Zn is found in small amounts within the animal body. A pig weighing 100 kg contains less than 2000 mg Zn( Reference Mahan and Shields 1 ); however, Zn plays important roles in several metabolic functions. Most enzymes in animals require Zn to maintain their normal structure and function, and thousands of transcription factors contain one or more Zn atoms. Zn is a cofactor of more than 300 metalloenzymes, and it is required for at least one enzyme in all six enzyme classes( Reference Underwood and Suttle 2 ). Therefore, sufficient amount of Zn is essential for animal growth and development. Usually, the amount of Zn supplied to pig diets largely exceeds the physiological requirements. Several studies( Reference Poulsen 3 – Reference Pluske, Hansen, Payne, Paterson and Barker 7 ) have indicated that pharmacological levels of dietary ZnO (1000–5000 mg/kg) could improve growth performance and reduce the prevalence of diarrhoea in weaning piglets. However, the subsequent application of too much Zn on soil may be toxic to plants and micro-organisms. Extensive feeding of high dietary Zn has led to an environmental concern due to the high amount of Zn excreted in the faeces( Reference Poulsen and Larsen 8 , Reference Case and Carlson 9 ). Thus, there is a need to find an effective and environmentally safe alternative.
Previously, it has been shown that bioavailability of organic Zn was higher than that of inorganic Zn in pigs( Reference Case and Carlson 9 – Reference Wang, Tang and Ma 11 ). Similarly, it has been reported that the bioavailability of Zn-lysine or Zn-methionine was higher than that of ZnO( Reference Schell and Kornegay 12 ). Zn is necessary in maintaining intestinal wall integrity, and it has beneficial effects on enteropathogenic Escherichia coli infection( Reference Crane, Naeher and Shulgina 13 ). The positive effects of Zn supplementation also include increasing the Th1 immune response and affecting intestinal bacterial populations( Reference Bhutta, Darmstadt and Hasan 14 ). Studies have shown that the microbiota in the gastrointestinal tract could reduce the quantity of Zn, as there is a competition for Zn utilisation by the microbiota and host gastrointestinal tract( Reference Gielda and DiRita 15 ). High levels of Zn as ZnO in diets of weanling pigs could increase intestinal tight junction protein expression and decrease intestinal permeability( Reference Zhang and Guo 16 ).
Chitosan (CS)-Zn is a chelate of Zn2+ with CS, and previous studies on the chelation of Zn2+ with CS were focused on its applications to the separation of metal ions or the treatment of waste water( Reference Mcafee, Gould and Nadeau 17 ). When CS binds to Zn2+ through N, O or a combination of both, the binding might enhance the biological activity of the chelate( Reference Wang, Du and Liu 18 ). Few studies have been reported on the application of CS–metal complexes in animal production. Our previous study has shown that CS-Zn could enhance growth performance and decrease the prevalence of diarrhoea in weaned piglets. The efficiency of CS-Zn at a level of 100 mg Zn/kg has been found to be as effective as 3000 mg Zn/kg as ZnO( Reference Xie, Zhu and Han 19 ), which would be beneficial for the environment and for sustaining swine production. Therefore, the objective of the present study was to investigate the impact of dietary CS-Zn on intestinal morphology, mucosal epithelial cell apoptosis and mucosal immune function in weanling pigs.
Materials and methods
Animals, housing and feeding
The present study followed the institutional and national guidelines for the care and use of animals. All experimental procedures involving animal care and sampling were approved by the Ethics Committee for Animal Experimentation of Zhejiang University. A total of 150 weanling barrows (25 (sem 2) d of age, Landrace × Yorkshire × Duroc) weighing 7·2 (sem 0·3) kg were randomly allocated to five treatments. Each treatment was replicated three times, with ten pigs per replicate (i.e. pen). The group fed a basal diet without Zn supplementation was used as the control. The other four groups were fed the control diet supplemented with 50 or 100 mg Zn/kg as CS-Zn, 100 mg Zn/kg as ZnSO4, 3000 mg Zn/kg as ZnO, respectively. CS-Zn, a Zn-CS chelate compound containing 16·1 % of Zn, was provided by Feed Science Institute of Zhejiang University. The basal diet contained 28·30 mg/kg of Zn, and all nutrients met or exceeded the National Research Council( 20 ) recommendations for weanling piglets. The basal diet for pigs contained 62 % maize, 17·5 % soyabean meal, 3 % extruded soyabean, 5 % fishmeal, 5 % whey powder, 1 % wheat bran, 0·5 % soyabean oil, 2 % glucose and 4 % mineral/vitamin premix. The nutrient levels of the diet were as follows: 13·7 MJ/kg calculated digestible energy, 18 % crude protein, 1·5 g Lys/kg, 0·9 g Met+Cys/kg. All pigs were housed in a curtain-sided pig barn with 3·0 × 4·0 m pens. The pens had concrete floors, and each pen was equipped with a feeder and nipple drinker. The pigs were given ad libitum access to feed and water. The feeding trial lasted 28 d after a 7 d adaptation period.
