Vitamin D status is influenced by a variety of factors including diet, sunlight exposure and underlying health conditions( Reference Prentice, Schoenmakers and Jones 1 ). In HIV-positive individuals, a high frequency of hypovitaminosis D has been reported, not only among populations at high latitudes but also among people living closer to the equator( Reference Allavena, Delpierre and Cuzin 2 – Reference Conrado, Miranda-Filho D de and Ximenes 6 ).
Vitamin D is an immunoregulatory hormone, and vitamin D deficiency and insufficiency in HIV-positive patients have been associated with clinical disease progression, treatment failure and mortality( Reference Havers, Smeaton and Gupte 7 – Reference Sudfeld, Wang and Aboud 9 ). Only a few studies have compared the vitamin D status of HIV-positive with HIV-negative individuals( Reference Dao, Patel and Overton 4 , Reference Lambert, Drummond and Mehta 10 , Reference Chotalia, Frontini and Tatini 11 ). Most previous vitamin D studies in HIV-positive patients are observational, and associations are based on a single measurement per patient. Moreover, only a few studies in HIV-positive patients have addressed the effect of vitamin D supplementation through a nutritional intervention trial with a placebo- or non-supplemented control group( Reference Bañón, Rosillo and Gómez 12 , Reference Stallings, Schall and Hediger 13 ).
In this trial, a lipid-based nutrient supplement (LNS) containing twice the daily recommended intake of vitamin D( Reference Olsen, Abdissa and Kæstel 14 ) was provided daily for 3 months to HIV patients initiating antiretroviral treatment (ART). We measured serum 25-hydroxyvitamin D (25(OH)D) before and 3 months after initiation of ART. We compared the level of serum 25(OH)D in HIV-positive and HIV-negative persons and investigated the role of nutritional supplementation and ART on serum 25(OH)D levels.
Methods
Study design and setting
A randomised nutritional supplementation trial was conducted in Jimma, south-west Ethiopia. The trial compared 200 g/d of LNS with either whey (LNS/w) or soya (LNS/s) protein with no supplementation during the first 3 months of ART. Both LNS/w and LNS/s provided 10 μg/d vitamin D3, which corresponds to twice the daily recommended intake. The details of the trial design and methods of recruitment have been described elsewhere( Reference Olsen, Abdissa and Kæstel 14 ). For the purpose of this study, those randomised to either LNS/w or LNS/s were categorised as supplemented and compared with non-supplemented participants.
Participants
HIV patients who were eligible for ART at Jimma University Specialized Hospital (JUSH), Jimma Health Centre and Agaro Health Centre were invited to participate in the trial from July 2010 to August 2012( Reference Olsen, Abdissa and Kæstel 14 ). Patients who were ≥18 years of age, not pregnant or lactating, having a BMI>16 kg/m2 with no current use of nutritional supplements and living within 50 km of the recruitment sites were enrolled to the trial. HIV patients who were not included in the trial because of low BMI were included in the baseline analysis of serum 25(OH)D. HIV patients with BMI of 16–17 kg/m2 received supplementation in the first 3 months, whereas participants with BMI>17 kg/m2 were randomised to early or delayed nutritional supplementation. The effect of nutritional supplementation was only assessed among HIV patients with BMI>17 kg/m2, as they were randomised to supplementation or no supplementation for the first 3 months. The HIV-negative reference group, age ≥18 years with no known acute or chronic diseases and matched for sex, age (±3 years) and BMI to the last 100 included HIV patients, were recruited among confirmed HIV-negative individuals from the voluntary counselling and testing service at JUSH.
Demographic and clinical data
Demographic data were collected using structured questionnaires in the local languages Amharic or Afaan Oromoo by trained study nurses. The Household Food Insecurity Access Scale with questions about experience of food insecurity in the previous 1 month was used to assess and categorise participants as food secure as well as mild, moderate and severe food insecure( Reference Coates, Swindale and Bilinsky 15 ). This scale has previously been validated in Ethiopia by other investigators( Reference Maes, Hadley and Tesfaye 16 ). Clinical data were collected by health professionals working in the ART clinics. Patients were considered tuberculosis (TB) co-infected if they were diagnosed with TB or were receiving TB treatment at study inclusion.
