The traditional Mediterranean diet (MD) is widely recognised as one of the healthiest in the world, and it is likely that more widespread adoption of this diet in non-Mediterranean countries would lead to a significant reduction in the incidence of many chronic diseases( Reference Sofi, Abbate and Gensini 1 ). Some health organisations in non-Mediterranean countries now recommend a MD. For example, in the UK, a MD is recommended by the NICE (National Institute for Health and Care Excellence) for secondary prevention following a myocardial infarction( 2 ). However, despite this type of targeted advice, there is only limited promotion of a MD to the general population in non-Mediterranean countries( Reference Piscopo 3 ), and campaigns for healthy eating tend to focus on promoting diets that are compatible with the cultural heritage of a people. For example, Public Health England promotes the Eatwell Plate, a dietary pattern modelled on a healthy UK-based diet( 4 ), and in Norway, the traditional Norwegian diet has been promoted as being more appropriate for this country than adopting a MD( Reference Bere and Brug 5 ).
Nevertheless, it can be argued that the well-proven health benefits of the MD justify it being more widely promoted in non-Mediterranean countries. Promoting a MD in non-Mediterranean countries is a viable public health approach since there is usually good compliance to this diet by non-Mediterranean individuals who adopt it, and, in general, eating habits in many countries are becoming more flexible( Reference Logan, Woodside and Young 6 , Reference Papadaki and Scott 7 ). In addition, local produce can be used, rather than foods that only grow in Mediterranean countries, since food choices for a MD are mostly based on food groups, such as ‘fruits’ or ‘vegetables’, rather than on specific foods( Reference Hoffman and Gerber 8 ). Indeed, it has been argued that many features of recommended dietary patterns in Northern Europe, such as high consumption of fruit and vegetables and low consumption of meat, are quite similar to the MD( Reference Bere and Brug 9 ).
One exception to the generalised recommendation of food groups, rather than specific foods, is to consume olive oil (OO) as the main source of added fat. Indeed, it is the consumption of OO – more than any other single factor – that distinguishes the traditional MD from other dietary patterns( Reference Lopez-Miranda, Perez-Jimenez and Ros 10 ). However, adopting OO as the main dietary fat as part of a MD in non-Mediterranean populations may present an obstacle since it is relatively costly compared with other cooking oils, and consumption of OO in non-Mediterranean populations is low( Reference Linseisen, Welch and Ocke 11 ). Consuming large quantities of OO in non-Mediterranean countries also raises the issues of food security. The food security agenda aims to increase the production of foods within national borders in order to guarantee food production independent of international influences. Since olive trees only grow in Mediterranean-type climates, this may not be compatible with food security issues, although this is less of an issue between European Union countries that share interdependent policies.
The health benefits of OO are attributed both to its high content of the MUFA oleic acid( Reference Bermudez, Lopez and Ortega 12 ) and to various minor components( Reference Cicerale, Conlan and Sinclair 13 ). Rapeseed oil (RO) (known as canola oil in the USA, Canada and some other countries) is a potential substitute for OO since it has a similar MUFA content to that of OO and its overall fatty acid (FA) profile is favourable due to a low content of SFA and a high content of PUFA, including α-linolenic acid (ALA). Consumption of RO is now high in many non-Mediterranean countries, partly due to the low cost, and also because it is perceived as being a healthy oil. There is increasing substitution of RO for OO, such as in recipes for the home cook, and in the UK, the NICE do not specify OO in their description of a MD but instead refer to ‘vegetable oil’, which in the UK generally refers to RO( 2 ). Hence, perhaps not surprisingly, consumption of RO in the UK may now be starting to displace that of OO since OO sales have seen their first fall in over 20 years( 14 ).
Rapeseeds are widely grown, both for biofuel and for human consumption, in many European Union countries, Canada, China, Australia and India( Reference Przybylski and Gunstone 15 ). In the UK, rapeseeds are the only oilseeds harvested in significant quantities. In view of the relatively low cost and the ready availability of RO, we examine whether the health benefits of RO justify it replacing OO as part of wider recommendations for consumption of a MD in non-Mediterranean countries, and so ask whether RO can be regarded as an ersatz ‘Northern OO’ for the domestic consumer.
Methods
We used a narrative review approach, and searched electronic databases such as PubMed and Scopus up until April 2014. Keywords such as ‘olive oil’, ‘virgin olive oil’, ‘rapeseed oil’ and ‘Canola’ were used in combination with keywords such as ‘composition’ (and related words such as ‘phenolics’, ‘antioxidants’), ‘cardiovascular disease’ (and related words such as ‘coronary heart disease’ and ‘myocardial infarction’), ‘cancer’ and ‘neurodegenerative disease’ (and related words such as ‘Alzheimer's disease’ and ‘dementia’) and the study method (such as ‘cohort’ and ‘meta analysis’).