Sample collection
At the end of the feeding trial, thirty pigs (without feed for 12 h, two piglets per pen and six piglets per treatment) were randomly selected and then killed with sodium pentobarbital (50 mg/kg body weight) to collect intestinal samples. Blood samples were collected from the anterior vena cava of pigs, and centrifuged at 4°C for 15 min at 3000 g , and serum was obtained. Serum was transferred to Eppendorf tubes, respectively, snap-frozen in liquid N2 and stored at − 70°C until chemical analysis. Ileal tissue was removed, dissected and washed carefully with normal saline. Ileal mucus was collected with a smooth glass rod and transferred to an Eppendorf tube. The samples were snap-frozen in liquid N2 and stored at − 70°C.
Sample preparation
The duodenal (about 5 cm from the pyloric–duodenal junction), mid-jejunal and ileal (about 10 cm from the ileal–caecal junction) segments were sampled and fixed in buffered formalin (10 %) at 4°C for morphometric analysis. Each sample was embedded with paraffin wax and sectioned at 5 μm on a rotary microtome. Then, the sections were stained with haematoxylin and eosin. Villus height and crypt depth were measured according to the technique of Goodlad et al. ( Reference Goodlad, Levi and Lee 21 ). Well-oriented crypt–villus units were selected for each intestinal cross-section, and the average of fifteen measurements was recorded. The sections were stained with haematoxylin and eosin. Evaluations were made under a light microscope using a 1/100 ocular scale (Olympus). Morphological indices were determined using an image processing and analysis system (version 1; Leica Imaging Systems Limited).
Tissue processing and immunohistochemistry
Formalin-fixed, paraffin wax-embedded ileal samples from treatments were sectioned at 5–7 μm on a rotary microtome and analysed for apoptosis in mucosal epithelial cells with a modified terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end labelling (TUNEL) method( Reference Gavrieli, Sherman and Ren Sasson 22 ). In brief, sections were deparaffinised in xylene and rehydrated with distilled water, and rinsed in PBS (0·1 mol/l, pH 7·2). Endogenous peroxidase expression was inactivated by incubation of tissue sections with 0·3 % H2O2 in methanol for 30 min at room temperature. After treatment with proteinase K, the sections were incubated with 3′-terminal transferase in the presence of biotin-labelled deoxyuridine triphosphate (dUTP) in a moist chamber at 37°C for 1 h. Then, the sections were incubated with sheep anti-fluorescein antibody conjugated with horseradish peroxidase at 37°C for 30 min, washed with PBS (3 × , 5 min) and treated with 3,3′-diaminobenzidine/H2O2 (0·5 mg/ml, 3,3′-diaminobenzidine with 0·003 % H2O2 in 0·05 m-Tris buffer, pH 7·6) at room temperature for 10 min. They were determined either without staining or after faint counterstaining with haematoxylin.
Quantitative analysis
The number of TUNEL-stained epithelial cells in ileal mucosa was counted in fifteen to twenty selected sections from each treatment. The sections were examined under a light microscope. The apoptotic index for each field was calculated as the number of positive TUNEL-stained epithelial cells divided by the total number of epithelial cells counted per field. Means and standard errors were calculated from these counts.