Body composition
Weight and height were measured with calibrated scales and stadiometers, respectively, with the participant barefoot and wearing minimal clothing; BMI was calculated as weight (kg)/(height (m))2. Body composition was assessed using the 2H dilution method. After collection of pre-dose saliva samples, 30 g of deuterium oxide (99·8 % 2H; Sercon) weighed with 0·01 g precision was given orally( 17 ). Post-dose saliva samples were collected after 4 h of equilibration. Saliva enrichment of 2H was determined by Fourier Transform Infrared Spectrometry (IRAffinity-1; Shimadzu). Total body water was calculated from post-dose 2H enrichment with adjustment for pre-dose enrichment, using a factor of 1·041 to adjust for proton exchange. Lean body mass was calculated on the basis of an assumed hydration factor of 73·2 %( 17 ).
Laboratory
Laboratory personnel collected 10 ml of fasting venous blood in EDTA tubes, and 10 ml in plain tubes at 0 and 3 months. Next, 1 ml of whole blood from the EDTA blood collection tubes was transferred to small tubes, and CD4+ T cells were enumerated using the Facscount® (Becton-Dickinson). After centrifugation, serum or plasma was separated and transferred to cryotubes in aliquots of 1 ml and stored at −80°C. Samples were shipped to the International Clinical laboratory in Addis Ababa, Ethiopia, for viral load quantification and to Denmark for analysis of C-reactive protein (CRP) and 25(OH)D, at University of Copenhagen and Aalborg University Hospital, respectively.
HIV-1 load was quantified using a commercial PCR assay (RealTime HIV-1; Abbott Laboratories) using automated extraction system (m2000 Real Time System; Abbott Laboratories). Viral RNA was amplified on the m2000rt platform (Abbott Laboratories). CRP was measured in serum using an immunoturbidimetric assay (HORIBA ABX A11A01611; Horiba Ltd) for Pentra 400 (HORIBA ABC; Horiba Ltd). The results are given in mg/l, and the precision of the assay was 8·3 CV% based on repeated measurements of a normal serum sample in each run (mean 0·71 (sd 0·06) mg/l).
Total serum 25(OH)D (D2+D3) was quantified using a chemiluminescence immunoassay (LIAISON code 310600) for DiaSorin Liaison® and expressed as nmol/l. The accuracy of the assay was monitored by participating in a Danish national quality control programme for clinical laboratories as well as running kit-specific high and low controls (LIAISON code 310601). Serum 25(OH)D<25, 25–50 and >50 nmol/l were defined as vitamin D deficiency, insufficiency and normal, respectively.
Statistical analysis
Descriptive characteristics of continuous and categorical variables are summarised as medians (interquartile range (IQR)) and percentages, respectively. Demographic and clinical characteristics were compared between HIV-positive and HIV-negative individuals using Wilcoxon’s rank-sum test or Pearson’s χ 2 test, as appropriate. Linear mixed models were used to investigate the relationships between 25(OH)D levels with socio-demographic characteristics (sex, age, religion, occupation and food security status), clinical parameters (HIV status, BMI, WHO stage, viral load, CD4 count and CRP), nutritional intervention and type of ART regimen. Season was classified into four categories on the basis of rainfall – that is, harvest (September–November), light rain (December–February), dry (March–May) and heavy rain seasons (June–August) with the dry season being the most sunny time of the year. As data were collected over 3 years, seasonal differences between years were adjusted for using season-year random effects and were included to capture changes in climatic conditions. Normal probability and residual plots were used for model checking. Variables with skewed distributions such as HIV RNA and serum 25(OH)D were logarithm transformed before analysis, and the results are reported after back-transformation. Stata version 11.2 (StataCorp LP) was used for all analyses.
Ethical consideration
Written informed consent was obtained from all the participants. Individuals randomised to no supplementation in the first 3 months were provided the nutritional supplement in the subsequent 3 months. Patients who were excluded because of severe acute malnutrition were referred to standard treatment according to national guidelines( 18 ). Ethics approval was obtained from the Ethiopian National Health Research Ethical Review Committee and Jimma University Ethical Review committee. A consultative approval was obtained from the Danish National Committee on Biomedical Research Ethics.
Results
A total of 348 HIV-positive persons were included in the trial, whereas the HIV-negative reference group comprised 100 persons. The median age of HIV-positive and HIV-negative persons was 30 (IQR 27; 38) and 29 (IQR 23; 37) years, respectively. HIV-positive patients had lower levels of education (P<0·001), lower median BMI (18·8 v. 20·5 kg/m2, P<0·001) and were more food insecure (P<0·001) compared with HIV-negative persons (Table 1).
