Healthy human pregnancy is associated with a number of changes in the maternal physiology and metabolism, which are closely linked to the nutritional needs of mother and foetus. However, available knowledge is limited regarding the physiological basis of these changes. In this context, the so-called adipokines (i.e. leptin, adiponectin and resistin) are of interest due to evidence suggesting that these hormones are involved in the regulation of food intake, body fatness, energy expenditure and insulin resistance(Reference Arner1–Reference Zavalza-Gómez, Anaya-Prado and Rincón-Sánchez4), i.e. variables well known to be affected during pregnancy and important in relation to the nutritional situation of women during gestation. A number of studies on pregnant women have been published regarding serum concentrations of these adipokines(Reference Highman, Friedman and Huston5–Reference Palik, Baranyi and Melczer8) as well as the relationship between such concentrations, on the one hand, and energy expenditure, body fatness and insulin resistance on the other hand. However, as indicated below, many of these studies included women with gestational diabetes, while others were conducted using less appropriate methods to assess body fatness. The value of studies with these limitations may be questionable when the purpose is to understand the physiological and biological basis for the kind of changes during pregnancy that are mentioned above. To adequately assess the impact of the maternal nutritional situation on offspring growth, development and health, such understanding is a requirement.
Serum concentrations of leptin correlate with the total body fat (TBF) content in pregnant(Reference Highman, Friedman and Huston5) and non-pregnant individuals(Reference Highman, Friedman and Huston5, Reference Silha, Nyomba and Leslie9, Reference Considine10) and with insulin resistance in non-pregnant women(Reference Silha, Nyomba and Leslie9). A correlation between leptin in serum and insulin resistance has also been found in a group of pregnant women including women with gestational diabetes(Reference McLachlan, O'Neal and Jenkins11) and in healthy women in the first trimester(Reference Mastorakos, Valsamakis and Papatheodorou12). Results from studies regarding relationships between serum concentrations of adiponectin and body fatness are contradictory in pregnant(Reference Fuglsang, Skjaerbaek and Frystyk6, Reference Mazaki-Tovi, Kanety and Pariente7, Reference Vitoratos, Delivelotou and Vlahos13) and non-pregnant(Reference Silha, Nyomba and Leslie9, Reference Jürimäe and Jürimäe14) women, probably because body fatness is not an independent predictor of adiponectin in serum(Reference Jürimäe and Jürimäe14). An inverse correlation between serum adiponectin and insulin resistance has been found in non-pregnant women(Reference Silha, Nyomba and Leslie9, Reference Jürimäe and Jürimäe14, Reference Gavrila, Chan and Yiannakouris15) and in groups of pregnant women including subjects with gestational diabetes(Reference Vitoratos, Delivelotou and Vlahos13, Reference Altinova, Toruner and Bozkurt16). With respect to resistin, Silha et al. (Reference Silha, Nyomba and Leslie9) found no correlation between body fatness and serum concentrations in non-pregnant women. We found no reports on relationships between resistin in serum and TBF or homeostatic model of insulin resistance (HOMA-IR) in pregnant women.
We previously assessed body fat and energy metabolism in healthy women in a longitudinal study covering a reproductive cycle and using a valid method to assess body composition(Reference Löf and Forsum17, Reference Löf and Forsum18). This provided an opportunity to explore relationships before, during and after pregnancy between energy expenditure, TBF, insulin resistance and serum concentrations of leptin, adiponectin and resistin. Results from such a study have not been reported previously.
Body fatness is known to be linked to insulin resistance, and Silha et al. (Reference Silha, Nyomba and Leslie9) found a significant relationship between TBF and HOMA-IR in non-pregnant women. This relationship is apparently also important during pregnancy, since gestational diabetes is more common in obese than in lean women(Reference King19). The design and methodology used in the present study provided an opportunity to study the effect of pregnancy on the relationship between TBF and HOMA-IR.
Materials and methods
Study outline
The women arrived at the hospital in the morning for collection of blood samples and for the assessment of body weight, body composition and BMR. Blood sampling and recording of body weight were conducted before pregnancy in gestational weeks 8, 14, 20, 32 and 35, and 2 weeks postpartum, while body composition and BMR were assessed before pregnancy in gestational weeks 14 and 32 and 2 weeks postpartum. BMR was assessed using indirect calorimetry as previously described(Reference Löf and Forsum18). The study was conducted according to the guidelines laid down in the declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the University of Linköping, Sweden. Verbal informed consent, witnessed and formally recorded, was obtained from all subjects.