Determination of intestinal mucus secretory IgA
Mucus secretory IgA (sIgA) was measured using a double-antibody sandwich ELISA kit (Bethyl). In brief, 1 ml of 0·01 m-PBS was added to an Eppendorf tube containing 0·1 g mucus. The sample was centrifuged at 4°C for 10 min at 3000 g . The supernatant was collected and the content of sIgA was analysed according to the manufacturer's instructions.
Chemical analysis
Serum diamine oxidase (DAO) activities were determined by enzymatic spectrophotometry, as described by Li et al. ( Reference Li, Lu and Hu 23 ). d-Lactate concentrations in serum were measured by enzymatic spectrophotometric assay using a Cobas Fara centrifugal analyser at 30°C (Hoffmann-La Roche), according to the method described previously( Reference Brandt, Siegel and Waters 24 ). Quantification of endotoxin in serum was determined by the chromogenic Limulus amebocyte lysate test using a chromogenic substrate and individual standard curves for each sample, as described previously( Reference Hurley, Tosolini and Louis 25 ).
Statistical analyses
Results are expressed as means with their standard errors. The data of serum parameter, apoptotic index and sIgA content were analysed by the one-way ANOVA procedure of SPSS 16.0 (SPSS, Inc.). For villus height, crypt depth and crypt depth:villus height ratio, the mean value was calculated in each segment. These data were analysed as repeated measures using the mixed procedure of SPSS 16.0 (SPSS, Inc.) according to the following model:
where μ is the general effect; α i is the main effect of the ith treatment; β j is the main effect of the jth segment; (αβ) ij is the treatment × segment interaction; ɛ ijk is the residual error. The α level for determination of significance was 0·05. Differences among means were tested by Duncan's multiple range test.
Results
Intestinal mucosal permeability
Fig. 1 shows DAO activities, d-lactate levels and endotoxin contents in serum. Compared with pigs that received the control or 100 mg Zn/kg as ZnSO4 diet, serum DAO activities (P= 0·0001) and d-lactate levels (P= 0·0001) decreased in pigs fed dietary 100 mg Zn/kg as CS-Zn or 3000 mg Zn/kg as ZnO. However, compared with pigs that received the control diet, serum endotoxin contents decreased in pigs that received dietary 100 mg Zn/kg as CS-Zn (P= 0·005) or 3000 mg Zn/kg (P= 0·04) as ZnO. No difference was observed for these indices between the pigs fed the diet containing 100 mg Zn/kg as CS-Zn or 3000 mg Zn/kg as ZnO. The pigs fed dietary 50 mg Zn/kg as CS-Zn (P= 0·0001) or 100 mg Zn/kg as ZnSO4 (P= 0·0001) showed lower serum d-lactate levels than those fed the control diet. There was no significant difference in serum endotoxin contents between the pigs that received dietary ZnSO4 and those fed the control diet.

Fig. 1 (A) Serum d-lactate level, (B) diamine oxidase (DAO) activity and (C) endotoxin concentration of weaned pigs fed the control, 50 or 100 mg zinc/kg as chitosan (CS)-Zn, 100 mg zinc/kg as ZnSO4 or 3000 mg zinc/kg as ZnO diet (n 6 pigs per treatment). a,b,c,dMean values with unlike letters were significantly different (P< 0·05). EU, endotoxin units.
Morphological measurements of small-intestinal mucosa
Morphological measurements of small-intestinal mucosa in pigs are shown in Table 1. The villus height and villus height:crypt depth ratio of duodenal and jejunal mucosa were higher in pigs fed the diet supplemented with 100 mg Zn/kg as CS-Zn or the high level of ZnO than in pigs fed the control diet. The crypt depth of the duodenal and jejunal mucosa of these groups was decreased compared with those of the control group. Compared with pigs fed dietary ZnSO4, the villus height and villus height:crypt depth ratio of duodenal mucosa increased in pigs fed the diet supplemented with 100 mg Zn/kg as CS-Zn, while the crypt depth of the duodenum decreased. The villus height:crypt depth ratio of the jejunum was higher in pigs fed dietary 100 mg Zn/kg as CS-Zn than in those fed dietary ZnSO4, and the crypt depth of the jejunum was lower than that in pigs fed dietary ZnSO4. Compared with pigs fed the control diet, the villus height and villus height:crypt depth ratio of ileal mucosa increased in pigs fed dietary 100 mg Zn/kg as CS-Zn, while the crypt depth of the duodenum decreased. The crypt depth of ileal mucosa was lower in pigs fed the diet supplemented with the high level of ZnO than in those fed the control diet, and the villus height:crypt depth ratio was higher than that in pigs that received the control diet. As shown in Table 1, treatment had an effect on villus height (P= 0·05), crypt depth (P= 0·02) and villus height:crypt depth ratio (P= 0·008). The villus height (p< 0·001), crypt depth (P= 0·04) and villus height:crypt depth ratio (P= 0·006) were significantly affected by the segment in the small intestine, with the duodenum having the highest villus height. There was a significant treatment × segment interaction (P< 0·001).
