Choline functions in several important structural and cell signalling roles, which are integral in the formation of VLDL, phospholipids (phosphatidylcholine and sphingomyelin) and the neurotransmitter acetylcholine (ACh)(Reference Ueland1). In addition, choline functions as a methyl donor and is crucial for DNA regulation and repair, protein function and intermediary metabolism. Following cellular uptake, choline is phosphorylated to phosphocholine, or irreversibly oxidised to betaine, which functions to donate methyl groups to homocysteine, producing the essential amino acid methionine(Reference Zeisel, Mar and Howe2). Choline can be synthesised endogenously by methylation of phosphatidylethanolamine, a process which occurs primarily in the liver(Reference Ridgway, Yao and Vance3), but also occurs in neuronal cells(Reference Blusztajn, Liscovitch and Richardson4). However, de novo synthesis alone is not sufficient to meet human requirements(Reference Zeisel and da Costa5). Choline is found naturally in a wide range of foods in the free and esterified form and betaine is also available directly from the diet(6).
A large body of evidence from animal studies suggests that choline supplementation during development improves cognitive and neurological function in offspring(Reference McCann, Hudes and Ames7). Evidence from animal feeding studies suggests a role for choline in hippocampal changes during brain development(Reference Albright, Mar and Friedrich8–Reference Wong-Goodrich, Glenn and Mellott10). The hippocampus is critical for the development and consolidation of memory, which along with attention, reasoning, language, perception and construction is a crucial component of human intelligence(Reference Baron11). In human subjects, substantial brain development occurs prenatally and continues to be rapid during the first few years of life. Brain development includes neurogenesis, axonal and dendritic growth, synaptogenesis, cell death, synaptic pruning, myelination and gliogenesis(Reference Grantham-McGregor, Cheung and Cueto12). A number of animal experiments have reported cognitive or other neurological benefits of dietary choline provided either at critical prenatal windows or throughout pregnancy to weaning (for a review, see McCann et al. (Reference McCann, Hudes and Ames7)). Animal studies have also manipulated intake postnatally and reported beneficial effects in the offspring(Reference Meck, Williams and Cermak13–Reference Ward, Kolodny and Nag15). In addition, these studies indicate that in animals the effects of choline supplementation or deficiency during development persist to later in life(Reference McCann, Hudes and Ames7, Reference Meck, Williams and Cermak13, Reference Zeisel16).
Despite the widely accepted importance of choline in development of the nervous system, the possible role of choline status on neurodevelopment in children has been investigated in few human studies to date. Signore et al.(Reference Signore, Ueland and Troendle17) prospectively studied 400 mother–child pairs recruited in Birmingham, Alabama, and reported no association at the age of 5 years between cord blood choline concentration and intelligence quotient scores measured using the Wechsler Preschool and Primary Scale of Intelligence-Revised. However, the present study reported scores well below the national norms on the developmental tests among both mothers and their children, and thereby raised questions about the study's ability to identify beneficial effects of individual nutrients, such as choline, in the face of poor overall nutrition or other environmental variables that might have contributed to the low scores. Wu et al. (Reference Wu, Dyer and King18) found that maternal choline status in the first half of pregnancy was significantly associated with cognitive development among healthy term gestation infants. To date, no study has examined the impact of the child's, rather than the mother's, choline status (and other biomarkers of one-carbon metabolism) on neurodevelopment.
As the brain continues to develop rapidly during childhood, nutrition is likely to continue to have an impact on neurodevelopment in the preschool years(Reference Rosales, Reznick and Zeisel19). Plasma free choline, for example, is a precursor of the endogenous neurotransmitter ACh and disturbances of the ACh system may impair hippocampus-related cognitive and emotional function(Reference Busche, Bagorda and Lehmann20). Also, epigenetic mechanisms, including DNA methylation, function in the neurobiology of cognition(Reference Sweatt21, Reference Roth, Roth and Sweatt22) and provide plausible mechanisms for a role for choline in development. We postulated that choline exposure in early life could affect hippocampal development, thereby affecting memory and intelligence in children. Therefore, we examined the relationship between biological markers of choline status (and other biomarkers of one-carbon metabolism) and neurodevelopment at 5 years of age in the Seychelles Child Development Nutrition Study.
