The prevalence of metabolic disorders, such as obesity, hyperlipidaemia and hyperglycaemia, is rising dramatically in developing and industrialised nations. Obesity is reaching epidemic proportions worldwide(Reference Baena Diez, del Val Garcia and Tomas1) and is an established risk factor for various comorbidities, such as type 2 diabetes mellitus and CVD(Reference Anderson and Konz2–Reference Unwin, Gan and Whiting4). The development of obesity induces systemic oxidative stress(Reference Furukawa, Fujita and Shimabukuro5) and affects the inflammatory state(Reference Gregor and Hotamisligil6). The constant increase in fat intake that is linked with sedentary lifestyles is the chief cause of this phenomenon(Reference Anderson and Konz2).
Developing preventive and therapeutic solutions that impede the rise in metabolic disorders has become a primary goal in the past decade. In addition to pharmaceutical approaches, the use of natural products in physiological doses has been recognised as an effective regimen to improve several health conditions(Reference Jouad, Haloui and Rhiouani7–Reference Balunas and Kinghorn9). Plant-based treatments have been validated as strategies in the prevention of obesity and type 2 diabetes mellitus(Reference Ye10).
Rosemary (Rosmarinus officinalis L.) extracts are natural antioxidants that are used in food, food supplements and cosmetic applications(Reference Panda11–Reference Ibarra, Cases and Bily15). Recently, rosemary extracts that have been standardised for carnosic acid and carnosol attained antioxidant status, garnering an additive E classification from the European Food Safety Authority, confirming its importance as a natural preservative in foods and beverages(Reference Aguilar, Autrup and Barlow13).
Carnosic acid-rich rosemary extract has been reported to have antioxidant activity in vitro by oxygen radical absorbance capacity and ferric-reducing/antioxidant power assays, and it inhibits the oxidation of Cu2+-induced LDL ex vivo (Reference Ibarra, Cases and Bily15). These antioxidant effects have been recapitulated in vivo. Consequently, carnosic acid-rich rosemary extract reduces oxidative stress in aged rats(Reference Posadas, Caz and Largo16). Carnosic acid has anti-inflammatory effects in cellular(Reference Yu, Lin and Chan17) and animal(Reference Mengoni, Vichera and Rigano18) models. Furthermore, carnosic acid has promising anti-obesity and anti-glycaemic effects.
In in vitro trials, carnosic acid inhibits pancreatic lipase(Reference Ninomiya, Matsuda and Shimoda19), activates PPAR-γ(Reference Rau, Wurglics and Paulke20) and prevents the differentiation of mouse pre-adipocytes into adipocytes(Reference Takahashi, Tabuchi and Tamaki21) – all of which are important mechanisms in glucose and lipid homeostasis. The capacity of rosemary to regulate weight gain(Reference Harach, Aprikian and Monnard22, Reference Wang, Takikawa and Satoh23) and glycaemia(Reference Harach, Aprikian and Monnard22–Reference Erenmemisoglu, Saraymen and Ustun24) has been observed in vivo. Nevertheless, no randomised clinical trials have been reported using rosemary extracts to control obesity and hyperglycaemia, but this evidence encourages further study of carnosic acid-rich rosemary extracts to prevent the development of metabolic disorders.
In the present study, we aimed to determine the preventive effects of a rosemary extract that was standardised to contain 20 % carnosic acid (RE) on weight gain, glycaemia levels and lipid homeostasis in mice that were started on a high-fat diet (HFD) as juveniles. The animals were given a low-fat diet (LFD), a HFD or a HFD with 500 mg RE/kg body weight per d (mpk) (HFD-RE). Physiological and biochemical parameters were measured throughout the 16 weeks of treatment, and the effects on pancreatic lipase and PPAR-γ agonist activity in vitro were examined.
Experimental methods
Rosemary leaf extract
RE was prepared as described by Ibarra et al. (Reference Ibarra, Cases and Bily15).
Animals and diet
Male C57BL/6J mice, aged 4 weeks, were purchased from Elevage Janvier (CERJ, Le Genest Saint Isle, France). All mice were housed in a cage on a 12 h light–12 h dark cycle in a temperature-controlled environment during a 2-week acclimatisation, with ad libitum access to water and a control standard diet – an energy-balanced diet. After acclimatisation, the mice were randomised by body weight into three groups of eight animals. Each group was fed an experimental diet (Research Diets, Inc., New Brunswick, NJ, USA) for 16 weeks, as described in Table 1 (LFD, HFD and HFD-RE). Body weight was measured twice per week, and food intake was recorded once per week. All procedures were performed as per French guidelines for the care and use of experimental animals.