Subjects
Twenty-three women aged 30 (sd 4) years and planning pregnancy were recruited through the health care system, or by advertising in the local press(Reference Löf and Forsum18). Before pregnancy, all the women were healthy with normal blood glucose (4·2 (sd 0·5) mmol/l) and serum insulin (7·4 (sd 2·4) mU/l) concentrations. Their dietary intake was assessed before pregnancy using three 24 h dietary recalls as previously described(Reference Löf and Forsum20). None of the women developed gestational diabetes. Two women had BMI values >30 kg/m2 before conception. The women conceived 8–524 d after the pre-pregnant measurement, and gestational age was calculated on the basis of an ultrasound examination in gestational week 12–14. During pregnancy, they gained 18 (sd 7) kg of body weight. They all delivered one healthy baby (gestational age 280 (sd 10) d) with a birth weight of 3740 (sd 510) g. Information about birth weight was obtained from hospital records. The women were asked to come to the hospital in a fasting state for all measurements, and upon arrival they were asked when they had last eaten any food. Three women reported not being in a strictly fasting state on one or two measurement occasions. We were able to assess blood glucose and serum insulin in seventeen (the HOMA-IR subgroup) out of the twenty-three women before pregnancy in gestational weeks 14 and 32 and 2 weeks postpartum. Women in this subgroup were all in the fasting state on these measurement occasions.
Body composition
Body weight of the women was recorded using the KCC150 scale (Mettler-Toledo, Albstadt, Germany). Total body water was assessed using 2H dilution as previously described(Reference Löf and Forsum17). To calculate fat-free mass, total body water was divided by the appropriate hydration fraction, which was 0·718 before pregnancy, 0·723 at week 14, 0·747 at week 32 and 0·734 at postpartum(Reference Löf and Forsum17). These figures were assessed in seventeen out of the twenty-three women using data for body weight, total body water and body volume assessed by means of underwater weighing(Reference Löf and Forsum17). TBF was calculated as body weight minus fat-free mass.
Analyses of blood and serum sample
Blood was collected from a cubital vein and its glucose content was measured colorimetrically using the HemoCue analyser (HemoCue AB, Ängelholm, Sweden). The blood was kept at +4°C for 4 h and thereafter centrifuged at 1500 g for 10 min. Serum was harvested and stored at − 70°C. Serum insulin was measured by means of RIA (Pharmacia Insulin RIA 100, Pharmacia & UpJohn Diagostics, Uppsala, Sweden). Insulin resistance (HOMA-IR) was calculated according to Matthews et al. (Reference Matthews, Hosker and Rudenski21). Serum glucose concentration was estimated by multiplying the blood glucose concentration by 1·11. Adipokines were analysed using kits from Linco Research (St Charles, MO, USA) i.e. the human leptin ELISA kit (catalogue no. EZHL-80SK), the human adiponectin ELISA kit (catalogue no. EZHADP-61K) and the human resistin ELISA kit (catalogue no. EZHR-95K). According to the manufacturer, these ELISA kits measure leptin in free and bound forms, all multimeric forms of adiponectin and both hexameric and trimeric forms of resistin, respectively. However, for resistin, it has not been possible to definitely confirm which isoforms the assay actually measures.
Statistics
Values are given as means and standard deviations. Significant differences were identified using multiple regression analysis with TBF (%) and individual variables for stage of gestation as indicator variables. Interaction between TBF (%) and stage of gestation was used to test whether the effect of TBF (%) on the dependent variable differed significantly between different stages of gestation. This test was followed by a Bonferroni correction to adjust for multiple comparisons. ANOVA followed by Tukey's test was also used to identify significant differences. Non-normally distributed variables were log transformed before ANOVA. Linear regression analysis was also used. When neither the dependent nor the independent variable was normally distributed, Spearman's r is given. Otherwise Pearsons's r is reported. Significance was accepted when P < 0·05. Statistical analyses were carried out using Statistica software, version 7.1 (StatSOFT, Scandinavia AB, Uppsala, Sweden) or Minitab, version 15 (Minitab Inc., State College, PA, USA).
