Globally, breast cancer ranks first for cancer incidence and fifth for cancer mortality in women( Reference Fitzmaurice and Dicker 1 ). Dietary and lifestyle factors may have an important role in the development of breast cancer( Reference Gerber, Müller and Reimer 2 – Reference Singletary and Gapstur 4 ), among which Ca intake has been suggested as a potential protective factor in mounting experimental research( Reference Whitfield, Boynton and MacManus 5 – Reference Kumar, Kumar and Ghosh 10 ) and several observational studies( Reference Knekt, Järvinen and Seppänen 11 – Reference Lin, Manson and Lee 13 ). A meta-analysis by Chen et al.( Reference Chen, Hu and Xie 14 ) involving six prospective cohorts and nine case–control studies suggested a significant inverse association between Ca intake and risk of breast cancer, with a summary relative risk (RR) of 0·81 (95 % CI 0·72, 0·90) for the highest compared with the lowest intake of Ca, with a significant publication bias. Their results became statistically non-significant after correcting for publication bias. The meta-analysis was followed by several subsequent prospective studies( Reference Park, Leitzmann and Subar 15 – Reference Abbas, Linseisen and Rohrmann 19 ) that also focused on the same topic, but their findings continued to be inconsistent. To clarify the association between Ca intake and risk of breast cancer, we performed an updated meta-analysis of prospective studies. Given the fact that high amounts of Ca, particularly from supplements, might increase risks of certain diseases, such as CVD( Reference Xiao, Murphy and Houston 20 – Reference Pentti, Tuppurainen and Honkanen 24 ) and kidney stones( Reference Jackson, LaCroix and Gass 25 , Reference Curhan, Willett and Speizer 26 ), we also attempted to explore the shape of the dose–response association between Ca intake and breast cancer that has not been investigated in the previous meta-analysis.
Methods
Search strategy
This meta-analysis was planned, conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendation( Reference Moher, Liberati and Tetzlaff 27 ). PubMed and Embase databases were searched for studies assessing the association between Ca intake and breast cancer up to March 2016. The following search terms were used to retrieve the relevant literature in the databases: (‘calcium’ OR ‘dairy products’ OR ‘dairy’ OR ‘milk’ OR ‘cheese’ OR ‘yogurt’ OR ‘butter’ OR ‘cream’) AND (‘breast cancer’ OR ‘mammary gland cancer’ OR ‘breast neoplasms’ OR ‘mammary gland neoplasms’ OR ‘neoplasm of the breast’ OR ‘neoplasm of the mammary gland’) AND (‘cohort’ OR ‘prospective’ OR ‘nested case-control’ OR ‘case-cohort’ OR ‘observational study’). The search strategy had no language, publication date or publication type restriction. In addition, the reference lists of retrieved full publications and previous meta-analysis were reviewed to complement the search and to identify relevant studies that were missed during electronic database search. We also contacted the authors of the primary studies for further information.
Study selection
To be included in this meta-analysis, the studies had to meet the following inclusion criteria: (a) the study design was a prospective study (including a prospective cohort study, nested case–control study and a case–cohort study); (b) the exposure of interest was Ca intake (dietary and/or supplemental Ca); (c) the outcome of interest was breast cancer incidence; (d) female participants; and (e) risk estimates with corresponding 95 % CI were available. Accordingly, retrospective studies, or studies on breast cancer mortality or recurrence, were excluded. If one study was reported in overlapping publications, the publication containing more detailed information (i.e. reporting data for subgroup or dose–response analyses) was selected.
Data extraction and quality assessment
Using a standardised data-collection form, the following data were abstracted from each study: the first author’s last name, publication year, study population, duration of the study, country, length of follow-up, number of cases, dietary assessment method, sources of Ca intake (diet and/or supplement), the multivariable-adjusted risk estimates with their corresponding 95 % CI for each category of Ca intake and statistical adjustment for potential confounding factors. The study quality was assessed using the nine-star Newcastle–Ottawa Scale (NOS)( Reference Wells, Shea and O’Connell 28 ), in which each study was judged based on the selection of the study groups, the comparability of the groups and the ascertainment of exposure and outcome. Two investigators (K. H. and G.-C. C.) participated in literature search, study selection and data extraction independently. Any discrepancies regarding inclusion were solved through group discussion.