Table 1 Composition of the experimental low-fat diet (LFD), high-fat diet (HFD) or a HFD plus rosemary extract (RE) and macronutrient content (%)

mpk, mg rosemary extract/kg body weight per d.
Blood biochemistry
Blood was collected from the retro-orbital sinus into EDTA-coated tubes under isoflurane anaesthesia after overnight fasting. Samples were collected at the beginning of the study (day 0) and after 16 weeks on the experimental diets. Blood samples were centrifuged at 4000 rpm for 15 min at 4°C to recover the plasma.
Biochemical levels were measured using commercial kits. Total cholesterol, TAG, glucose (kits CH3810, TR3823 and GL3815; Randox Laboratories Limited, Newbury, Berkshire, UK) and NEFA (kit 434-91717; Wako Pure Chemical Industries Limited, Osaka, Japan) were measured by spectroscopy. Insulin (kit INSKR020; Crystal Chem, Inc., Downers Grove, IL, USA) was measured by ELISA.
Faecal lipid measurements
Faeces were collected at weeks 0, 8 and 16, frozen at − 80°C and pulverised. For each condition, faeces from eight mice, harvested during a 24 h period, were pooled. Total lipids were extracted from 100 mg of dried faeces as described(Reference Folch, Lees and Stanley25). Total lipid levels from several independent extractions were estimated by traditional gravimetric analysis: 500 μl of total lipids in chloroform were dried by evaporation and weighed.
The amount of faecal fat energy that was excreted, expressed in kJ/animal per d, was calculated in the lyophilised total fat that was excreted and collected throughout the experiment, assuming that 1 g lipid equals 37·7 kJ.
Pancreatic lipase activity assay
Human pancreatic lipase was purchased from Lee Biosolutions, Inc. (St Louis, MO, USA). Orlistat (tetrahydrolipstatin, a pancreatic lipase inhibitor) was purchased from Sigma Chemical Company (St Louis, MO, USA). Other chemicals were of reagent grade. The pancreatic lipase was diluted in dimethyl sulfoxide to obtain a final activity of 0·1 × 106 U/l. Orlistat was tested at two concentrations in dimethyl sulfoxide.
Lipase activity was measured using the ENZYLINETM Lipase Colour Assay kit (Biomérieux, Marcy-l'Etoile, France) according to the manufacturer's instructions. Briefly, pancreatic lipase, substrate and the test sample were mixed gently, and incubated for 5 min at 37°C. Activator reagent was added, and the mixtures were incubated again for 6 min at 37°C. The recorded rate of increase in absorbance at 550 nm, due to the formation of quinone diimine dye, reflected pancreatic lipase activity.
PPAR-γ assay
PPAR-γ activation was measured in a cell-based luciferase assay. COS-7 cells (African Green Monkey SV40-transformed kidney fibroblast cell line), cultured in Dulbecco's modified Eagle's medium that was supplemented with 10 % fetal calf serum, were transiently transfected with a fusion protein GAL4/PPAR-γ and a DNA construct that harboured the gene reporter. The plasmid pGal5-TK-pGL3 was obtained by inserting five copies of the Gal4 (a yeast transcription factor) DNA-binding site upstream of the thymidine kinase promoter in pTK-pGL3.
The plasmid pGal4-human PPAR-γ was constructed by PCR amplification of the human PPAR-γ DEF domain (nuclear receptor Hinge region (D)+ligand binding domain (E)+C-terminal domain (F)) (aa 318–505). The resulting amplicons were cloned into pBD-Gal4 (Stratagene, La Jolla, CA, USA), and the chimera was subsequently subcloned into pCDNA3.
After transfection, the COS-7 cells were incubated for 24 h with RE to assess its capacity to activate PPAR-γ. Dimethyl sulfoxide was used as the reference control, and rosiglitazone was used as a positive control. The activation of PPAR-γ by RE induced the expression of luciferase and a consequent increase in luminescence.
After 24 h, the cells were collected, and the luciferase assay was performed according to the manufacturer's instructions (SteadyGlow; Promega, Charbonnières, France). Luminescence was measured on a Tecan Ultra spectrophotometer (Tecan, Männedorf, Switzerland). All experiments were performed in quadruplicate. Relative luciferase activity of a sample was calculated as the ratio of mean luciferase activity in the test cells to that in the control cells, and PPAR-γ ligand-binding activity was expressed as the ratio of relative luciferase activity to that of the reference control.
Analysis of results
The animals were randomised based on total body weight by principal component analysis (GENFIT, Loos, France), resulting in groups of animals between which no statistical difference was observed for any parameter.