Results
Composition
Fats
In addition to a high MUFA content (mainly oleic acid), OO also contains a range of other FA( Reference Boskou and Gunstone 16 ). The levels of various FA in OO vary quite widely between oils depending on factors such as the type of olive tree cultivar used for oil production (see Table 1). RO also has a high MUFA content, as well as considerably higher levels of ALA than OO (see Table 1). Consumption of ALA is linked to cardioprotective benefits (see below). However, RO also contains approximately 1 % trans isomers of ALA, which are produced during the deodorisation step of oil production( Reference Gladine, Meunier and Blot 17 , Reference Vermunt, Beaufrere and Riemersma 18 ). There is a well-established link between trans-fatty acid consumption and the increased risk of CHD( Reference Bendsen, Christensen and Bartels 19 ), and although the level in RO does not in itself constitute a health risk, it is desirable to keep the levels of trans-fatty acids to a minimum.
Table 1 Compositions of rapeseed oil and olive oil

RO is very low in SFA, comprising only approximately 6 % of the total FA. This is about half the average content of SFA in OO, and it has been argued that this gives RO an advantage over OO( 20 ). However, the quite low proportion of SFA even in OO means that it would not normally be a significant daily source of SFA compared with other dietary sources such as meat or dairy products. For example, 20 ml OO contains 128 mg SFA, giving 9·62 kJ (2·3 kcal) of energy as SFA. The current UK intake of SFA is 12·7 % of the total energy intake( Reference Levy 21 ). Hence, consumption of 20 ml OO represents less than 1 % of the average daily intake of energy in the UK from SFA (0·9 % total energy in women based on an intake of 8368 kJ (2000 kcal) and 0·7 % total energy in men based on an intake of 10 460 kJ (2500 kcal)).
Minor components
There are significant differences between the minor components in RO and extra-virgin olive oil (EVOO), due not only to the source of the oil but also to production methods. EVOO is produced using mild conditions that include pressing olive fruits at a low temperature, washing with water, filtration and centrifugation. These conditions retain many of the original components of the olives. The most abundant minor component is the hydrocarbon squalene, and there are smaller quantities of carotenoids, triterpenoids, phytosterols (e.g. β-sitosterol, Δ5-avenasterol and campesterol) and tocopherols (approximately 95 % α-tocopherol) (Table 1). EVOO also contains a wide variety of phenolic compounds including secoiridoids (e.g. oleuropein) and their phenolic derivatives (e.g. tyrosol and hydroxytyrosol), flavonoids (e.g. luteolin and apigenin) and lignans (e.g. pinoresinol and acetoxypinoresinol). EVOO is the best quality OO and must meet predefined criteria in terms of sensory qualities and limits of acidity. Other OO have substantially lower levels of most of the minor components, and phenolic compounds, in particular, are reduced( Reference Boskou and Gunstone 16 ).
Many potentially beneficial biological actions have been described for the minor components in EVOO. Phenolic compounds of EVOO reduce the markers of inflammation and oxidative stress in vitro and in vivo ( Reference Servili, Sordini and Esposto 22 , Reference Cicerale, Lucas and Keast 23 ). Squalene reduces oxidative stress in human mammary epithelial cells( Reference Warleta, Campos and Allouche 24 ). Lignans are phyto-oestrogens with possible anticancer activity( Reference Landete 25 ), and it is noteworthy that OO (both EVOO and other OO) has been found to be the major dietary source of lignans in participants of the Prevención con Dieta Mediterránea (PREDIMED) study( Reference Tresserra-Rimbau, Medina-Remon and Perez-Jimenez 26 ). Secoiridoids such as oleuropein and its derivatives are of particular interest in relation to the health benefits of EVOO since they are not found in other food plants.
Standard production of RO requires a far higher level of processing including solvent extraction of the oil from the pressed seeds, and refining by degumming, neutralisation, bleaching and deodorisation. As a consequence, most of the minor constituents that were originally present in the rapeseeds are significantly depleted in the oil. Some of the phytosterols (including β-sitosterol, campesterol and brassicasterol) and tocopherols (mainly α- and γ-tocopherol, in a ratio of approximately 1:2) are lost, as are most or all of the phenolic compounds originally present (including a high proportion of sinapic acid and its derivatives)( Reference Zacchi and Eggers 27 ). Phytosterols are best known for their ability to reduce cholesterol uptake from the gut, although some, such as Δ5-avenasterol, possess antioxidant activity.