Statistical analysis
RR was chosen as the common measure of association across this study, and hazard ratio was directly considered as RR. A DerSimonian & Laird random-effects model( Reference DerSimonian and Laird 29 ) was used to calculate the summary risk estimates. The degree of heterogeneity in the relationship between Ca intake and breast cancer across studies was assessed using Q and I 2 statistics. For the Q statistic, P<0·1 was considered statistically significant, and for the I 2 statistic the following conventional cut-off points were used: <25 % (low heterogeneity), 25–50 % (moderate heterogeneity) and >75 % (severe heterogeneity). Both Begg’s rank correlation test and Egger’s linear regression test were performed to investigate potential publication bias( Reference Egger, Davey Smith and Schneider 30 ). If evidence of publication bias was observed, the trim and fill method was applied to correct the bias( Reference Duval and Tweedie 31 ).
To explore potential sources of heterogeneity, subgroup and meta-regression analyses were performed according to geographic region, duration of follow-up, sources of Ca, menopausal status and quality scores. To investigate the impacts of individual studies on the overall results, we also performed a sensitivity analysis by omitting one study in each turn while pooling results from the remainder. We performed a linear dose–response analysis examining the association between Ca intake and breast cancer risk according to the method proposed by Greenland & Longnecker( Reference Greenland and Longnecker 32 ) and Orsini et al.( Reference Orsini, Bellocco and Greenland 33 ). This method requires the number of cases and person-years and the risk estimates with their variance estimates for at least three quantitative exposure categories. For the studies that did not provide the number of cases and/or person-years in each exposure category, we estimated these data from the total number of cases and person-years. For each study, the median or mean level of intake for each category was assigned to each corresponding risk estimate. When the median or mean intake per category was not provided, we considered the midpoint of the upper and lower boundaries in each category as average intake. If the highest or lowest category was open-ended, we assumed the width of the interval to be the same as in the closest category. Forest plots of the linear dose–response meta-analysis were presented for RR for each 300 mg/d increment of Ca intake (the unit equivalent to Ca content in 250 ml or one serving of milk). Potential non-linear dose–response relationship between Ca intake and breast cancer risk was examined by modelling exposure levels using restricted cubic splines with three knots at percentiles 10, 50 and 90 % of the whole Ca distribution( Reference Orsini, Li and Wolk 34 ). The P value for non-linearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to 0. All statistical analyses were performed using the STATA software, version 11.0 (StataCorp. LP). All P values were two-sided, and the level of significance was at <0·05, unless explicitly stated.
Results
Study characteristics
A flow chart of study selection, including reasons for exclusion, is presented in Fig. 1. We included eleven studies( Reference Knekt, Järvinen and Seppänen 11 – Reference Lin, Manson and Lee 13 , Reference Park, Leitzmann and Subar 15 – Reference Abbas, Linseisen and Rohrmann 19 , Reference Shin, Holmes and Hankinson 35 – Reference Larsson, Bergkvist and Wolk 37 ) that fully met our inclusion criteria for this meta-analysis. The characteristics of the included studies are summarised in Table 1. These studies were published between 2002 and 2013, with a total of 26 606 breast cancer cases diagnosed among 872 895 participants. Ten studies were conducted in Western populations (five in the USA, one in Finland, one in France, one in Norway, one in Sweden and one in ten European countries), and one study consisted of Singaporean Chinese. The duration of follow-up ranged from 7 to 25 years. One study( Reference McCullough, Rodriguez and Diver 36 ) was conducted among postmenopausal women only, six studies( Reference Kesse-Guyot, Bertrais and Duperray 12 , Reference Lin, Manson and Lee 13 , Reference Hjartåker, Thoresen and Engeset 16 , Reference Li, Koh and Jin 17 , Reference Abbas, Linseisen and Rohrmann 19 , Reference Shin, Holmes and Hankinson 35 ) reported results by menopausal status and four studies( Reference Knekt, Järvinen and Seppänen 11 , Reference Park, Leitzmann and Subar 15 , Reference Genkinger, Makambi and Palmer 18 , Reference Larsson, Bergkvist and Wolk 37 ) combined premenopausal and postmenopausal breast cancer. One study( Reference Larsson, Bergkvist and Wolk 37 ) further reported results by oestrogen receptor (ER) and progesterone receptor (PR) status of the tumour( Reference Larsson, Bergkvist and Wolk 37 ). Either FFQ or 24-h recall was used as a dietary assessment tool. Ca intakes in the highest categories across studies ranged from >345 to >1750 mg/d, and the intakes in the lowest categories ranged from <203·2 to <807 mg/d. Most individual studies adjusted for a wide range of potential confounding factors, such as age, BMI, family history of breast cancer, hormone replacement therapy use and total energy intake. The details of quality assessment according to the nine-star NOS are presented in the online Supplementary Table S1. Nine of these studies were given scores of ≥7.