Subjects and methods
Study population
Participants were recruited from an ongoing study investigating maternal exposure to methylmercury through fish consumption and developmental outcomes in the Republic of Seychelles, an archipelago of over 100 islands in the Indian Ocean, about 1500 km off the coast of East Africa. In 2001, we recruited, at their first antenatal visit (gestational age range 14–24 weeks), 300 healthy pregnant women on the island of Mahé to participate in the study. Of those initially recruited, 256 children completed the 5-year evaluation and complete data on choline variables; endpoints and a priori selected covariates were available for 210 children (106 boys and 104 girls).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Institutional Review Boards of the University of Rochester and the Ministry of Health in the Republic of Seychelles. Written informed consent was obtained from all the participants.
Demographic anthropometric and neurodevelopmental assessments
Birth outcome data, such as birth weight, were obtained from hospital records. When the children were nearing the age 5 years, they were recalled for an evaluation. At the evaluation, mothers completed a questionnaire providing demographic data and the Child Behaviour Checklist (total T-score)(23). The children completed a test battery, which included anthropometry and a series of cognitive, motor and language tests. Children's height and weight were measured by trained nurses and used to calculate BMI z-score based on WHO cut-offs(Reference de Onis, Onyango and Borghi24). All measuring equipment was calibrated prior to initiation of the study, and regularly throughout the study, by the Seychelles Bureau of Standards.
The neurodevelopmental test battery included the following: Finger Tapping (total taps, dominant and non-dominant hand), the Preschool Language Scale (PLS)-Revised (total language, auditory comprehension and verbal knowledge)(Reference Zimmerman, Steiner and Pond25), the Woodcock–Johnson Test of Scholastic Achievement (applied problems, letter-word recognition)(Reference Woodcock and Johnson26), The Child Behaviour Checklist (total T-score)(23) and two of three subtests of the Kaufman Brief Intelligence Test (verbal reasoning, matrices)(Reference Kaufman and Kaufman27). Testing was accomplished by J. H., a Maternal and Child Health Nurse specially trained at the University of Rochester to administer the test battery. All tests were translated to Creole, the language spoken at home in the Republic of Seychelles by the Kreole Institute in Seychelles. All tests were translated from English to Creole and then back-translated. Pilot testing was conducted to verify that test results were in line with the test norms.
Blood sampling and biochemical measurements
Children's venous non-fasting blood samples were collected in EDTA-containing tubes for plasma separation after completion of the 5-year developmental assessment. Samples were stored in the dark at 4°C until separation, which was performed within 0·5–2·5 h of the time of sampling. Plasma and whole blood samples were stored (for a maximum of 1 year) at − 70°C until batch analysis at the end of the study. Plasma free choline, betaine, dimethylglycine (DMG), total homocysteine (tHcy) and methionine were analysed at the laboratory of Bevital AS (http://www.bevital.no) by liquid chromatography-tandem MS (liquid chromatography-MS/MS), as described previously(Reference Holm, Ueland and Kvalheim28).
Covariates
Analyses controlled for the following covariates known to be associated with child development(Reference Davidson, Myers and Cox29): birth weight (continuous), child's age at testing (continuous), socio-economic status (the Hollingshead Four-Factor Socioeconomic Status modified for use in the Seychelles; continuous), home environment (the Paediatric Review of Children's Environmental Support and Stimulation continuous), maternal intelligence (measured on the Matrices subtest of the Kaufman Brief Intelligence Test at the child's 19-month evaluation; continuous), sex of the child, the number of nuclear family members living with the child at the time of the 5-year evaluation (living with both parents v. other situations) and maternal age (continuous).