Results
Characteristics of women and serum concentration of adipokines
Table 1 shows body weight, body fat and BMR before, during and after pregnancy for all twenty-three women in the study. The corresponding values for the seventeen women in the HOMA-IR subgroup did not differ in any important way from those shown in Table 1. Table 2 shows the dietary intake of the women before pregnancy. Table 3 shows blood glucose, serum insulin and HOMA-IR before, during and after pregnancy for women in the HOMA-IR subgroup. Table 4 shows serum concentrations of leptin, adiponectin and resistin before, during and after pregnancy for all twenty-three women in the study. These results for individual women are shown in Fig. 1.
Table 1 Body weight, total body fat and BMR before and during pregnancy and postpartum for the twenty-three women in the study
(Mean values and standard deviations)

Table 2 Daily intakes of energy and some nutrients by twenty-three healthy Swedish women before pregnancy
(Mean values and standard deviations)
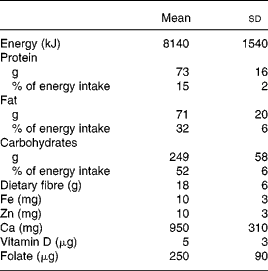
Table 3 Blood glucose, serum insulin and homeostasic model of insulin resistance (HOMA-IR) before pregnancy in gestational weeks 14 and 32 and 2 weeks postpartum in seventeen Swedish women (the HOMA-IR subgroup)
(Mean values and standard deviations)
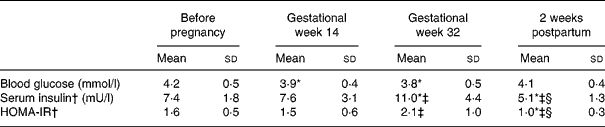
* Significantly different (P < 0·05) from the corresponding value before pregnancy using ANOVA followed by Tukey's test.
† Values were log transformed before ANOVA.
‡ Significantly different (P < 0·05) from the corresponding value in gestational week 14 using ANOVA followed by Tukey's test.
§ Significantly different (P < 0·05) from the corresponding value in gestational week 32 using ANOVA followed by Tukey's test.
Table 4 Serum concentrations of leptin, adiponectin and resistin before and during pregnancy and postpartum in twenty-three Swedish women
(Mean values and standard deviations)
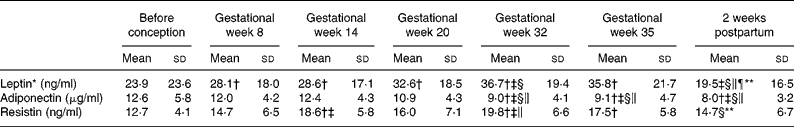
* Values were log transformed before ANOVA.
** Significantly different (P < 0·05) from the corresponding value in gestational week 35 using ANOVA and Tukey's test.
† Significantly different (P < 0·05) from the corresponding value before pregnancy using ANOVA and Tukey's test.
‡ Significantly different (P < 0·05) from the corresponding value in gestational week 8 using ANOVA and Tukey's test.
§ Significantly different (P < 0·05) from the corresponding value in gestational week 14 using ANOVA and Tukey's test.
∥ Significantly different (P < 0·05) from the corresponding value in gestational week 20 using ANOVA and Tukey's test.
¶ Significantly different (P < 0·05) from the corresponding value in gestational week 32 using ANOVA and Tukey's test.

Fig. 1 Serum concentrations of leptin (a), adiponectin (b) and resistin (c) for twenty-three women before pregnancy in gestational weeks 8, 14, 20, 32 and 35 and 2 weeks postpartum. Each subject is represented by one line.