Cooking
Consumption of raw EVOO is often quite high in a Mediterranean cuisine, and this may be important since compositional changes can occur to oils during cooking (see below). Raw EVOO is used as a salad dressing or simply poured on bread, as a main ingredient in many dips and sauces and as an addition to stews at the end of cooking to enhance flavour. Whereas some people prize EVOO for its flavour, it is unclear whether the flavour of raw RO would be an acceptable substitute. OO is also consumed after frying and baking due to the oil being absorbed into the cooked food. Large quantities of OO are consumed in the lathera dishes of some eastern Mediterranean countries since the cooking oil in which vegetables are cooked is consumed as an integral part of the dish. OO is more commonly used for shallow frying (which typically requires an oil temperature of 140–160°C) rather than deep frying (180–190°C) due to its relatively low smoke point.
There can be significant thermal degradation of FA and minor components in oils during cooking, and this may potentially have detrimental health effects. Undesirable changes include the hydrolysis and polymerisation of TAG, the oxidation of FA and sterols, and the generation of trans-fatty acids. Lipid oxidation is influenced by various factors such as the type of food present, the proportion of the oil exposed to the air, and the amount of unsaturated fats in the oil. Oxidation increases with the degree of unsaturation: ALA (18 : 3n-3) is 2·4 times more reactive than linoleic acid (18 : 2n-6), which is forty times more reactive than oleic acid (18 : 1n-9)( Reference Frankel 28 ). This is of potential concern for RO due to its high ALA content. Prolonged and repeated deep frying with RO, as may occur in commercial establishments, can also lead to the generation of quite high levels of trans-fatty acids( Reference Roe, Pinchen and Church 29 ).
Loss of antioxidants
During frying, antioxidants in oils are lost due to both direct thermal degradation and to being consumed during the thermal oxidation of unsaturated fats( Reference Roman, Heyd and Broyart 30 ). EVOO contains a favourable ratio of antioxidants:PUFA compared with other types of oils, and this reduces both the rate at which antioxidants are lost and the rate of lipid oxidation that occurs during frying( Reference Santos, Cruz and Cunha 31 , Reference Sacchi, Paduano and Savarese 32 ). Antioxidants in EVOO deplete at different rates, as demonstrated in a study by Gomez-Alonso et al. ( Reference Gomez-Alonso, Fregapane and Salvador 33 ), who found that hydroxytyrosol was depleted to a far greater extent than tyrosol when EVOO was used for frying potatoes at 180°C for 10 min. Phenolic compounds in EVOO help stabilise vitamin E during heating, and vitamin E, in turn, helps protect PUFA from oxidative degradation( Reference Santos, Cruz and Cunha 31 ).
Despite the losses of minor components due to frying, heated virgin olive oil (VOO) has been shown to retain beneficial effects on postprandial inflammation. VOO repeatedly heated to 180°C has been shown to suppress postprandial inflammation in obese subjects (determined as NF-κB activation in peripheral blood monocytes) compared with a seed oil with a similar fat content (a blend of high-oleic acid sunflower oil and RO)( Reference Perez-Herrera, Delgado-Lista and Torres-Sanchez 34 ). Although the heating protocol completely depleted hydroxytyrosol in VOO, other minor components, including some phenolic compounds, were retained.
In summary, although antioxidants in EVOO are reduced during frying, using EVOO rather than other types of OO for frying may be justified as a means to minimise the oxidation of the relatively low content of PUFA and to reduce postprandial inflammation. Antioxidants in EVOO have also been to shown to migrate into the food during cooking, and so may confer health benefits in the body( Reference Chiou, Kalogeropoulos and Boskou 35 , Reference Kalogeropoulos, Chiou and Mylona 36 ).
Antioxidants in RO include phytosterols, vitamin E and Coenzyme Q, although levels of phenolic compounds are very low compared with those in EVOO (see Table 1). Vitamin E content was reduced by two-thirds when RO was heated at 150°C for 6 h( Reference Roman, Heyd and Broyart 30 ), and vitamin E was also significantly depleted using conditions designed to replicate RO being used for deep frying( Reference Przybylski, Gruczynska and Aladedunye 37 ). The concentration of ALA in RO is a major determinant of the extent of FA oxidation( Reference Warner and Mounts 38 ). The relatively low ratio of antioxidants:PUFA in RO may lead to significant losses of antioxidants and increase lipid peroxidation, although this will depend on the time period and temperature used for frying. The more favourable balance between antioxidants and PUFA in EVOO may retain more antioxidants.