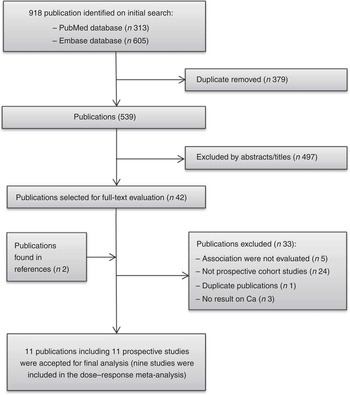
Fig. 1 Flow chart of study selection.
Table 1 Prospective cohort studies of calcium intake and breast cancer risk (Adjusted relative risks (RR) and 95 % confidence intervals)

PRM, premenopausal women; POM ,postmenopausal women; BBD, benign breast disease; HRT, hormone replacement therapy; ER/PR, oestrogen and progesterone receptors.
Calcium intake and breast cancer risk, high v. low intake
The combined multivariable-adjusted RR for the highest v. lowest Ca intake was 0·92 (95 % CI 0·86, 0·99) (online Supplementary Fig. S1), with evidence of moderate heterogeneity (P=0·026, I 2=44·2 %). Both the Begg’s rank correlation test and Egger’s linear regression test suggested the presence of publication bias (Begg, P=0·029; Egger, P=0·016). However, because no missing studies were detected to be filled, the results remained unchanged despite the fact that the trim and fill method was performed to correct the bias.
Subgroup and sensitivity analyses
The results of subgroup analyses stratified by geographic area, duration of follow-up, type of Ca intake, source of Ca, menopausal status and quality scores are presented in Table 2. The inverse association between Ca intake and breast cancer risk was not significantly affected by these factors (P difference >0·130). By menopausal status, the summary RR were 0·75 (95 % CI 0·59, 0·96) for premenopausal breast cancer and 0·94 (95 % CI 0·87, 1·01) for postmenopausal breast cancer. By sources of Ca, the summary RR were 0·93 (95 % CI 0·84, 1·03) for total Ca, 0·90 (95 % CI 0·84, 0·97) for dietary Ca and 0·98 (95 % CI 0·92, 1·03) for supplemental Ca. Few studies( Reference Kesse-Guyot, Bertrais and Duperray 12 , Reference Shin, Holmes and Hankinson 35 ) also reported results for dairy Ca intake, and the summary RR were 0·80 (95 % CI 0·53, 1·21) for dairy Ca and 1·00 (95 % CI 0·78, 1·29) for non-dairy Ca. Results of the sensitivity analysis indicated that the overall risk estimates were not dominated by any single study, with summary RR ranging from 0·90 (95 % CI 0·83, 0·98) to 0·93 (95 % CI 0·87, 0·99). The summary RR was 0·91 (95 % CI 0·84, 0·98) after an exclusion of the only Asian study. In addition, one study( Reference Larsson, Bergkvist and Wolk 37 ) reported results by ER and PR status of the tumour, and the inverse association of Ca intake with breast cancer risk appeared to be restricted to women with ER-negative and PR-negative tumours (RR 0·66; 95 % CI 0·44, 0·99).
Table 2 Subgroup analysis of breast cancer in relation to calcium intake (Relative risks (RR) and 95 % confidence intervals)
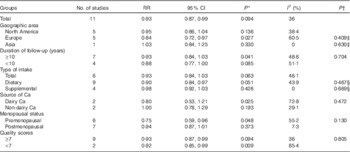
* P value for heterogeneity among studies.
† P value for heterogeneity between groups according to meta-regression.