Leptin, total body fat, homeostasic model of insulin resistance and BMR
Fig. 2 shows results obtained when leptin in serum was regressed on TBF (%) before, during and after pregnancy. The ratio between serum leptin and TBF (%) is also given. TBF (%) was found to be significantly correlated with serum leptin before pregnancy in gestational weeks 14 and 32 as well as postpartum. The equations obtained before pregnancy in gestational weeks 14 and 32 as well as postpartum when serum leptin was regressed on TBF (%) are also given in Fig. 2. The slopes of these regression lines (2·11–2·66) showed little variation between measurements and were not significantly different. However, the leptin/TBF ratio increased significantly during pregnancy, especially in gestational week 32. Leptin in serum was significantly correlated with HOMA-IR before pregnancy and in gestational weeks 14 and 32 (n 17, r 0·53–0·70, 0·002 < P < 0·05), but not postpartum. The increase in TBF (4·4 (sd 3·6) kg, n 23) during the first 32 weeks of pregnancy correlated significantly (r 0·58, P < 0·01) with the increase in serum leptin (12·7 (sd 16·1) ng/ml, n 23) during this part of pregnancy. Furthermore, the change in TBF between the measurements postpartum and before pregnancy (4·1 (sd 3·8) kg, n 23) correlated significantly (r 0·52, P < 0·05) with the corresponding change in serum leptin ( − 4·4 (sd 14·8) ng/ml, n 23). The increase in TBF (1·4 (sd 2·6) kg, n 23) during the first 14 weeks of pregnancy did not correlate with the increase in serum leptin (4·6 (sd 11·8) ng/ml) during this part of gestation. Leptin concentrations in serum were neither correlated with the BMR (kJ/24 h) of the women, nor with BMR/fat-free mass (kJ/24 h per kg) at any of the measurement occasions before, during or after pregnancy.

Fig. 2 Relationships between leptin in serum (ng/ml) (y) and total body fat (TBF) (%) (x) before pregnancy (a) in gestational weeks 14 (b) and 32 (c) as well as 2 weeks postpartum (d) in twenty-three Swedish women. The linear regressions are: y = 2·66x − 62·9, r 0·88 (P < 0·001) (a); y = 2·11x − 42·1, r 0·87 (P < 0·001) (b); y = 2·26x − 38·4, r 0·85 (P < 0·001) (c); y = 2·33x − 63·0, r 0·85 (P < 0·001) (d). Leptin (ng/ml)/TBF (%) was 0·64* (sd 0·36; before pregnancy), 0·80† (sd 0·35; gestational week 14), 1·04 (sd 0·40; gestational week 32) and 0·50‡ (sd 0·46; 2 weeks postpartum). Using ANOVA followed by Tukey's test, the following significant (P < 0·05) differences were found: * different from gestational weeks 14 and 32; † different from gestational week 32 and 2 weeks postpartum; ‡ different from gestational week 32.
Adiponectin, total body fat and homeostasic model of insulin resistance
Neither TBF (%) nor TBF (kg) correlated with adiponectin in serum at any of the measurement occasions before, during or after pregnancy. However, adiponectin in serum was negatively correlated with HOMA-IR in gestational week 32 (n 17, r − 0·52, P < 0·05). Concentrations of adiponectin in serum and of glucose in blood (mmol/l) were correlated before pregnancy (n 17, r − 0·57, P < 0·05), but not during or after pregnancy. The changes in serum adiponectin (μg/ml) were not correlated with the corresponding changes in TBF (kg) during the first 14 or 32 weeks of pregnancy, or with changes between the measurements postpartum and before pregnancy.
Resistin, total body fat and homeostasic model of insulin resistance
Serum concentrations of resistin did not correlate with TBF (%) or TBF (kg) either before pregnancy or in gestational weeks 14 or 32 or postpartum. Resistin in serum did not correlate with HOMA-IR (n 17) either before pregnancy or in gestational weeks 14 or 32 or postpartum. The changes in serum resistin (ng/ml) were not correlated with the corresponding changes in TBF (kg) during the first 14 or 32 weeks of pregnancy, or between the postpartum measurements and the measurements before pregnancy.
Homeostasic model of insulin resistance and total body fat
Fig. 3 shows regression analysis with HOMA-IR as the dependent variable and TBF (%) as the independent variable before pregnancy in gestational weeks 14 and 32 and postpartum. These variables were significantly correlated before pregnancy in gestational weeks 14 and 32, but not postpartum. Furthermore, the slope of the regression line in gestational week 32 (0·111) was significantly steeper than the corresponding value (0·046) before pregnancy.