Generation of toxic compounds
Insufficient protection of PUFA from oxidation leads to their conversion to hydroperoxides, and these may break down to various volatile compounds( Reference Moya Moreno, Mendoza Olivares and Amezquita Lopez 39 ). Some compounds, such as acetaldehyde and acrolein (2-propenal), are toxic. Acetaldehyde is classified as a carcinogen by the European Union, whereas the main health effect of exposure to acrolein is irritation of the eyes, the mucosae and the skin( 40 ). It is therefore desirable to minimise the exposure to toxic volatile compounds present in cooking fumes produced during frying. Fullana et al. ( Reference Fullana, Carbonell-Barrachina and Sidhu 41 ) reported that acetaldehyde production at 180°C was twice as high for RO as for either OO or VOO, although the levels from all oils were low, and no acetaldehyde emissions were detected by Katragadda et al. ( Reference Katragadda, Fullana and Sidhu 42 ) at 180°C. Production of acrolein by RO at 180°C was found to be approximately five times higher than acrolein production by either EVOO or OO( Reference Fullana, Carbonell-Barrachina and Sidhu 41 , Reference Katragadda, Fullana and Sidhu 42 ). This is probably due to the high ALA content of RO since recent studies have indicated that thermal degradation of ALA is the main source of acrolein in RO( Reference Ewert, Granvogl and Schieberle 43 , Reference Endo, Chieko and Yamanaka 44 ). The presence of antioxidants in EVOO such as chlorophylls, pheophytins and carotenoids may also reduce acrolein formation compared with RO( Reference Procida, Cichelli and Compagnone 45 ). Despite the generation of some toxic volatile compounds, especially by RO, there is no evidence that, under normal domestic conditions, using fresh RO for shallow frying is likely to pose a health risk through inhalation.
In summary, there exists a clear advantage for EVOO over RO in terms of the former's richer composition, limited processing without solvent extraction and deodorisation, and safety of use in cooking.
Health
Various studies have assessed the health benefits of OO and RO. Several expert committees have described the basis for making a robust judgement of a causal relationship between a nutrient or food and disease risk( 46 , 47 ). Consistency between several observational studies is necessary, with prospective studies being favoured over case–control studies. When available, there should be randomised controlled trials of sufficient size and duration, with more weight being given to disease incidence as an endpoint rather than to biological markers. Experimental studies, both in vivo and in vitro, can provide biological plausibility. We follow these guidelines for assessing the respective health benefits of OO and RO. Epidemiological studies are summarised in Tables 2 and 3.
Table 2 Recent epidemiological studies on the health effects of olive oil (OO)

Med diet, Mediterranean-style diet; PA, physical activity; EPIC, European Prospective Investigation into Cancer and Nutrition; EVOO, extra-virgin olive oil; CV, cardiovascular; Goldberg exclusion, exclusion of participants with poor concordance of energy intake with energy expenditure identified using the Goldberg criteria.
Table 3 Epidemiological studies on the health effects of dietary α-linolenic acid (ALA)
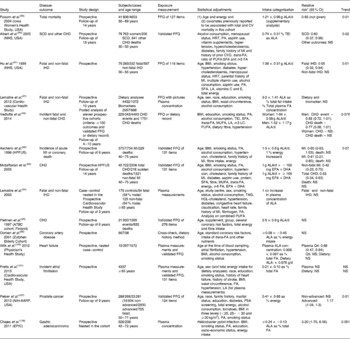
CV, cardiovascular; NHS, Nurse's Health Study; SCD, sudden cardiac death; HRT, hormone replacement therapy; PA, physical activity; MI, myocardial infarction; FA, fatty acids; TEI, total energy intake; LA, linoleic acid; LC, long chain; HPFUS, Health Professional Follow-up Study; Q, quintile; NIH-AARP National Institute of Health Aged American Retired Persons; PSA, prostate-specific antigen; EPIC, European Prospective Investigation into Cancer and Nutrition.
* When used as a nested case–control study.
† P for sex interaction.
Olive oil and health
CVD
Many epidemiological studies, including randomised controlled trials, have shown that a Mediterranean dietary pattern that includes OO is convincingly associated with a reduced risk of CVD, and is probably associated with a reduced risk of certain cancers and neurodegenerative diseases (reviewed in Hoffman & Gerber( Reference Hoffman and Gerber 48 )). Only a few of these epidemiological studies have focused on the specific effect of OO. Ancel Keys, the pioneer advocate of the MD, first proposed that it was the ratio of MUFA:SFA that was the key component for the health benefits of the MD( Reference Keys, Menotti and Aravanis 49 ). Although this suggested that the importance of OO was to provide MUFA, later on it was established that MUFA from sources other than OO (animal fat containing 40–45 % of MUFA) did not have the same beneficial effect( Reference Jakobsen, O'Reilly and Heitmann 50 ).
Consequently, studies were undertaken to decipher the specific effect of OO. In the Three-City Study, individuals with intensive use of OO showed a lower risk of stroke compared with those who never used OO( Reference Samieri, Feart and Proust-Lima 51 ). In the Italian-EPIC cohort, women with a high consumption of OO had reduced incidence risk of non-fatal and fatal myocardial infarction( Reference Bendinelli, Masala and Saieva 52 ), although it should be noted that this study has been criticised because it was not fully adjusted. In another analysis conducted on the EPIC population in Spain, a high intake of OO decreased the risk of overall mortality by 26 % and of CVD deaths by 44 %( Reference Buckland, Mayen and Agudo 53 ). A recent meta-analysis by Martinez-Gonzalez et al. ( Reference Martinez-Gonzalez, Dominguez and Delgado-Rodriguez 54 ) comparing high v. low intake of OO found a significant risk reduction for stroke, but the risk reduction for CHD was not significant (Table 2).