Table 1 Characteristics of 342 HIV-positive study participants and a healthy reference group of 100 HIV-negative persons (Numbers and percentages; medians and interquartile ranges (IQR))
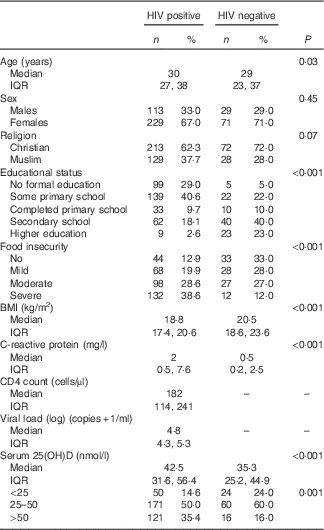
The median serum 25(OH)D level was 40·8 (IQR 29·0; 53·4) nmol/l with 16·7 and 52·3 % having deficient and insufficient levels, respectively. The median baseline serum 25(OH)D levels were higher in HIV-positive than in HIV-negative persons (42·5 v. 35·3 nmol/l, P<0·001). Moreover, 64 % of HIV-positive and 84 % of HIV-negative persons had serum 25(OH)D<50 nmol/l (P<0·001).
Factors associated with serum 25-hydroxyvitamin D at the time of initiating antiretroviral treatment
In a mixed model including HIV status, sex, age, religion, occupation and food insecurity and after controlling for random effects of season-year, HIV-positive patients had 17 % (95 % CI 6, 33 %) higher serum 25(OH)D than HIV-negative persons. Females had 24 % (95 % CI 16, 31 %) lower serum 25(OH)D than males, and Muslims had 12 % (95 % CI 5, 19 %) lower serum 25(OH)D than Christians. The age groups 35–45 and >45 years had 12 % (95 % CI 1, 22 %) and 18 % (95 % CI 4, 30 %) lower serum 25(OH)D, respectively, than the age group 18–25 years. Farmers and daily labourers had 37 % (95 % CI 11, 70 %) and 17 % (95 % CI 3, 33 %) higher serum 25(OH)D, respectively, than persons who had governmental jobs. Serum 25(OH)D was not associated with the level of food insecurity (Table 2).
Table 2 Factors associated with baseline serum 25-hydroxyvitamin D level in 342 HIV-positive and 100 HIV-negative persons (Percentage differences β and 95 % confidence intervals)
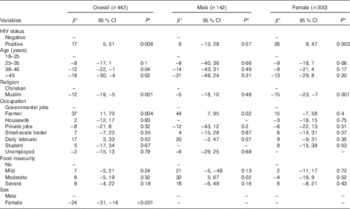
* Values are adjusted for all variables in the table as fixed effects with season-year as the random effect.
As there were interactions between sex and religion and sex and occupation, we further analysed stratifying by sex. In males, there was no significant difference in serum 25(OH)D between HIV-positive and HIV-negative persons, religion and different age groups (P>0·5). However, male farmers and daily labourers had 44 % (95 % CI 7, 95 %) and 20 % (95 % CI −2, 47 %), respectively, higher serum 25(OH)D than male participants who had governmental jobs. Moreover, male participants with moderate food insecurity had 32 % (95 % CI 5, 67 %) higher serum 25(OH)D than participants with no food insecurity. HIV-positive females had 26 % (95 % CI 8, 47 %) higher serum 25(OH)D than HIV-negative females, and Muslims had 15 % (95 % CI 7, 23 %) lower values compared with Christians. No differences in serum 25(OH)D were seen between the age groups, occupation and status of food insecurity in females (P>0·5) (Table 2).
We assessed the association of the clinical parameters of HIV patients at baseline with serum 25(OH)D level using a mixed model adjusting for fixed effects of variables associated with baseline serum 25(OH)D (Table 2), as well as for the random effect of season-year. HIV-positive persons with WHO stage IV at baseline had 17 % (95 % CI 1, 30 %) lower 25(OH)D level compared with WHO stage I, and participants on TB treatment had 14 % (95 % CI 1, 26 %) lower 25(OH)D level than participants with no TB. We found no association between serum 25(OH)D and CRP, viral load or CD4 count (Table 3).
Table 3 Clinical factors associated with baseline serum 25-hydroxyvitamin D level in 342 HIV-positive persons at antiretroviral treatment initiation (Values are percentage difference β and 95 % confidence intervals and P value)

* Adjusted for sex, age, occupation and religion as fixed effect and for season-year as random effect.