Fig. 3 Relationships between (homeostasis model assessment of insulin resistance HOMA-IR) (y) and total body fat (TBF) (%) (x) before pregnancy (a) in gestational weeks 14 (b) and 32 (c) as well as 2 weeks postpartum (d) in seventeen Swedish women. The linear regressions are: y = 0·046x+0·10, r 0·678 (P < 0·01) (a); y = 0·067x − 0·75, r 0·788 (P < 0·001) (b); y = 0·111*x − 1·46, r 0·789 (P < 0·001) (c); y = 0·024x+0·17, r 0·456 (P>0·05) (d); * significantly (P < 0·05) different from the corresponding values obtained before pregnancy and 2 weeks postpartum using multiple regression analysis followed by a Bonferroni correction. (HOMA-IR/TBF (%)) 100 was 4·89† (sd 1·21; before pregnancy), 4·35† (sd 1·22; gestational week 14), 6·37‡ (sd 2·08; gestational week 32) and 2·93 (sd 0·88; 2 weeks postpartum). Using ANOVA followed by Tukey's test, the following significant (P < 0·05) differences were found: † different from gestational week 32 and 2 weeks postpartum; ‡ different from 2 weeks postpartum.
Discussion
Although our subjects were not randomly selected, they were in many respects (BMI before pregnancy(Reference Bostrom22) and increases in TBF and BMR during pregnancy(23)) comparable with other Western women. The intake of energy and nutrients of our women was very similar to that reported for other Swedish women of comparable age(Reference Becker and Pearson24). The dietary intake of our women was also in agreement with that recently recorded for 259 Swedish pregnant women (M Löf, unpublished results). Three women in the present study had ingested a small amount of nutrients before blood sampling. Excluding these women from the analysis did not change the results and conclusions of the present study.
The serum concentrations of leptin and the findings that such concentrations are correlated with body fatness before, during and after pregnancy, as observed in the present study, are consistent with previous results(Reference Highman, Friedman and Huston5, Reference Butte, Hopkinson and Nicolson25). However, significant relationships between HOMA-IR and serum leptin before pregnancy as well as in gestational weeks 14 and 32 have not been reported previously in one group of healthy women. Nor have correlations between changes in body fat and changes in serum leptin during pregnancy been identified before. Highman et al. (Reference Highman, Friedman and Huston5) were unable to find such correlations possibly due to the small size of their study. Bajoria et al. (Reference Bajoria, Sooranna and Ward26) considered that factors other than fat mass alone, such as leptin production by the placenta(Reference Zavalza-Gómez, Anaya-Prado and Rincón-Sánchez4), cause the increased serum leptin concentrations in human pregnancy. This suggestion is supported by the fact that leptin concentrations in serum decrease after delivery. However, Bajoria et al. (Reference Bajoria, Sooranna and Ward26) considered that this suggestion was also supported by the lack of correlations between changes in BMI and serum leptin during pregnancy, and by the finding that during gestation, increases in serum leptin predate the increase in adipose tissue mass. Our data show that serum leptin was already increased in gestational week 8, when only very small increases in body fat can have occurred and when the placenta is not yet fully established. Also, during pregnancy, changes in TBF were correlated with changes in serum leptin. Our findings thus tend to suggest that the increased serum concentration of leptin during pregnancy is not only due to the increased fat mass and leptin production by the placenta, but may also have other as yet unknown explanations. Bajoria et al. (Reference Bajoria, Sooranna and Ward26) and Zavalza-Gómez et al. (Reference Zavalza-Gómez, Anaya-Prado and Rincón-Sánchez4) suggested that a state of leptin resistance develops during pregnancy. We demonstrated that the slopes of regression lines relating TBF (%) and leptin in serum showed only small and non-significant differences when comparing values obtained before, during and after pregnancy. These observations do not support the concept of leptin resistance during pregnancy. A well-established role of leptin is its participation in the regulation of energy homeostasis, with effects on food intake and energy expenditure(Reference Henry and Clarke3). However, we found no relationships between serum concentrations of leptin and BMR. Highman et al. (Reference Highman, Friedman and Huston5) investigated relationships between serum leptin and oxygen consumption before and during pregnancy. Their observations suggested a role for leptin in regulating the changes in energy expenditure occurring during pregnancy. In the present study, we were unable to find support for such a role for leptin.