In the studies included in the meta-analysis by Martinez-Gonzalez et al. ( Reference Martinez-Gonzalez, Dominguez and Delgado-Rodriguez 54 ), only that by Buckland et al. ( Reference Buckland, Travier and Barricarte 55 ) distinguished between OO and EVOO. In this well-conducted study from Spain, there was a reduction in CVD incidence of 7 % for each 10 g increase of OO per 8·4 MJ ingested, and this effect was greater for EVOO (risk reduction 14 %). The role of EVOO was examined in the PREDIMED randomised control trial. Participants at high vascular risk were randomly allocated to three groups. Of these groups, two received a typical MD supplemented with either EVOO (1 litre/week) or mixed nuts (30 g/d). The third control group was advised to follow a low-fat diet. In the two groups that received advice on the MD, the risk of CVD (myocardial infarction, stroke or death from CVD) was reduced by approximately 30 %( Reference Estruch, Ros and Salas-Salvado 56 ). Recent additional analysis of the PREDIMED study provides further evidence for a superior beneficial effect of EVOO v. non-virgin OO on CVD risk. This observational prospective cohort analysis was based on baseline consumption of OO, i.e. before randomisation into groups. In individuals at high cardiovascular risk, there was a statistically significant reduction in total cardiovascular risk and stroke (but not myocardial infarction) for total OO consumption or for consumption of EVOO, but not for consumption of non-virgin OO( Reference Guasch-Ferré, Hu and Martinez-Gonzalez 57 ) (see Table 2). These results remained even after adjusting for adherence to a MD. The results highlight the possible important contribution of minor components in EVOO to cardiovascular protection.
Short-term studies with cardiovascular risk factors as end-points have also suggested that phenolic compounds are important for the cardiovascular benefits of VOO. For example, the EUROLIVE (the effect of olive oil consumption on oxidative damage in European countries) study, comparing OO high and low in phenolic compounds, found a linear increase in HDL-cholesterol levels for low-, medium- and high-polyphenol OO, and a linear decrease in oxidised LDL levels( Reference Covas, Nyyssonen and Poulsen 58 ). A reduction in LDL oxidation for EVOO with a minimum hydroxytyrosol content is the basis for a recent health claim issued by the European Food Safety Authority for the health benefits of OO( 59 ). VOO, as part of a Mediterranean diet, has also been shown to reduce the levels of circulating inflammatory molecules associated with increased cardiovascular risk( Reference Urpi-Sarda, Casas and Chiva-Blanch 60 ).
Experimental models, both in vitro and in vivo, have suggested that VOO can favourably alter many stages in atherosclerosis. VOO has been shown to reduce atherosclerosis in apoE-deficient mice and hamsters( Reference Lou-Bonafonte, Arnal and Navarro 61 ). Anti-inflammatory activities of minor components in VOO include reducing prostacyclin synthesis in human vascular smooth muscle cells, inhibiting cyclo-oxygenases( Reference Lucas, Russell and Keast 62 ), and inhibiting endothelial adhesion molecule expression( Reference Carluccio, Siculella and Ancora 63 ). Phenolic compounds also have favourable effects on haemostasis( Reference Delgado-Lista, Garcia-Rios and Perez-Martinez 64 ).
Although many studies have indicated that cardiovascular risk is reduced when MUFA replaces dietary SFA or carbohydrates( Reference Gillingham, Harris-Janz and Jones 65 ), epidemiological evidence for a specific contribution of oleic acid in OO to cardiovascular protection is limited. However, short-term feeding studies in human subjects have suggested that one benefit of diets rich in OO is that they do not have the adverse effects on postprandial inflammation and haemostasis compared with diets rich in SFA( Reference Bermudez, Lopez and Ortega 12 ). OO has also been shown to have beneficial hypotensive effects in short-term feeding studies( Reference Bermudez, Lopez and Ortega 12 ), and oleic acid has been implicated in these effects since, in rat models, triolein (the main TAG in OO, consisting of three oleic acid moieties) has been shown to reduce blood pressure as effectively as VOO( Reference Teres, Barcelo-Coblijn and Benet 66 ).