Data analysis
Bivariate relationships among choline, tHcy and related measures and between these measures and neurodevelopmental endpoints were evaluated using correlation coefficients (Pearson or Spearman depending on variable distribution) and scatter plots. χ2 Tests (for categorical variables) and t tests (for continuous variables) were performed to compare covariate values among the 210 subjects with complete data with those of excluded subjects (for whom some covariate data were not available). Analyses were conducted using R 2.12.0 (R Foundation for Statistical Computing) and SAS version 9.2 (SAS Institute, Inc.). All statistical tests were evaluated using a two-sided P= 0·05 significance level. Non-parametric statistical tests (including the Mann–Whitney test) were used where data could not be normalised (plasma DMG and methionine concentrations) by log-transformation and analysed using parametric tests.
The association of each of the five choline-related measures with each of the ten neurodevelopmental endpoints was estimated using separate multiple linear regression analyses, adjusted for all covariates outlined earlier. As choline metabolism may vary by sex(Reference Chew, Jiang and Yan30), interaction terms between each of the choline measures and sex were included in the models. If the interaction term was not statistically significant, it was excluded and the analysis was rerun. Within each model, we used a two-tailed α-level of 0·05 to determine the significance of interactions and independent variable effects.
Regression assumptions were checked for each model. For models in which the assumption of normally distributed errors with constant variance was violated, we log-transformed the dependent variable to stabilise the variance and produce more normally distributed errors. Variance inflation factors were used as a check for collinearity among variables(Reference Weisberg31). Statistical outliers (defined as observations with standardised residuals greater than 3 in absolute value) and influential points (defined as observations with a Cook's distance larger than 0·50)(Reference Weisberg31) were identified for each model and affected models were then run with and without these values. When results differed substantially with the inclusion or exclusion of outliers or influential points, the differences were noted. Although multiple measures of exposures and cognitive outcomes are included, statistical models are not independent and address the same hypothesis and the interpretation of results is guided by prior data reported in previous cohorts(Reference Strain, Davidson and Thurston32). The present study, therefore, does not correct for multiple testing, which is overly conservative and suffers from numerous limitations(Reference Glantz33, Reference Perneger34).
Results
Relative to the 210 subjects with complete data, excluded subjects (owing to incomplete covariate data, n 46) did not differ significantly in terms of the child's age, sex or birth weight; the mother's age, intelligence or socio-economic status; or the family's home environment. The child's development was assessed at a mean age of 5·61 (sd 0·3) years. In total, 50 % of the subjects were boys. The mean BMI of children at time of testing was 15 (sd 2·1) kg/m2. Characteristics of the study cohort, including concentrations of free choline, betaine, DMG and tHcy, are summarised in Table 1. The concentration of free choline and its related metabolites did not differ significantly between boys and girls. However, sex differences were evident in a number of neurodevelopmental endpoints. Boys performed better than girls in Finger Tapping (total, dominant and non-dominant), while girls performed better than boys in the PLS (total language and verbal knowledge) (Table 1). Free choline concentration was significantly correlated with concentration of betaine (r 0·24; P= 0·0006), DMG (r 0·15; P= 0·03), methionine (r 0·24; P= 0·0005) and tHcy (r 0·19; P= 0·006). Betaine concentration was positively correlated with the logarithm of DMG concentration (r 0·28; P< 0·0001) and negatively correlated with tHcy concentration (r − 0·18; P= 0·01). DMG concentration was positively correlated with methionine concentration (r 0·30; P< 0·001).
Table 1 Summary statistics for predictors, cognitive endpoints and covariates in 5 year-old Seychellois children (Mean values and standard deviations)

PLS, Preschool Language Scale; WJ, Woodcock–Johnson; CBCL, Child Behaviour Checklist; K-BIT, Kaufman Brief Intelligence Test; PROCESS, Paediatric Review of Children's Environmental Support and Stimulation.
* Comparison between boys and girls using t test or Mann–Whitney test as appropriate.
The results of multiple linear regression analyses examining the association between choline measures and neurodevelopmental endpoints are presented in Table 2. There was no interaction between the choline measures and sex in any model. The PLS-total language score improved with increasing plasma betaine concentration (i.e. 0·7-point increase in PLS per standard deviation of betaine concentration (‘standard deviation of betaine’ multiplied by ‘β value for PLS-total language score’)), but there were no other associations present among choline, DMG, methionine or tHcy concentration and any of the endpoints. Results were similar when choline measures were log-transformed.