Our data regarding serum concentrations of adiponectin during pregnancy are in agreement with previous reports(Reference Fuglsang, Skjaerbaek and Frystyk6, Reference Mazaki-Tovi, Kanety and Pariente7). We have not been able to find any study in healthy pregnant women reporting a negative correlation between serum adiponectin and HOMA-IR. In the present study of women with an insulin resistance typical for healthy women, we found no correlations between TBF and serum adiponectin, or between changes during pregnancy in these two variables, confirming the assertion that body fatness is not an independent predictor of adiponectin in serum(Reference Jürimäe and Jürimäe14). Regarding serum concentrations of resistin during pregnancy, the present results were not in complete agreement with previously published data(Reference Palik, Baranyi and Melczer8, Reference Chen, Dong and Fang27). We were not able to identify any significant relationships between serum concentrations of resistin, on the one hand, and the TBF content or HOMA-IR, on the other hand, before, during or after pregnancy. Cortelazzi et al. (Reference Cortelazzi, Corbetta and Ronzoni28) were of the opinion that resistin seems to have a minor role in relation to insulin resistance during pregnancy and our data support this conclusion.
The present results show that body fatness and insulin resistance are related in pregnant and non-pregnant healthy women, and that pregnancy affects the slope of the regression line obtained when HOMA-IR is regressed on TBF (%). Thus, a woman in the second half of pregnancy tends to be more insulin resistant than a non-pregnant woman with the same TBF content (%). A high insulin resistance during pregnancy tends to direct glucose to the foetus rather than to maternal tissues. Thus, it may be advantageous for the foetus if the mother gains fat during pregnancy, since this helps it to compete favourably for available nourishment. Note that our data demonstrate this enhancing effect of pregnancy in healthy non-diabetic women. We consider this to be a new and interesting observation. Previous investigators(Reference Mastorakos, Valsamakis and Papatheodorou12) have pointed out that body fat retention may be a causal factor for the decrease in insulin sensitivity during normal pregnancy and the relationship between body fat and insulin resistance is well established. The new finding in the present paper is that healthy pregnancy enhances this relationship. The mechanism behind this enhancing effect is unknown. Our data suggest that adiponectin may have a role for the physiological increase in insulin resistance during pregnancy. Published data(Reference Antuna-Puente, Feve and Fellahi2) indicate that leptin is also involved when insulin resistance is established. Therefore, it is interesting to note that serum concentrations of leptin correlated with HOMA-IR in our healthy women before and during pregnancy. This information together with the well-established relationship between leptin and body fatness makes it relevant to propose that leptin has a role regarding the enhancing effect of pregnancy on the relationship between body fatness and insulin resistance as identified in the present study.
The present findings can be reconciled with data showing a relationship between the maternal TBF content and birth weight(Reference Forsum, Löf and Olausson29) and between the maternal pre-pregnant BMI and birth weight(Reference Baker, Michaelsen and Rasmussen30). These reports suggest that a high-fat content of the mother stimulates foetal growth. Our observation regarding an enhancing effect of pregnancy on the relationship between body fatness and insulin resistance may represent an important physiological explanation for the strong tendency by pregnant women to retain body fat. It is conceivable that the maternal fat content has a role in the regulation of foetal growth, which could be very useful biologically since fat mothers tend to live in an environment where food is plentiful, while the opposite is true for lean mothers. Body size is an important factor determining the amount of dietary energy required by man and other mammals. We speculate that the present results represent a mechanism by which offspring size is regulated in response to the availability of dietary energy in the environment in which the mother is living.
Acknowledgements
The present study was designed by E. F. and conducted by M. L. and H. O. B. E. was responsible for the analysis of adipokines and compiled the data in the manuscript. B. E. and E. F. wrote the manuscript that was reviewed by the other authors. The authors' thanks go to all the women in the study, to Anna Christina Granath for help with the analysis of adipokines and to Olle Eriksson for help with statistical analysis. None of the authors had any conflict of interest. The present study was supported by the Swedish Research Council projects no. 12 172 and 15 402 and the County Council of Östergötland.