Cancers
A beneficial effect of adherence to a MD (as assessed by a MD score) and reduced cancer risk is found to be greater in Mediterranean, rather than non-Mediterranean, populations( Reference Hoffman and Gerber 8 ). The overall cancer mortality in the above-quoted Spanish study showed a relative risk of < 1, but was non-significant( Reference Buckland, Mayen and Agudo 53 ). In the PREDIMED study, no statistically significant associations were found between consumption of any type of OO and mortality from all types of cancer( Reference Guasch-Ferré, Hu and Martinez-Gonzalez 57 ). However, different cancer sites are characterised by different risk factors, and for some types of cancer, there are indications of a specific effect of OO, and this is supported by several in vitro and in vivo experimental studies( Reference Casaburi, Puoci and Chimento 67 ). A meta-analysis of twenty-five studies reported risk reduction for upper digestive and respiratory tract cancers, breast and, possibly, colorectal and other cancer sites( Reference Pelucchi, Bosetti and Negri 68 ). Similarly, a posteriori dietary pattern analysis has demonstrated a greater risk reduction in breast cancer when OO was present in the pattern( Reference Bessaoud, Daures and Gerber 69 – Reference Siari, Scali and Richard 71 ). A more recent study addressed the question of OO and breast cancer in the Mediterranean countries of the EPIC (European Prospective Investigation into Cancer and Nutrition) study and observed a non-significant risk reduction in oestrogen receptor-negative (ER − ) and progesterone receptor-negative breast cancers for a high intake of OO( Reference Buckland, Travier and Agudo 72 ). These cancers are independent from hormonal factors and differ from ER+ breast cancers in terms of risk factors. However, they represent only 25 to 30 % of all breast cancers, and the lack of statistical power might explain the large CI observed in this study (see Table 2). This epidemiological observation has been supported by an experimental model showing that the OO phytochemical oleuropein is more cytotoxic for basal-like ER − MDA-MB-231 cells than for luminal ER+ MCF-7 cells( Reference Elamin, Daghestani and Omer 73 ).
Neurodegenerative diseases
In the prospective Three-City Study, OO was associated with a decrease in cognitive impairment( Reference Berr, Portet and Carriere 74 ). In participants of the PREDIMED study, consumption of some foods was independently associated with better cognitive function. Among them, total OO positively correlated with immediate verbal memory and EVOO with delayed verbal memory( Reference Valls-Pedret, Lamuela-Raventos and Medina-Remon 75 ). More recently, in the PREDIMED-Navarra trial, 285 participants at high vascular risk were randomly allocated to three groups: a MD supplemented with EVOO; a MD supplemented with mixed nuts; a low-fat diet. Lower mild cognitive impairment was observed in the EVOO group compared with the control group( Reference Martínez-Lapiscina, Clavero and Toledo 76 ). Participants assigned to the MD+nuts group did not differ from the control group. Various antioxidant and anti-inflammatory phenolic compounds in EVOO may contribute to these beneficial effects since oxidative stress and inflammation are associated with neurodegeneration( Reference Gorelick 77 ). More specific effects have also been described for phenolic compounds of EVOO. Tyrosol and hydroxytyrosol have been shown to decrease activation by β-amyloid of the pro-inflammatory transcription factor NF-κB in cultured neuroblastoma cells( Reference St-Laurent-Thibault, Arseneault and Longpre 78 ). In mouse models of Alzheimer's disease where there is increased levels of β-amyloid, the EVOO phenolic compounds oleocanthal and oleuropein reduced β-amyloid levels and plaque deposits( Reference Grossi, Rigacci and Ambrosini 79 , Reference Abuznait, Qosa and Busnena 80 ), and improved memory( Reference Farr, Price and Dominguez 81 ).
The severity of skin photo-ageing was significantly attenuated by the consumption of MUFA from OO in subjects of the Suppléments en Vitamines et Minéraux Antioxydants (SUVIMAX) cohort( Reference Latreille, Kesse-Guyot and Malvy 82 ). Only MUFA from OO was efficient, suggesting that phenolic compounds or squalene in OO might be responsible for the beneficial effect on skin photo-ageing.
In summary, based on the recognised criteria of evidence in human studies, the level of evidence for the relationship of EVOO with CVD can be qualified as ‘convincing’, and for cancers as ‘limited-suggestive’, especially ER − breast cancer. For ageing and cognitive impairment, fewer data exist in favour of a specific beneficial effect of OO, and require confirmation. There is good evidence from both human and experimental studies that phenolic compounds present in EVOO are important for cardiovascular benefits. More limited experimental studies have also suggested that phenolic compounds are important for the anti-cancer and neuroprotective effects of EVOO.
Rapeseed oil and health
Whereas many studies have examined the relationship of OO with disease incidence or mortality as well as biomarkers for disease, studies with RO are mainly limited to outcomes based on biomarkers. Funding from the food industry and the RO industry was received by two recent reviews( Reference Harland 83 , Reference Lin, Allemekinders and Dansby 84 ), hence leading to possible conflicts of interest( Reference Bes-Rastrollo, Schulze and Ruiz-Canela 85 , Reference Smith 86 ). Most studies with RO have used raw RO. This limits the interpretation of these studies since most RO is consumed after frying, and this can cause significant changes in composition, especially of ALA, as discussed previously.