Table 2 Associations between choline and its related metabolites and neurodevelopmental endpoints in 5 year-old Seychellois children (β Coefficients and their standard errors from adjusted* multiple linear regression analyses)†
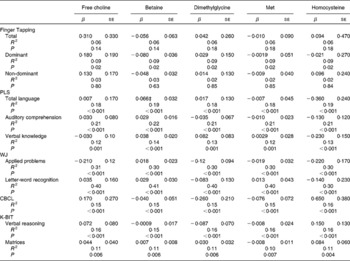
PLS, Preschool Language Scale; WJ, Woodcock–Johnson; CBCL, Child Behaviour Checklist; K-BIT, Kaufman Brief Intelligence Test.
* Adjusted for birth weight, child's age at testing, socio-economic status, home environment, maternal intelligence, sex of the child, the number of nuclear family members living with the child and maternal age.
† For all models, n 210 and df = 200.
‡ Significant association (P= 0·04).
Discussion
The major finding from the present study is the lack of significant associations in covariate-adjusted models between choline measures (and other biomarkers of one-carbon metabolism) and various cognitive outcomes in the 5-year-old children in the Seychelles Child Development Nutrition Cohort. We did not find a significant association between the plasma concentration of free choline, or its related metabolites, and children's intelligence assessed by the Kaufman Brief Intelligence Test, a test which measures both verbal and non-verbal intelligence and provides an indication of visual perception, cognitive ability and receptive vocabulary. The Woodcock–Johnson Test of Achievement, which assesses the overall level of scholastic achievement, was also non-significantly associated with choline concentration. In addition, we included measures of neurodevelopment that may be more specifically related to choline. For example, the Finger Tapping Test, which provides a measure of fine motor speed, may be relevant in light of reports that postnatal dietary choline supplementation improves gross motor locomotion in mice(Reference Nag and Berger-Sweeney35). The PLS and Child Behaviour Checklist provide a more specific assessment of visuospatial and auditory memory and social or emotional responses, domains which may be particularly sensitive to choline(Reference Meck, Smith and Williams36, Reference Cheng, MacDonald and Williams37), potentially owing to the influence of ACh on hippocampal plasticity(Reference Busche, Bagorda and Lehmann20). Nevertheless, we did not find any consistent relationships between the endpoints measured and the plasma concentration of choline, DMG, methionine or tHcy. However, plasma betaine concentration was positively associated with the PLS-total language score. A previous intervention study in healthy elderly adults reported a positive effect of betaine on memory performance, which the authors speculated was explained by the greater availability of choline metabolites for synthesis of ACh and structural phospholipids, such as phosphatidylcholine and sphingomyelin(Reference Eussen, Ueland and Clarke38). Such a mechanism is also plausible in children, particularly as the early years are a time of rapid brain growth, with the peak time for development between 5 months gestation and 4 years of age(Reference Shen, Wu and Lin39). Albeit scientifically plausible, the present finding that the PLS-total language score was significantly associated with betaine requires confirmation in future research, as it may be owing to chance.
To our knowledge, the present study is the first to examine the relationship between the plasma concentration of choline and other biomarkers of one-carbon metabolism and concomitant cognitive performance in children. Animal studies, however, have consistently shown a cause-and-effect relationship between postnatal choline intake (and other biomarkers of one-carbon metabolism) and subsequent performance in offspring(Reference McCann, Hudes and Ames7), with beneficial effects reported to be long term and evident in adulthood and old age(Reference Meck, Williams and Cermak13). The majority of research investigating choline intake and performance has been undertaken in animal models, specifically in rodents(Reference McCann, Hudes and Ames7). The growth and development of the rat and human brain progress at different rates, making direct extrapolation of animal data to human subjects difficult. However, similar to the rat, it has been reported that a large proportion (80 %) of the human brain growth spurt is postnatal(Reference Dobbing and Sands40). This observation, together with the evidence that neural plasticity in response to learning continues throughout childhood, would suggest that requirements for brain-related nutrients, including choline, would be important in early childhood(Reference Rosales, Reznick and Zeisel19). The essentiality of nutrients will be related to the timing of their delivery, compared with the critical periods during brain development. However, unlike the prenatal period, the windows of exposure for brain development during the pre-school years are relatively broad(Reference Rosales and Zeisel41), while, furthermore, the capacity for plasticity within the human brain may allow it to compensate for fluctuations in nutritional status.