‡ Studies conducted in the North America as a reference group.
§ P Total Ca intake data as a reference group.
Dose–response analysis
Two studies( Reference Knekt, Järvinen and Seppänen 11 , Reference Genkinger, Makambi and Palmer 18 ) that did not report sufficient data for the dose–response analysis were excluded, and the remaining nine studies were eligible to be included in this analysis. In the linear dose–response analysis (Fig. 2), the summary RR for every 300 mg/d increase in Ca intake was 0·98 (95 % CI 0·96, 0·99, P heterogeneity=0·123, I 2=30·8 %) for all women, without evidence of a non-linear relationship (P non-linearity=0·17), although the reduction in breast cancer risk appeared somewhat steeper in the lower range of Ca intake (<800 mg/d) than in the higher range (online Supplementary Fig. S2). By menopausal status (Fig. 2), the summary RR were 0·92 (95 % CI 0·87, 0·98) for premenopausal breast cancer and 0·98 (95 % CI 0·97, 0·99) for postmenopausal breast cancer. By sources of Ca, the summary RR were 0·97 (95 % CI 0·95, 0·98) for dietary Ca and 0·99 (95 % CI 0·97, 1·01) for supplemental Ca (online Supplementary Fig. S3).

Fig. 2 Dose–response meta-analysis of calcium intake (per 300 mg/d) and breast cancer risk. RR, relative risks.
Discussion
The present meta-analysis of eleven prospective studies supports an inverse association between Ca intake and breast cancer. Dose–response analysis revealed that each 300 mg/d increase in Ca intake was significantly associated with 2, 8 and 2 % reduced risk of total, premenopausal and postmenopausal breast cancer, respectively.
Although the exact mechanisms by which Ca may reduce the risk of breast cancer remain unclear, the ability of Ca in regulating cell proliferation, differentiation and apoptosis makes it biologically plausible as a potential protective factor against breast cancer( Reference Whitfield, Boynton and MacManus 5 – Reference Sergeev 7 ). Evidence from animal studies suggests that Ca has anti-proliferative and pro-differentiation actions on mammary gland cells of rats fed the high-fat diet, and can reduce the incidence of mammary tumours in rats( Reference Jacobson, James and Newmark 8 , Reference Abou-Issa, Moeschberger and el-Masry 9 ). Much of the evidence indicates that the anti-carcinogenic potential of Ca relies on its interrelation and correlation with vitamin D. However, experimental evidence suggests that an increased level of Ca alone is sufficient to trigger apoptosis( Reference Kumar, Kumar and Ghosh 10 ). Increased risk of breast cancer has been linked with several chronic diseases such as diabetes, obesity and the metabolic syndrome( Reference Porto, Lora and Soares 38 – Reference Eliassen, Colditz and Rosner 41 ), all of which have been suggested to be inversely associated with Ca intake( Reference Moore-Schiltz, Albert and Singer 42 – Reference Lee, Cho and Lee 44 ). Therefore, Ca intake may indirectly be associated with lower breast cancer risk through its association with these disorders.
With additional five large prospective studies( Reference Park, Leitzmann and Subar 15 – Reference Abbas, Linseisen and Rohrmann 19 ) included and comprehensive analyses conducted, our findings are generally consistent with those from the previous meta-analysis( Reference Chen, Hu and Xie 14 ), and thereby further support a potentially beneficial role of Ca in the development of breast cancer. Indeed, a possible U-shaped association between Ca intake and health outcomes has been widely considered( Reference Larsson, Orsini and Wolk 45 , Reference Wang, Chen and Ouyang 46 ) Numerous studies also suggested that a high intake of Ca, particularly from supplements, may be associated with increased risks of CVD( Reference Abbas, Linseisen and Rohrmann 19 – Reference Li, Kaaks and Linseisen 23 ) and kidney stones( Reference Pentti, Tuppurainen and Honkanen 24 , Reference Jackson, LaCroix and Gass 25 ). Given concerns about adverse risks of ingesting a high dose of Ca on health, the maximum daily Ca intake from diet and/or dietary supplement should be carefully considered( 47 ). Results of our dose–response analysis showed that the inverse association of Ca intake with breast cancer risk remained when the intake was up to 1900 mg/d.