The data from the in vivo and in vitro studies are expressed as means and standard deviations. One-way ANOVA (one-way Bonferroni) and Student's t test were performed to compare groups using Sigma Plot 11.0 (2008) (Systat Software, Inc., Chicago, IL, USA). Statistical significance was considered at P < 0·05.
In the in vivo study, gains in the HFD-RE group are expressed as a percentage compared with those in the HFD and LFD control groups, which is calculated as:
In the in vitro studies, changes are expressed relative to their respective controls.
Results
Effect of rosemary extract on body and organ weight and food intake in mice fed a high-fat diet
Body weight between the HFD and LFD groups began to differ significantly after the first week of treatment. In HFD-RE animals, body weight differed significantly after day 80 (Fig. 1) compared with that in HFD mice and weight gain peaked at 69 % (P < 0·01) at the end of the study (Table 2). This effect was associated with a 79 % (P < 0·001) less of an increase in epididymal fat mass; liver weight was unaffected by the treatment. No significant changes in food or energy intake were observed between the groups (Table 2).

Fig. 1 Effects of rosemary extract (RE) standardised to 20 % carnosic acid on body weight after 16 weeks. Low-fat diet (![]() ), high-fat diet (HFD,
), high-fat diet (HFD, ![]() ) and HFD supplemented with RE (HFD-RE,
) and HFD supplemented with RE (HFD-RE, ![]() ) at a concentration equivalent to 500 mg RE/kg body weight per d. Values are means, with standard deviations represented by vertical bars. Mean values were significantly different from HFD control (ANOVA one-way Bonferroni): * P < 0·05, ** P < 0·01, *** P < 0·001.
) at a concentration equivalent to 500 mg RE/kg body weight per d. Values are means, with standard deviations represented by vertical bars. Mean values were significantly different from HFD control (ANOVA one-way Bonferroni): * P < 0·05, ** P < 0·01, *** P < 0·001.
Table 2 Effects of chronic administration of rosemary extract (RE) standardised to 20 % carnosic acid on nutritional and weight parameters in mice fed a low-fat diet (LFD), a high-fat diet (HFD) or a HFD plus RE after 16 weeks
(Mean values and standard deviations)
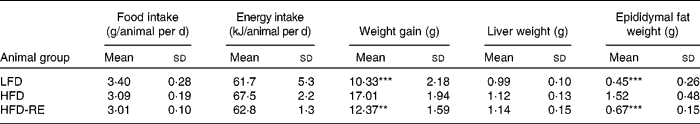
Mean values were significantly different from HFD control (ANOVA one-way Bonferroni): ** P < 0·01 and *** P < 0·001.
Effects of rosemary extract on serum biochemical parameters
At 16 weeks of treatment, total fasting glycaemia, total cholesterol and NEFA levels rose significantly in the HFD group compared with the LFD animals. HFD-RE mice experienced 72 % (P < 0·01) less increase in plasma glucose levels and 68 % (P < 0·001) less an a rise in total cholesterol compared with HFD mice. No significant effects were observed in NEFA or TAG levels in HFD-RE mice compared with the HFD group (Table 3).
Table 3 Effects of chronic administration of rosemary extract (RE) standardised to 20 % carnosic acid on plasma lipid and glucose levels in mice fed a low-fat diet (LFD), a high-fat diet (HFD) or a HFD plus RE after 16 weeks
(Mean values and standard deviations)

Mean values were significantly different from HFD control (ANOVA one-way Bonferroni): * P < 0·05, ** P < 0·01 and *** P < 0·001.
Fasting insulinaemia was also monitored; insulin levels remained low during the entire experiment, and no significant differences were observed between the groups (data not shown).
Effect of rosemary extract on faecal fat excretion
HFD animals had higher faecal fat excretion values (7·63 (sd 1·27) mg/100 mg) compared with LFD mice (2·74 (sd 0·10) mg/100 mg, P < 0·001) after 16 weeks. Moreover, RE-treated mice experienced a significant 1·2-fold increase (P < 0·01) in total faecal content compared with HFD animals (Fig. 2).

Fig. 2 Effects of rosemary extract (RE) standardised to 20 % carnosic acid on total faecal lipid content at weeks 0, 8 and 16. Low-fat diet (LFD), high-fat diet (HFD) and HFD supplemented with RE (HFD-RE) at a concentration equivalent to 500 mg RE/kg body weight per d. Values are means, with standard deviations represented by vertical bars of pooled data (n 6; except for LFD, n 3). Mean values were significantly different from HFD control (ANOVA one-way Bonferroni): ** P < 0·01, *** P < 0·001.