CVD
A number of reports comparing the effect of RO with a source of SFA on the biomarkers of CVD risk (total cholesterol, LDL-cholesterol, HDL-cholesterol and TAG, lipid peroxidation and inflammatory biomarkers) have found that RO is relatively beneficial, as it is an oil low in SFA and high in MUFA+PUFA( Reference Lin, Allemekinders and Dansby 84 ). As the US Food and Drug Administration put it in the qualified health claim for RO in 2006: ‘Limited and not conclusive scientific evidence suggests that eating about 1·5 tablespoons (19 g) of RO daily may reduce the risk of CHD due to the unsaturated fat content in RO. To achieve this possible benefit, RO is to replace a similar amount of saturated fat and not increase the total number of calories you eat in a day.’( 87 )
It is the generally accepted view that the benefits to heart health are greater when SFA is replaced with PUFA, rather than when SFA is substituted with MUFA( Reference Jakobsen, O'Reilly and Heitmann 50 ). Since there are no observational studies with RO, a review of epidemiological studies of the specific effect of ALA is relevant, albeit with the proviso of possible changes due to frying. These are summarised in Table 3. A review by the Afssa expert group in 2008 concluded that results on mortality were inconsistent( 88 ). Whereas Folsom & Demissie( Reference Folsom and Demissie 89 ) observed a modest risk reduction of total mortality in the Iowa Women's Health Study, two studies from the Nurse's Health Study cohort found an effect on mortality only from a sudden cardiac event( Reference Albert, Oh and Whang 90 , Reference Hu, Stampfer and Manson 91 ). Similarly, two studies( Reference Ascherio, Rimm and Giovannucci 92 , Reference Mozaffarian, Ascherio and Hu 93 ) from the Health Professional Study showed a risk reduction in myocardial infarction. An interesting finding was the observation that there was a risk reduction by ALA when EPA+DHA consumption was < 100 mg/d, and that this effect was lost when EPA+DHA consumption was ≥ 100 mg/d with a significant interaction (P= 0·003 for myocardial infarction and P= 0·006 for total CVD) between the two intakes. Similarly, the risk reduction observed for fatal IHD in a prospective study based on the measurement of ALA in phospholipids was abolished after adjusting for EPA+DHA( Reference Lemaitre, King and Mozaffarian 94 ). In two prospective studies based on ALA intake and conducted in Northern Europe, the Alpha-Tocopherol, Beta-Carotene (ATBC) study( Reference Pietinen, Ascherio and Korhonen 95 ) and the Zutphen study( Reference Oomen, Ocke and Feskens 96 ), no significant association has been observed.
More recently, another study based on circulating and dietary ALA found no effect of this FA on congestive heart failure( Reference Lemaitre, Sitlani and Song 97 ). In a meta-analysis published in 2012, there was a borderline significant risk reduction for CVD, and only fatal CHD was significant( Reference Pan, Chen and Chowdhury 98 ). A large unexplained heterogeneity was present in this meta-analysis, casting doubts on the results. A more recent analysis using a pooled study design found a non-significant inverse association between ALA intake and CHD risk in men, but found no consistent association in women( Reference Vedtofte, Jakobsen and Lauritzen 99 ). There has also been a report of a moderate non-linear association between ALA and heart failure( Reference Wilk, Tsai and Hanson 100 ), and another showing no association of ALA with atrial fibrillation( Reference Fretts, Mozaffarian and Siscovick 101 ).
Several studies have compared the effect of RO with that of OO on risk factors for CVD. A hypoenergetic RO-containing diet (supplied as oil and margarine) reduced systolic blood pressure, and total and LDL-cholesterol to a comparable extent as a refined OO diet, and also resulted in a greater reduction in diastolic blood pressure, probably because of the higher ALA content of the RO diet( Reference Baxheinrich, Stratmann and Lee-Barkey 102 ). In another study, RO resulted in a reduction of total cholesterol of 12 v. 5·4 % for OO, but HDL-cholesterol was also significantly reduced in the RO group, but not in the OO group( Reference Lichtenstein, Ausman and Carrasco 103 ). In a further study, eighteen subjects in six experimental cross-over groups received 50 g oil/10 MJ in a diet of 15 MJ. After 3 weeks, there was a significant reduction of LDL-cholesterol in the RO group, which is expected since RO contains 21 % PUFA( Reference Pedersen, Baumstark and Marckmann 104 ). All other biomarkers were not significantly different. With the same study design, the same group later published the results on TAG. After 3 weeks, fasting TAG concentrations were significantly higher for the OO regimen, with no difference on either postprandial TAG or susceptibility to lipoprotein oxidation( Reference Nielsen, Pedersen and Sandstrom 105 ).
