. (C) Colonic epithelial apoptosis as revealed by terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay of control, EWS- and ERS-fed rats (light microscope, 400× magnification) and quantification of apoptotic cells by TUNEL labelling index (%). Values are means (n 8 rats/group), with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (at least P<0·05) between groups.
Systemic effects of sorghum diets in growing Wistar rats (lipid profile and oxidative status) are shown in the online Supplementary material.
Conclusions
This study provides evidence of potential in vivo mechanisms for the protective and AO effects of diets rich in extruded sorghum. The consumption of extruded WG sorghum exerted physiological effects on the organism, both locally in the intestinal tract and systemically. Both EWS and ERS effectively showed a satiety effect reducing BW and decreasing caecal pH and caecal β-glucosidase and β-glucuronidase activities. In addition, ERS consumption increased sIgA, probably because of the higher content of polyphenols. Moreover, extruded sorghum exerted an additional favourable effect on the AO enzymatic system of both colon segments promoted by Nrf-2 nuclear translocation. A differential impact of the sorghum diets on the colonic mucosa has been demonstrated. Although ERS diet may have protective effects against colorectal cancer by decreasing proliferation, EWS induced hyperproliferation and also increased apoptosis of epithelial cells, ensuring the re-establishment of homoeostasis of the colonic mucosa. Although modest effects at the systemic level and lipid profile have been found, sorghum intake might be beneficial for preventing hypertriglyceridaemia. Taken together, these findings suggest that the consumption of extruded sorghum, mainly ERS, may prevent against certain diseases of the intestine by enhancing AO status and could be considered as a novel functional food for colon health.
Acknowledgements
The authors are thankful to the National Agency of Scientific and Technological Support (SECyT, PICT-1282) and the Universidad Nacional del Litoral (CAI + D 2011-PI-0367) for their financial support.
S. R. D. formulated the research questions and designed the entire study; E. E. L., R. E. C. and M. M. E. L.-O. carried out analytical determinations; Z. A. and A. W. designed diets and carried out animal study; M. M. E. L.-O., E. E. L. and S. R. D. analysed the data and wrote the article; M. M. E. L.-O. and S. R. D. had primary responsibility for final content; all authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517002513