Effect of supplementation
A total of 282 study participants with BMI>17 kg/m2 were randomised to either the supplemented (n 189) or the non-supplemented group (n 93) during the first 3 months. Of these, baseline 25(OH)D was available for 186 participants with BMI>17 kg/m2 who received LNS and for ninety participants who did not receive LNS during the first 3 months of ART. We found no major differences between these two groups regarding demographic or baseline clinical characteristics, ART regimen initiation and baseline serum 25(OH)D level. Of 276 participants who had baseline 25(OH)D data, 249 (90 %) completed the 3-month follow-up visit, with 89 % in the supplemented group and 92 % in the non-supplemented group( Reference Olsen, Abdissa and Kæstel 14 ). A total of fifty-one (29 %) participants had ‘poor adherence’, which was defined as intake of LNS<75 %. The supplemented group had a 4·1 (95 % CI 1·7, 6·4) nmol/l increase in serum 25(OH)D level, whereas the non-supplemented group had a 10·8 (95 % CI 7·8, 13·9) nmol/l decrease at the 3rd month (Fig. 1).

Fig. 1 Baseline and 3rd-month 25-hydroxyvitamin D (25(OH)D) levels by supplementation group. ![]() , Baseline 25(OH)D (nmol/l);
, Baseline 25(OH)D (nmol/l); ![]() , 3rd month 25(OH)D (nmol/l).
, 3rd month 25(OH)D (nmol/l).
To further understand these differences, we explored the role of fat mass changes. The median fat mass change in the supplemented group and the non-supplemented group was 1·73 (IQR 0·18; 3·6) kg and 0·52 (IQR −0·87; 1·75) kg, respectively. No association was found between change in fat mass and change in serum 25(OH)D (data not shown).
In a mixed model adjusting for baseline serum 25(OH)D, change in fat mass and fat-free mass and for variables shown to influence baseline 25(OH)D (Tables 2 and 3), the supplemented group had 43 % (95 % CI 31, 55 %) higher serum 25(OH)D than the non-supplemented group at 3 months (Table 4). Females had 16 % (95 % CI 7, 23 %) lower serum 25(OH)D than males. Participants who received TDF/3TC/EFV and zidovudine (AZT)/3TC/NVP treatment regimens had 14 % (95 % CI 2, 25 %) and 15 % (95 % CI 1, 28 %), respectively, lower serum 25(OH)D than participants who had been initiated with the TDF/3TC/NVP regimen. However, no difference was found when we classified ART regimens as efavirenz- and nevirapine-based regimen or tenofovir- and non-tenofovir-based regimen (data not shown). Serum 25(OH)D declined in both supplemented and non-supplemented participants with higher baseline serum 25(OH)D (Fig. 2). The overall median change in serum 25(OH)D was 10·1 (95 % CI 3·48, 20), −0·5 (95 % CI −2·51, 2·01) and −7·25 (95 % CI −12·46, −3·78) for participants with baseline serum 25(OH)D level <25, 25–50 and >50 nmol/l, respectively.

Fig. 2 Change in serum 25-hydroxyvitamin D (25(OH)D) from baseline to 3 months by baseline 25(OH)D level and supplementation group.
Table 4 Factors associated with serum 25-hydroxyvitamin D level after 3 months of nutritional supplementation and antiretroviral treatment among 282 HIV-positive persons (Values are percentage difference β and 95 % confidence intervals and P value)

TDF, tenofovir-DF; 3TC, lamivudine; NVP, nevirapine; EFV, efavirenz; AZT, zidovudine.
* Values are with each variable adjusted for age, sex, religion, occupation, baseline tuberculosis treatment, baseline serum 25(OH)D, change in fat mass and fat-free mass and all variables in the table as fixed effect and season-year at 3 months as the random effect.
Discussion
The classical effects of low vitamin D status are rickets and osteomalacia, but current evidence suggests that low serum 25(OH)D is also linked to other health consequences such as diabetes, cancer and susceptibility to infections( Reference Prentice, Schoenmakers and Jones 1 ). In HIV-positive patients, low vitamin D has been associated with clinical disease progression, treatment failure and mortality( Reference Havers, Smeaton and Gupte 7 – Reference Sudfeld, Wang and Aboud 9 ). The present study shows a low serum 25(OH)D in both HIV-positive and HIV-negative individuals in Ethiopia. Moreover, further reduction in serum 25(OH)D was observed after ART initiation in HIV patients who did not receive vitamin D-enriched nutritional supplementation.