In conclusion, despite limited evidence of the beneficial effects of RO in short-term studies on the biomarkers of risk factors for CVD, there are currently no observational and intervention studies to suggest that RO has the cardiovascular benefits of EVOO. Any benefits of RO are likely to be due to ALA.
Cancer
ALA has been associated with an increased risk of prostate cancer, but results have been inconsistent. A meta-analysis did not find an association between dietary ALA intake and prostate cancer risk( Reference Carleton, Sievenpiper and de Souza 106 ), although a more recent study has found that ALA intake increased the risk of advanced prostate cancer in elderly men( Reference Pelser, Mondul and Hollenbeck 107 ) (Table 3). There are indications of risk for gastric cancer( Reference Chajes, Jenab and Romieu 108 ). Inhalation of the vapours from unrefined RO with a high content of ALA used for cooking was associated with cancers in China( Reference Shields, Xu and Blot 109 ).
We did not conduct searches for the effects of RO on other diseases.
Discussion
Recent developments
The increased susceptibility of ALA to oxidation has led to the commercial development of modified RO with decreased ALA. These include a low-linolenic acid RO, which has increased linoleic acid content, and high-oleic acid RO( Reference Przybylski and Gunstone 15 ). These modified oils have better heat stability( Reference Przybylski, Gruczynska and Aladedunye 37 ), but they are more expensive than the standard RO. Currently, there are no clinical studies on their effects on health. However, as noted above, reducing ALA and increasing MUFA may reduce the possible cardioprotective benefits of RO.
A second approach has been to increase the level of antioxidant phytochemicals in RO. In 2006, the European Union-funded project ‘Optim'Oils’ was initiated with the aim of improving production methods for RO. An oil with significantly lower 18 : 3 trans and improved phytochemical composition (minimised losses of phytosterols, tocopherols and phenolics) was successfully developed( Reference Gladine, Meunier and Blot 17 ). In a clinical study, total/HDL-cholesterol and LDL/HDL-cholesterol concentrations were increased by 4 % (P< 0·05) with the consumption of raw standard RO, and there were also non-significant increases in oxidised LDL. These increases were not observed with the optimised oil( Reference Gladine, Combe and Vaysse 110 ), and hence there were modest benefits of the optimised RO compared with the standard RO.
Another interesting way forward is to incorporate olive phenolics into RO. The waste water from OO production (olive mill waste water, OMWW) contains high levels of some olive phenolics( Reference Obied, Allen and Bedgood 111 ), and disposal of OMWW is of major environmental concern( Reference Dermechea, Nadoura and Larrocheb 112 ). An OMWW extract has been used to improve the oxidative stabilities of lard( Reference De Leonardis, Macciola and Lembo 113 ), sunflower oil( Reference Lafka, Lazou and Sinanoglou 114 ) and refined OO( Reference Fki, Allouche and Sayadi 115 ). A seed oil comprising 30 % high-oleic sunflower oil and 70 % RO enriched with OMWW was found to reduce postprandial inflammation in obese subjects as effectively as VOO, even after twenty cycles of heating the oils at 180°C( Reference Perez-Herrera, Delgado-Lista and Torres-Sanchez 34 ). Incorporation of phenolic compounds from OMWW also has the potential to improve the cardiovascular health benefits of RO since OMWW, which has high levels of hydroxytyrosol, has been shown to reduce LDL oxidation( Reference Visioli, Romani and Mulinacci 116 ).
Conclusions
The extensive evidence for the health benefits of EVOO is not matched by similar data for RO, and based on current evidence, RO cannot be recommended as equivalent in terms of health benefits compared with EVOO. There are significant losses of minor constituents during the processing of standard RO, and there may also be deleterious changes in FA composition when RO is used for cooking. New initiatives to alter the production methods and composition of RO are addressing some of these issues and could lead to a far healthier, albeit more expensive, product for the consumer in the future. Nevertheless, RO lacks many of the constituents in EVOO, such as secoiridoids and their derivatives, which are thought to be important for its health benefits and desirable stability during cooking. The use of OMWW to stabilise RO and improve its health benefits may be of mutual benefit to both industries by using an environmentally polluting waste product from the OO industry to the benefit of producing a healthier product for the RO industry. However, the current high fungicide usage on the oilseed rape crop is also of concern( Reference Barnes, Wreford and Butterworth 117 ).
Acknowledgements
The present review received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors’ contributions were as follows: M. G. was responsible for the Health sections; R. H. conceived the article and was responsible for the remainder of the content and editing of the article.
Neither of the authors has any conflicts of interest to declare.