Table 1 Intestinal villus, crypt depth and villus height:crypt depth ratio of weaned pigs fed the control, 50 or 100 mg zinc/kg as chitosan (CS)-Zn, 100 mg zinc/kg as ZnSO4 or 3000 mg zinc/kg as ZnO diet (Mean values with their standard errors, n 6 pigs per treatment for each parameter)
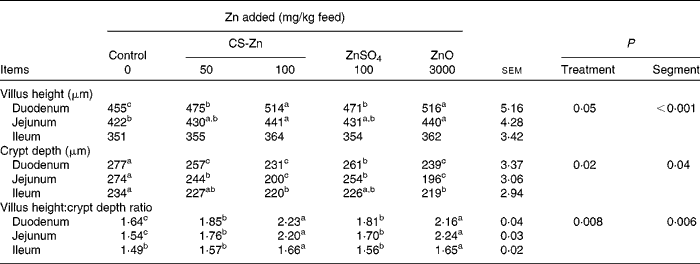
a,b,cMean values within a column with unlike superscript letters were significantly different (P< 0·05).
TUNEL staining and immunocytochemistry
Following in situ labelling of ileal mucosal epithelium, microscopic examination revealed stained epithelial cells undergoing apoptosis of ileum in pigs fed the control diet. Apparent TUNEL-stained cells were also found in pigs fed the diet supplemented with 50 mg Zn/kg as CS-Zn or ZnSO4. These phenomena were not found in pigs that received the diet containing 100 mg Zn/kg as CS-Zn or the high level of Zn as ZnO. The calculation of the apoptotic index from the quantification of TUNEL-stained cells indicated a significant decrease in ileal epithelial cell apoptosis in pigs fed the diets containing Zn compared with those fed the control diet (Fig. 2). A statistically significant decrease was found between the pigs fed the diet supplemented with 50 mg Zn/kg as CS-Zn or ZnSO4 and those that received the diet containing 100 mg Zn/kg as CS-Zn or the high level of Zn as ZnO. There was no significant difference in the apoptotic index of ileal epithelial cells observed between the pigs fed dietary 100 mg Zn/kg as CS-Zn and those fed dietary ZnO.

Fig. 2 Apoptotic index calculated for ileal epithelium of weaned pigs fed the control, 50 or 100 mg zinc/kg as chitosan (CS)-Zn, 100 mg zinc/kg as ZnSO4 or 3000 mg zinc/kg as ZnO diet (n 6 pigs per treatment). a,b,cMean values with unlike letters were significantly different: a significant decrease of apoptosis (P <0·05) was found in pigs fed the diets supplemented with zinc compared with those fed the control diet.
Content of intestinal mucus secretory IgA
Compared with pigs that received the control or ZnSO4 diet, the level of sIgA in ileal mucus was increased in pigs fed the diet containing 100 mg Zn/kg as CS-Zn or the high level of Zn as ZnO (Fig. 3). The sIgA content in ileal mucus was higher in pigs fed the diet supplemented with 50 mg Zn/kg as CS-Zn than in those fed the control diet. However, there was no significant difference between the pigs fed the control diet and those fed the diet supplemented with ZnSO4.

Fig. 3 Secretory IgA (sIgA) concentration in the ileal mucus of weaned pigs fed the control, 50 or 100 mg zinc/kg as chitosan (CS)-Zn, 100 mg zinc/kg as ZnSO4 or 3000 mg zinc/kg as ZnO diet (n 6 pigs per treatment for each value). a,b,cMean values with unlike letters were significantly different (P< 0·05).