Choline circulates in a bound form, mostly in the form of phosphatidylcholine, or in a free form initially reported as a likely mechanism supplying choline to the brain(Reference Klein, Koppen and Loffelholz42). Although an accepted and often used biomarker of choline status, plasma free choline represents only a fraction of the total choline pool(Reference Zeisel43) and may be a poor marker of ACh synthesis and status in the brain(Reference Amenta and Tayebati44). Indeed, another study that reported a positive association between betaine status and memory also observed no association with plasma free choline concentration(Reference Eussen, Ueland and Clarke38). Our lack of association may also be attributed to the high plasma choline concentration (status) of the children in the Seychelles. A previous study only observed a relationship between plasma choline concentration and leucocyte ACh concentration in children who had low choline status(Reference Innis, Davidson and Bay45); no relationship was evident in children who had plasma concentrations of choline, betaine and DMG, similar to those reported in the present analysis. The lack of significant associations may also be owing to the small sample size of the present study.
The present study has a number of strengths. The cohort of 300 mothers initially recruited to the present study represented one-fifth of total annual deliveries in the Seychelles and 75 % of all women booking at antenatal clinics during the enrolment period, and was therefore considered to be a representative sample of the population(Reference Bonham, Duffy and Robson46). In addition, the children were sampled and tested early in childhood within a period of significant brain development, and the test battery used specific and reproducible measures of pre-school neurological function. Extensive data on additional factors that influence child development were also collected and controlled for in the present analysis. Furthermore, choline, betaine, DMG and tHcy were assessed using a sensitive method based on liquid chromatography-MS/MS(Reference Holm, Ueland and Kvalheim28), and plasma free choline concentrations were in keeping with values reported previously in US children(Reference Signore, Ueland and Troendle17, Reference Innis, Davidson and Bay45). However, the study also has limitations. The cohort size was not adequate to examine interactions (between choline and the other biomarkers of one-carbon metabolism on neurodevelopmental endpoints) and other unmeasured covariates, such as folate and vitamin B12, which may have been significant. In addition, the critical period, if any, for human postnatal development with respect to choline may not correspond to 5 years of age. As non-fasting samples were collected from the children, plasma choline concentrations may have been sensitive to recent dietary intakes.
In summary, we found few significant associations between the concentration of free choline (within the normal physiological range for children), or its metabolites, and cognitive outcome in children at 5 years of age. However, the experimental evidence suggesting that choline status is positively associated with cognitive outcome in animals supplemented with choline in the postnatal period suggests that this issue needs further investigation.
Acknowledgements
We thank all the women and children who participated in the study and the nursing staff in the Seychelles for their assistance with data collection. The present study was supported by the US National Institute of Environmental Health Sciences, National Institutes of Health (R01-ES010219, R01-ES015578, P30-ES001247 and T32-ES007271), the European Union (Sixth Framework Programme; PHIME; FOOD-CT-2006-016253) and by the Government of Seychelles. The contents reflect only the authors' views; the European Union is not liable for any use that may be made of the information. The authors' responsibilities were as follows: J. M. W. W., M. P. B., E. M. Mc. S. and J. J. S. were involved in the hypothesis generation, organisation of the study, statistical analysis, and data interpretation; E. v. W., R. W. K. and S. W. T. assisted in the statistical analysis and data interpretation; P. W. D., G. J. M., G. E. W. and C. F. S. contributed to the study design and implementation; M. S. M., A. J. Mc. A. and J. H. were involved in the sample analysis and data interpretation; and P. M. U. contributed to the sample analysis; all authors contributed to the manuscript preparation. No author had any conflict of interest.