In this study, farmers and daily labourers who were assumed to be exposed to sunlight had better vitamin D status; Muslims had lower vitamin D status than Christians. However, when we stratified by sex, we found an association with occupation only in males and with religion only in females. The latter finding may be because of the use of more covering clothes by female Muslims, which may reduce their sun exposure. In line with this, a study from Germany has shown that Turkish females with more covering clothes had lower vitamin D levels than Turkish females with conventional clothing( Reference Farahati, Nagarajah and Gilman 19 ). Thus, even when there is abundant sunshine, the extent of UVB skin exposure depends on clothing, living and working environments( Reference Prentice, Schoenmakers and Jones 1 ).
There was no association between food security level and vitamin D status in females, whereas in males moderate food insecurity was associated with better vitamin D status. This may indicate that food is not the main source of vitamin D as reported among pregnant women in southern Ethiopia( Reference Gebreegziabher and Stoecker 20 ). Therefore, the high prevalence of vitamin D deficiency and insufficiency in both HIV-positive and HIV-negative individuals in Ethiopia may be due to the combined effect of low sun exposure and low consumption of vitamin D-rich foods.
A number of studies have reported high proportion of vitamin D deficiency and insufficiency in HIV-positive patients( Reference Allavena, Delpierre and Cuzin 2 , Reference Wiboonchutikul, Sungkanuparph and Kiertiburanakul 5 , Reference Vescini, Cozzi-Lepri and Borderi 21 – Reference Gedela, Edwards and Benn 23 ). However, studies that compared vitamin D status of HIV-positive patients with HIV-negative controls or the general population have reported higher serum 25(OH)D in HIV-positive compared with HIV-negative persons( Reference Dao, Patel and Overton 4 , Reference Lambert, Drummond and Mehta 10 , Reference Chotalia, Frontini and Tatini 11 ). Higher serum 25(OH)D has also been reported in patients with TB when compared with healthy controls( Reference Friis, Range and Changalucha 24 ). We also found that HIV-positive patients had considerably higher serum 25(OH)D than HIV-negative individuals. When we stratified the analysis by sex, this association remained only in females. However, we found no interaction between HIV status and sex, and the absence of an association in males may be due to small number of male participants in our study. Previous studies have reported that HIV associated weight loss mainly consist of fat mass loss( Reference Forrester, Spiegelman and Woods 25 – Reference Mulligan, Tai and Schambelan 27 ). Studies have also documented that females lose disproportionately more fat than males during HIV infection( Reference Kotler, Thea and Heo 28 – Reference Swaminathan, Padmapriyadarsini and Sukumar 30 ). As vitamin D is stored in adipose tissue, an increase in serum 25(OH)D due to HIV-associated fat mass loss may have contributed to the higher level of vitamin D in antiretroviral-naive HIV patients compared with the HIV-negative controls. However, we cannot confidently exclude residual confounding by season, even though we adjusted our analyses for this.
We found that individuals with WHO clinical stage IV and those who were on TB treatment at ART initiation had lower serum 25(OH)D, but serum 25(OH)D was not associated with CD4 count, viral load and serum CRP. The lower serum 25(OH)D in patients with WHO clinical stage IV disease and TB may be due to the reduced dietary vitamin D intake because of the disease itself and/or due to reduced exposure to sun because of their reduced mobility or hospitalisation. Moreover, drugs used for TB treatment such as isoniazid and rifampicin may have contributed to the reduction in serum 25(OH)D, because of their direct biological effects on the metabolism of vitamin D( Reference Brodie, Boobis and Hillyard 31 ).
Serum 25(OH)D increased slightly in the supplemented patients, and declined in those not given a supplement. Other studies among HIV patients have also shown an increase in serum 25(OH)D with vitamin D supplementation( Reference Bañón, Rosillo and Gómez 12 , Reference Stallings, Schall and Hediger 13 , Reference Zhou, Zhu and Chen 32 , Reference Steenhoff, Schall and Samuel 33 ). Longitudinal studies in HIV patients without vitamin D supplementation( Reference Wohl, Orkin and Doroana 34 , Reference Havers, Detrick and Cardoso 35 ) have reported a decrease in serum 25(OH)D with ART initiation as observed in our study. The decline in serum 25(OH)D in the absence of supplementation may be due to sequestration of vitamin D during gain in fat mass observed with ART initiation( Reference Olsen, Abdissa and Kæstel 14 ). We found that patients with higher serum 25(OH)D (>50 nmol/l) at baseline had a larger decline, whereas supplemented patients with lower 25(OH)D (<25 nmol/l) at baseline had a larger increase in serum 25(OH)D at 3 months. This may be due to regression to the mean, or may suggest that patients who had higher 25(OH)D levels at baseline may be affected more by the fat mass change, whereas those with low serum 25(OH)D may have benefited more from the supplementation.