Throughout the experiment, there was no significant difference in fat energy intake between the HFD and HFD-RE groups. However, faecal fat energy excretion rose 1·3-fold (P < 0·05) in RE-treated mice (1·13 (sd 0·16) kJ/animal per d) v. the HFD group (0·87 (sd 0·15) kJ/animal per d). Faecal fat energy excretion differed between the LFD group (0·32 (sd 0·01) kJ/animal per d, P < 0·001) and HFD mice (Fig. 3).

Fig. 3 Effects of rosemary extract (RE) standardised to 20 % carnosic acid on total faecal energy excretion over 16 weeks. Low-fat diet (LFD), high-fat diet (HFD) and HFD supplemented with RE (HFD-RE) at a concentration equivalent to 500 mg RE/kg body weight per d. Values are means, with standard deviations represented by vertical bars of pooled data (n 6; except for LFD, n 3). Mean values were significantly different from HFD control (Student's t test): * P < 0·05, *** P < 0·001.
In vitro analysis of the mechanism of rosemary extract
As shown in Fig. 4, 100 μg/ml RE (P < 0·001) inhibited pancreatic lipase activity by 70 % compared with Orlistat. RE also activated PPAR-γ by 1·66-fold (P < 0·001) in a dose-dependent manner compared with the blank control at 30 μg/ml (Fig. 5), whereas activation in the positive control, rosiglitazone, was 4·35-fold (P < 0·001) that in the blank control at 10 nm. Therefore, 30 μg/ml RE are able to activate PPAR-γ by 19·70 % compared with the positive control.

Fig. 4 Effects of rosemary extract (RE) standardised to 20 % carnosic acid on pancreatic lipase inhibition in vitro. Values are means, with standard deviations represented by vertical bars expressed as a percentage of inhibition of three independent experiments. Mean values were significantly different from the blank control (Student's t test): *** P < 0·001.

Fig. 5 Effects of rosemary extract (RE) standardised to 20 % carnosic acid on PPAR-γ activation in vitro. Values are means, with standard deviations represented by vertical bars expressed as a percentage of inhibition of three independent experiments. Mean values were significantly different from the blank control (Student's t test): *** P < 0·001. DMSO, dimethyl sulfoxide.
Discussion
The production of RE, estimated to exceed 100 tonnes annually, has risen considerably in recent years due to its widespread use in food, beverage, flavour, food supplements and cosmetic applications(Reference Panda11–Reference Ibarra, Cases and Bily15). Consequently, technologies to develop standardised extracts from rosemary have evolved tremendously with regard to quality and reproducibility. Recently, we have demonstrated the importance of standardisation in determining the biological activity of plant extracts. Furthermore, depending on the content, we have observed that the antioxidant activities of various preparations of RE vary(Reference Ibarra, Cases and Bily15).
Rosemary exerts various biological activities with which preventive nutritional strategies against metabolic disorders, such as obesity, dyslipidaemia and diabetes, can be developed(Reference Ninomiya, Matsuda and Shimoda19–Reference Wang, Takikawa and Satoh23). Nevertheless, individual studies have examined specific extracts, the chemical description of which is not always available. Thus, it can be difficult to extrapolate the health benefits of a unique RE to another solely on the basis of published data.
In a previous study, we identified RE as the most potent antioxidative extract in an ex vivo LDL oxidation model(Reference Ibarra, Cases and Bily15), prompting us to determine whether it affects other metabolic parameters in a HFD mouse model. In the present study, the administration of a 44·92 % fat diet to 6-week-old C57BL/6J mice for 16 weeks resulted in significant increases in body weight, epididymal fat mass, glycaemia and cholesterol, compared with a LFD, confirming previous reports(Reference Surwit, Feinglos and Rodin26, Reference Black, Croom and Eisen27).
Treatment of mice with 500 mpk of RE reduced the gains in weight that were induced by the HFD without affecting food intake or fat energy intake. It also lowered epididymal fat tissue weight significantly compared with HFD mice. In addition, total faecal lipid content increased in HFD-RE mice compared with the HFD group, which correlates with the amount of total faecal fat energy that was excreted. Based on the present study and other reports(Reference Ninomiya, Matsuda and Shimoda19, Reference Harach, Aprikian and Monnard22), limiting lipid absorption in the intestine is a potential mechanism by which RE prevents weight gain.