Discussion
In the present study, we found that supplementation with dietary CS-Zn influenced intestinal morphology, ileal mucosal apoptosis and mucosal immune function in weanling pigs. Generally, weaning induces various problems in piglets( Reference Van Beers-Schreuers, Nabuurs and Kalsbeek-van der Valk 26 ). At the weaning stage, piglets encounter one of the most stressful events including transition of food, environment and maternal and littermate separation. Stress can suppress the immune system and change intestinal physiological function. As a result, a significant reduction in feed intake and performance, increased susceptibility to disease and post-weaning diarrhoea have usually been observed( Reference Madec, Bridoux and Bounaix 27 , Reference Boudry, Guerin and Malbert 28 ). Weaning is also associated with increased intestinal permeability and inflammation, villous atrophy of the small intestine, a decrease in the number of goblet cells and a reduced immunological response, contributing to reduced nutrient absorption and disease resistance( Reference Smith, Clark and Overman 29 – Reference Castillo, Martín-Orúe and Nofrarías 31 ). Zn is necessary in maintaining intestinal wall integrity and instrumental in maintaining a healthy immune system( Reference Prasad, Bao and Beck 32 ). A study has indicated that Zn supplementation could contribute significantly to heal the leaky gut( Reference Sturniolo, Di Leo and Ferronato 33 ).
Small-intestinal mucosa makes up the first defence barrier of the intestine, to protect against the entry of micro-organisms and potential pathogens. The small-intestinal mucosal barrier of piglets could be damaged due to the entry of harmful microbes resulting from changes in nutrients and living environment after weanling( Reference Wu, Meier and Knabe 34 ). Serum d-lactate level, DAO activity and endotoxin concentration are useful markers of the permeability of intestinal mucosa, intestinal injury and reperfusion insults( Reference Murray, Barbose and Cobb 35 ). In the present study, serum d-lactate level, DAO activity and endotoxin concentration were higher in the control group than in the other groups. The results indicated that small-intestinal integrity may be damaged and the permeability of intestinal mucosa increased. However, these markers were lower in pigs that received the diet supplemented with ZnO or 100 mg Zn/kg as CS-Zn but not in pigs fed dietary ZnSO4. The results showed that the high dietary ZnO level would alleviate the injury of intestinal integrity, which is consistent with a previous study( Reference Hu, Qian and Xu 36 ). Moreover, dietary 100 mg Zn/kg as CS-Zn could also protect the integrity of intestinal mucosa and alleviate stress resulting from weaning. This may be the reason that dietary 100 mg Zn/kg as CS-Zn could significantly reduce post-weaning diarrhoea( Reference Xie, Zhu and Han 19 ). Therefore, the body weight of pigs fed dietary 100 mg Zn/kg as CS-Zn or a high level of ZnO was similar in the study and was higher than that of pigs fed the control or ZnSO4 diet( Reference Xie, Zhu and Han 19 ).
The small intestine is the major site for digestion and absorption of nutrients, and intestinal mucosa plays a crucial role during these processes. There exists a positive correlation between intestinal morphology and gut health. The main function of the intestinal villus is nutrient absorption, and the ratio of villus height:crypt depth reflects the status of gut health. Therefore, good intestinal morphology is the physiological basis for nutrient absorption and animal growth. It is well known that changes occur in the gut morphology of piglets after weaning, which include villous atrophy and crypt hyperplasia( Reference Hedemann, Højsgaard and Jensen 37 ). These changes would lead to the dysfunction of nutrient digestion and absorption in weanling piglets. Some reports( Reference Carlson, Hoover and Hill 38 , Reference Li, van Kessel and Caine 39 ) have shown that a high level of dietary Zn as ZnO could increase the villus height and reduce the crypt depth in weanling pigs, and the present results are in conformity with these reports. Moreover, dietary 100 mg Zn/kg as CS-Zn had the same effects as the high level of Zn as ZnO and improved small-intestinal morphology. However, dietary ZnSO4 almost had no effect on either villus height or crypt depth. The improvement of intestinal morphology in pigs fed the diet supplemented with 100 mg Zn/kg as CS-Zn reflected intestinal integrity and better nutrient absorption, and increased growth performance. Thus, these findings are consistent with the above results of intestinal permeability and integrity.