ART has been associated with vitamin D deficiency in HIV patients( Reference Allavena, Delpierre and Cuzin 2 , Reference Conrado, Miranda-Filho D de and Ximenes 6 , Reference Welz, Childs and Post 36 – Reference Klassen, Martineau and Wilkinson 39 ). All patients in this study received a combination of non-nucleoside RT inhibitors and nucleoside RT inhibitors. Patients who were treated with a combination of TDF/3TC/EFV and AZT/3TC/NVP had lower serum 25(OH)D at 3 months compared with patients on TDF/3TC/NVP. Several studies have reported an association between efavirenz and vitamin D deficiency( Reference Allavena, Delpierre and Cuzin 2 , Reference Havers, Detrick and Cardoso 35 , Reference Theodorou, Sersté and Van Gossum 37 , Reference Brown and McComsey 40 , Reference Welz, Childs and Ibrahim 41 ). In the MONET trial, which was conducted among European HIV-positive adults on ART, efavirenz and zidovudine were associated with vitamin D deficiency. Moreover, an increase in vitamin D level was noted when study participants in the MONET trial were changed from efavirenz or zidovudine( Reference Fox, Peters and Prakash 42 ). Although the exact mechanism on how efavirenz affects vitamin D metabolism is not well known, it is hypothesised to be related to its effect on increased catabolism of 25(OH)D through induction of the cytochromes (CYP450, CYPA4, CYP24 and CYP2C9)( Reference Welz, Childs and Post 36 , Reference Brown and McComsey 40 , Reference Welz, Childs and Ibrahim 41 , Reference Pasquet, Viget and Ajana 43 ).
This study investigated early changes in serum 25(OH)D levels in HIV patients initiating ART and the effect of nutritional supplementation in adult HIV patients. This has not been reported in other studies in Africa or elsewhere in the tropics. In addition, we have found and demonstrated a high occurrence of low levels of serum 25(OH)D in people living in the tropics. However, our study has some limitations. The HIV-negative reference group was recruited over a short period of time and mainly after recruitment of participants for the trial, whereas HIV-positive participants were recruited over a 2-year time period. We also found differences in serum 25(OH)D level between the same seasons of different years, which we, however, controlled for by analysing season-year as a random effect. We were not able to assess the effect of supplementation on serum 25(OH)D among those with BMI<17 kg/m2, as those with BMI between 16 and 17 kg/m2 were not randomised to delayed supplementation, and those with BMI<16 kg/m2 were not included in the trial. As very few patients had switched ART regimen in the 3 months( Reference Abdissa, Olsen and Yilma 44 ), our analyses are based on the ART regimen used at initiation, and also the influence of ART could not be analysed in details because of a very uneven distribution of regimens and the use of second-line drugs by indication.
Conclusion
Our results demonstrated low levels of serum 25(OH)D in both HIV-positive and HIV-negative individuals in Ethiopia. Female participants, males with presumed low sun exposure, and participants with low BMI, WHO clinical stage IV disease and on TB treatment were at higher risk of low serum 25(OH)D. Moreover, further reduction in serum 25(OH)D after 3 months of ART was observed in HIV patients who did not receive nutritional supplement. We suggest that the changes in body composition in HIV patients that result from HIV itself before ART and from antiretroviral drugs after ART may contribute to the early change in serum 25(OH)D. Public awareness about the importance of sun exposure is necessary to improve serum 25(OH) levels. Vitamin D replenishment may be needed to prevent reduction in serum 25(OH)D level during ART.
Acknowledgements
The authors thank the study participants for their cooperation. The authors are grateful to Jimma University Specialized Hospital – HIV clinic staff and the study staff for their support during data acquisition.
The study was funded by US Dairy Export Council and Ministry of Foreign Affairs of Denmark. Nutriset developed the supplements and partially covered transportation expenses.
D. Y., A. B. A., O. K. and H. F. conceived the study. D. Y., P. K., C. M., K. F. M., A. B. A., O. K. and H. F. designed the study. D. Y., M. F. O., P. K., M. T., A. A., H. K. and T. G. contributed to data acquisition. D. Y., P. K., A. B. A., H. F. and C. R. contributed to the analysis of data. All authors contributed to data interpretation and reviewed the manuscript. All the authors have read and approved the final manuscript.
The authors declare no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114516003743