This hypothesis is strongly supported by evidence of the in vitro inhibitory effect of RE on pancreatic lipase activity, a key enzyme in the digestion and absorption of fat. Moreover, similar effects have recently been reported with an ethanolic extract of rosemary that contains rosmarinic acid, carnosol and carnosic acid, wherein the treatment of 15-week-old diet-induced obesity mice with 200 mpk of extract limited the weight gain that was induced by a 50 d HFD and increased the lipid faecal content by 2·2-fold. Ninomiya et al. (Reference Ninomiya, Matsuda and Shimoda19) observed that after 2 weeks of treatment with 20 mpk of carnosic acid alone, the weight of ddY (Deutschland, Derker, Yoker) mice fell by 7·6 % compared with the control group. Thus, in the present study, the effects of RE on faecal fat excretion and, consequently, faecal fat energy excretion partially explain the observed reductions in body weight.
In addition to its effects on physiological measures, RE significantly reduced elevated cholesterol levels that were induced by the HFD. Although these effects were not observed by Harach et al. (Reference Harach, Aprikian and Monnard22) or Ninomiya et al. (Reference Ninomiya, Matsuda and Shimoda19), they were observed in human subjects with Orlistat(Reference Micic, Ivkovic-Lazar and Dragojevic28–Reference Erdmann, Lippl and Klose30) and in animal models with other plant-based pancreatic lipase inhibitors(Reference Han, Zheng and Yoshikawa31, Reference Sheng, Qian and Zheng32). Dietary cholesterol absorption has been proposed to be associated with fat digestion; Young & Hui(Reference Young and Hui33) have shown that minimal TAG hydrolysis is sufficient to increase cholesterol transport significantly from lipid emulsions to intestinal cells. Consequently, pancreatic lipase inhibition has been proposed to be a target against which lipid malabsorption can be triggered to control TAG and cholesterol levels(Reference Young and Hui33).
In the present study, fasting glycaemia was reduced in animals in the HFD-RE group compared with the HFD control group. Few studies have evaluated the effect of rosemary on diabetes. Anti-hyperglycaemic effects can be induced by an ethanolic RE in the alloxan diabetic rat model(Reference Bakirel, Bakirel and Keles34) and by a water RE in a mouse model(Reference Erenmemisoglu, Saraymen and Ustun24), whereas no such effect has been observed in the diet-induced obesity mouse model with an extract that contains rosmarinic acid, carnosol and carnosic acid(Reference Harach, Aprikian and Monnard22). Based on these data, it appears that the anti-hyperglycaemic activity and preventive effects of an extract against type 2 diabetes mellitus depend on its composition and the animal model in which it is tested.
Recently, it has been reported that the glucose-lowering effect of rosemary is attributed to PPAR-γ activation, in which carnosic acid and carnosol were proposed be the active compounds(Reference Rau, Wurglics and Paulke20). Therefore, we examined the in vitro effects of RE on PPAR-γ activation, hypothesising that the glucose-lowering effects are mediated through this mechanism.
In the present study on C57BL/6J mice, we used an effective dose of RE – 500 mpk – that contains 100 mpk of carnosic acid. The European Food Safety Authority Panel on Food Additives has estimated the dietary exposure for adults and pre-school children (aged 1·5–4·5 years) to carnosol plus carnosic acid to be 0·04 and 0·11 mpk, respectively(Reference Aguilar, Autrup and Barlow13). Thus, considering the normal dietary exposure of carnosic acid, we used a pharmacological dose of RE.
In addition, the Panel also notes that the margin between the not observable adverse effect level of carnosol plus carnosic acid, as calculated in 90 d rat studies, is equivalent to 20–60 mpk, and the mean intake of carnosic acid-rich rosemary extracts is estimated to be 500–1500 mg/d in adults and 182–546 mg/d in pre-school children(Reference Aguilar, Autrup and Barlow13). Therefore, future randomised clinical trials that aim to confirm the efficacy of RE in humans should consider these values to establish an effective and safe dose.
In conclusion, we have demonstrated that a carnosic-standardised RE limits weight gain and improves plasma lipid and glucose levels in a HFD mouse model. These data confirm its potential for use in preventive strategies against metabolic disorders and encourage the initiation of further studies to recapitulate the physiological activity of RE in human subjects.
Acknowledgements
Naturex is involved in the research/development and marketing/sales of rosemary extracts as ingredients for the food, cosmetic and nutraceutical industries. Therefore, Naturex has a commercial interest in this publication. Naturalpha was paid by Naturex to perform and report the scientific work that formed the basis of this publication. Naturalpha and Naturex declare that the data in this report represent a true and faithful representation of the work that has been performed. The financial assistance from Naturex is gratefully acknowledged. A. I. and C. R. designed the protocol. J. C. and M. R. developed the sample of RE. The study was conducted under the supervision of A. C. A. C.-B. analysed the data and reviewed the manuscript.