Villous atrophy means the death or apoptosis of intestinal epithelial cells. Cell apoptosis is a different mode of death from programmed death of necrotic cells. Apoptosis is an autonomic ordered programmed cell death in order to maintain homeostasis, and these processes are controlled by genes. Numerous molecules and approaches are involved in these processes. Zn deficiency in the body could induce cell apoptosis in order to maintain the homeostasis of the internal environment( Reference Salgueiro, Zubillaga and Lysionek 40 ). The present data indicated that ileal epithelial cells in pigs fed the diets without Zn addition were subject to a greater degree of apoptosis. Moreover, pigs fed the diet containing ZnSO4 or 50 mg Zn/kg as CS-Zn also showed evident apoptosis of epithelial cells. However, the apoptosis was not obvious in pigs that received the diet containing the high level of Zn as ZnO or 100 mg Zn/kg as CS-Zn. These data indicated that a low dietary Zn level would speed epithelial cell apoptosis, leading to the injury of the intestinal mucosal barrier. The present results support the suggestion of Salgueiro et al. ( Reference Salgueiro, Zubillaga and Lysionek 40 ). The changes in cell apoptosis in the presence of a high level of dietary Zn as ZnO or 100 mg Zn/kg as CS-Zn might be responsible for the observed effects on small-intestinal morphology and mucosal integrity.
The gastrointestinal tract is not only an organ for digestion, absorption and excretion, but also the largest immunological organ of the body. It acts as the first line of defence against orally administered antigens (e.g. food proteins) and intestinal pathogens (e.g. bacteria, parasites)( Reference Scott and Koski 41 ). Weaning piglets are vulnerable to enteric pathogens, and this vulnerability results from factors such as an immature immune system. This can be partly explained by the presence of a low level of sIgA in the intestine( Reference Ushida, Kameue and Tsukahara 42 ). sIgA is a protective molecule of the mucosal immune system( Reference Snoeck, Peters and Cox 43 ). It has long been recognised as a first line of defence in protecting the intestinal epithelium from enteric pathogenic micro-organisms and toxins( Reference Mantis, Rol and Corthésy 44 ). The present study found that dietary supplementation of Zn as CS-Zn or a high level of Zn as ZnO increased the small-intestinal sIgA concentration, suggesting that CS-Zn or ZnO exerts a protective role against pathogen infection. These findings present some supporting evidence that Zn plays an important role in cell-mediated immune functions and also functions as an antioxidant and anti-inflammatory agent( Reference Prasad 45 ). The functions of sIgA in mucosal immunity and intestinal homoeostasis are not well known, and it is thought that sIgA acts primarily through receptor blockade, steric hindrance, induction of conformational changes and/or immune exclusion, or all of these( Reference Prasad 45 ). A healthy immune system can easily block out pathogens, but a weak one may be overrun. The present findings indicated that the increased concentration of intestinal sIgA might be associated with improved intestinal morphology and mucosal integrity. When the immune system is overactive for an extended period of time, a leaky gut can develop.
Conclusively, dietary supplementation of 100 mg Zn/kg as CS-Zn provided beneficial effects against apoptosis and on the maintenance of intestinal barrier and mucosal immune function, preventing intestinal atrophy and activation absorptive function. Dietary 100 mg Zn/kg as CS-Zn was found to be as effective as dietary 3000 mg Zn/kg as ZnO. The results demonstrated that CS-Zn might be a kind of new source of Zn for animals, and further research needs to be carried out.
Acknowledgements
We thank Bojing Liu and Ya-Li Zhang for their skilful technical assistance.
The present study was financed by the National Science Foundation (31272477). The funder contributed to the conduct of the study, the analysis of the samples and the preparation of the manuscript.
The authors' contributions are as follows: X.-Y. H. and L.-C. Q. designed the research; X.-Y. H., Y.-F. M., M.-Y. L. and Z.-P. W. conducted and analysed the data; X. H. wrote the paper; X.-Y. H. and L.-C. Q. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that there is no conflict of interest.