The inverse relation between Ca intake and breast cancer appeared to be stronger in the dietary Ca group (RR 0·90; 95 % CI 0·84, 0·97; n 9) than in the supplemental Ca group (RR 0·98; 95 % CI 0·92, 1·03; n 6). There were several explanations for these findings. First, it is possible that the interactions between Ca and other nutrient components in diets, such as vitamin D, conjugated linoleic acids and SFA, are necessary for Ca to exert its protection on breast cancer( Reference Dong, Zhang and He 48 ). Second, it is possible that the benefits of Ca may be restricted to the individuals with Ca deficiencies, and Ca supplementation may not bring additional benefits for those who have consumed enough Ca from foods. Third, it is also possible that the observed inverse association may be partly or completely explained by other beneficial nutrients (potential confounders) that share similar food sources with Ca.
Furthermore, the findings from our meta-analysis were consistent with previous meta-analysis showing that inverse association of Ca intake with breast cancer risk is limited to premenopausal women( Reference Chen, Hu and Xie 14 ). To date, convincing explanations for the menopause-related difference in the association of Ca intake with the risk of breast cancer have not yet been established. We considered several possible explanations for this difference. First, the complex interactions among Ca, vitamin D and insulin-like growth factors may promote growth inhibition in breast cancer cells( Reference Lin, Manson and Lee 13 , Reference Allen, Roddam and Allen 49 ). Second, Ca may serve as a potential regulator in oestrogen-driven cell proliferation( Reference Liu, Hu and Chakrabarty 50 ). Third, as Ca inadequacy is more prominent in postmenopausal women( Reference Malabanan and Holick 51 , Reference Meng, Kerr and Zhu 52 ), it is also possible that the beneficial effects of Ca in postmenopausal women might only occur in higher doses.
Strengths and limitations
The present study has several strengths, including incorporated evidence and relevant studies to the date. The enlarged sample size enhanced the power to detect a significant difference and provide more precise estimates of the effects. Most of the original studies included are of long follow-up durations, and all studies used a prospective design, which thereby reduced the likelihood of potential biases (e.g. recall and selection biases). We quantified the association between intake of Ca and risk of breast cancer by carrying out linear and non-linear dose–response analyses. Given the considerably distinct levels of the intake among different populations, a dose–response meta-analysis is necessary in addition to the comparison of the highest v. lowest categories of intake.
There are several potential limitations that are worthy of consideration in this meta-analysis. First, there was evidence of publication bias. Although the results did not change after using statistical methods to correct the bias, findings based on evidence of published data should always be interpreted with caution. Second, the strong interrelationship between Ca intake and vitamin D intake makes it difficult to identify the true effects of Ca intake on breast cancer risk as an independent variable in observational studies. Third, the present meta-analysis was unable to assess breast cancer subtypes by hormone receptor status because of the limited studies available. In clinical course of breast cancer, hormonal status is very important for predicting prognosis and efficacy of chemotherapy; thereby, it may also be important to assess whether Ca intake and risk of breast cancer is modified by ER/PR status of the tumour. Fourth, there was moderate heterogeneity across studies. The heterogeneity may be because of the variation in exposure definitions, exposure ranges, dietary assessment methods or population characteristics among studies. Our further analyses indicated that menopausal status was a major potential contributor to the variation in the strength of the association. Finally, although individual studies have considered a wide range of potential confounders in their analyses, the potential impacts of residual/unknown confounding factors on our findings cannot be completely excluded.
Conclusion
In summary, results from this meta-analysis of eleven prospective cohort studies suggest an inverse dose–response association between Ca intake and breast cancer. Additional large prospective studies focusing on the influence of hormone receptor status on this association are necessary to confirm our findings, and such studies would also be helpful for exploring potential mechanisms whereby Ca may reduce breast cancer.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
K. H. and G.-C. C. contributed to the study design, literature search, data extraction and data analyses. K. H. wrote the paper. R. Z. assisted with literature selection. R. Z., X. D. and S.-Y. Z. provided statistical support and created all tables and figures. L.-Q. Q. and B.-M. S. critically reviewed the manuscript for important intellectual content. All authors read and approved the final manuscript.
There are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/10.1017/S0007114516001768







